Automated Detection of Tetranychus urticae Koch in Citrus Leaves Based on Colour and VIS/NIR Hyperspectral Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaf Samples
2.2. Detection of T. urticae Damage Using Colour Imaging
2.2.1. Colour Image Acquisition and Segmentation
2.2.2. Definition of the Discrimination Parameters
- The number of damaged areas detected per leaf.
- The total damaged area (mm2) of the leaf, as the sum of the areas of all the objects found.
- The area (A), roundness (R), compactness (C), perimeter (P), and elongation (E) of each object found in the leaf.
2.3. Detection of T. urticae Damage Using Hyperspectral Imaging
2.3.1. Hyperspectral Image Acquisition
2.3.2. Multivariate Data Analysis
2.3.3. Model Performance Evaluation
3. Results and Discussion
3.1. Detection of T. urticae Damage Using Colour Imaging
3.1.1. Discrimination Parameters
3.1.2. Detection of the Damage
3.2. Detection of T. urticae Damage Using Hyperspectral Imaging
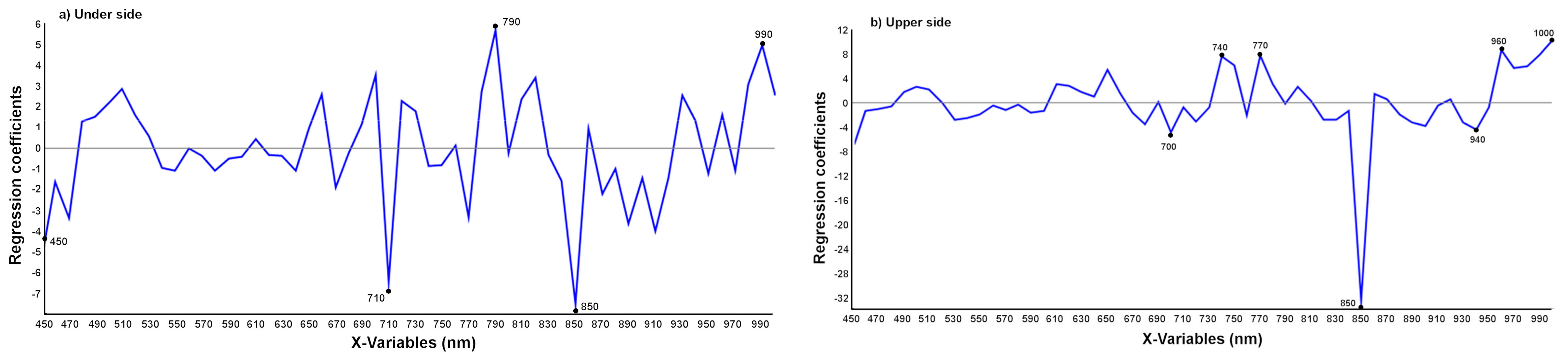
3.2.1. Detection of T. urticae Damage
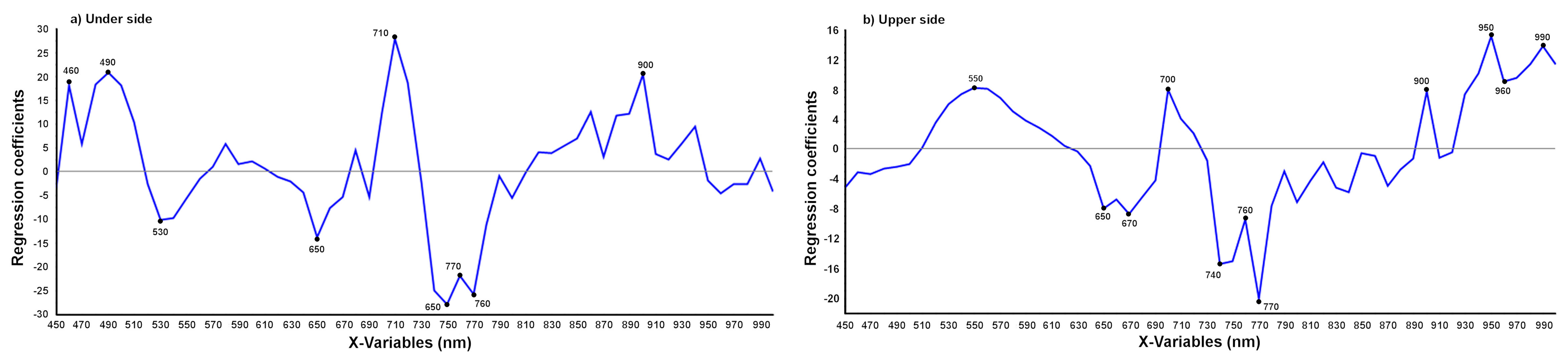
3.2.2. Detection of the Age of the T. urticae Damage
3.2.3. Discrimination between T. urticae and Other Damage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| C | Calibration |
| CV | Cross-validation |
| CCD | Charge-coupled device |
| LDA | Linear discriminant analysis |
| LV | Latent variables |
| NIR | Near-infrared |
| NP/NND | No pest/No nutritional deficiencies |
| P | Prediction |
| P/ND | Pest/Nutritional deficiencies |
| PC | Principal component |
| PCA | Principal component analysis |
| P.citrella | Phyllocnistis citrella |
| PLS | Partial least squares |
| PLS-DA | Partial least squares discriminant analysis |
| R2 | Coefficient of determination |
| RGB | Red–Green–Blue |
| RMSE | Root mean square error |
| ROI | Region of interest |
| T.urticae | Tetranychus urticae |
| VIS | Visible |
| VNIR | Visible and near infrared |
References
- FAOSTAT. Food and agriculture organisation of the United Nations 2021. Available online: http://faostat.fao.org (accessed on 2 May 2021).
- Hare, J.D. Sampling Arthropod Pests in Citrus. In Handbook of Sampling Methods for Arthropods in Agriculture; Pedigo, L.P., Buntin, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 417–431. [Google Scholar]
- Jacas, J.A.; Urbaneja, A. Biological Control in Citrus in Spain: From Classical to Conservation Biological Control. In Integrated Management of Arthropod Pests and Insect Borne Diseases; Springer Science and Business Media LLC: Berlin/Heisenberg, Germany, 2010; pp. 61–72. [Google Scholar]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef]
- Martínez-Ferrer, M.T.; Jacas, J.A.; Ripollés-Moles, J.L.; Aucejo-Romero, S. Approaches for Sampling the Twospotted Spider Mite (Acari: Tetranychidae) on Clementines in Spain. J. Econ. Èntomol. 2006, 99, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Aucejo, S.; Foó, M.; Ramis, M.; Troncho, P.; Gómez-Cadenas, A.; Jacas, J.A. Evaluación de la eficacia de algunos acaricidas contra la araña roja, Tetranychus urticae Koch (Acari: Tetranychidae), en clementino. Bol. San. Veg. Plagas. 2003, 29, 453–459. [Google Scholar]
- Ansaloni, T.; Pascual-Ruiz, S.; Hurtado, M.A.; Jacas, J.A.; Jaques, J.A. Can summer and fall vegetative growth regulate the incidence of Tetranychus urticae Koch on clementine fruit? Crop. Prot. 2008, 27, 459–464. [Google Scholar] [CrossRef]
- Fenollosa, E.A.; Gual, M.V.I.; Ruiz, M.H.; Hurtado, M.; Jacas, J. Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): Bottom-up regulation mechanisms. Biol. Control. 2011, 59, 158–170. [Google Scholar] [CrossRef]
- Aucejo-Romero, S.; Gómez-Cadenas, A.; Jacas-Miret, J.-A. Effects of NaCl-stressed citrus plants on life-history parameters of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2004, 33, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Ruiz, S.; Aguilar-Fenollosa, E.; Ibáñez-Gual, V.; Hurtado-Ruiz, M.A.; Martínez-Ferrer, M.T.; Jacas, J.A.; Jaques, J.A. Economic threshold for Tetranychus urticae (Acari: Tetranychidae) in clementine mandarins Citrus clementina. Exp. Appl. Acarol. 2013, 62, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja, A.; Pascual-Ruiz, S.; Pina, T.; Abad-Moyano, R.; Vanaclocha, P.; Montón, H.; Dembilio, Ó.; Castañera, P.; Jacas, J.A.; Jaques, J.A. Efficacy of five selected acaricides against Tetranychus urticae (Acari: Tetranychidae) and their side effects on relevant natural enemies occurring in citrus orchards. Pest Manag. Sci. 2008, 64, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Fonte, A.; Garcerá, C.; Tena, A.; Chueca, P. CitrusVol Validation for the Adjustment of Spray Volume in Treatments against Tetranychus urticae in Clementines. Agronomy 2019, 10, 32. [Google Scholar] [CrossRef]
- Soler-Salcedo, E.; Rodrigo, E.; Ferragut, F. Colonización, comportamiento alimenticio y producción de daños en las arañas rojas Tetranychus urticae y T. turkestani (Acari: Tetranychidae). Boletín Sanid. Veg. Plagas 2006, 32, 523–534. [Google Scholar]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA, 1975. [Google Scholar]
- Chueca, P.; Garcerá, C.; Moltó, E.; Jacas, J.A.; Urbaneja, A.; Pina, T. Spray Deposition and Efficacy of Four Petroleum-Derived Oils Used Against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Èntomol. 2010, 103, 386–393. [Google Scholar] [CrossRef]
- Rey, B.; Aleixos, N.; Cubero, S.; Blasco, J. Xf-Rovim. A Field Robot to Detect Olive Trees Infected by Xylella Fastidiosa Using Proximal Sensing. Remote. Sens. 2019, 11, 221. [Google Scholar] [CrossRef]
- Cubero, S.; Marco-Noales, E.; Aleixos, N.; Barbé, S.; Blasco, J. RobHortic: A Field Robot to Detect Pests and Diseases in Horticultural Crops by Proximal Sensing. Agriculture 2020, 10, 276. [Google Scholar] [CrossRef]
- Martin, D.E.; Latheef, M.A. Remote Sensing Evaluation of Two-spotted Spider Mite Damage on Greenhouse Cotton. J. Vis. Exp. 2017, 122, e54314. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.; Berenstein, M.; Sade, A.; Karnieli, A.; Bonfil, D.J.; Weintraub, P.G. Spectral monitoring of two-spotted spider mite damage to pepper leaves. Remote. Sens. Lett. 2012, 3, 277–283. [Google Scholar] [CrossRef]
- Fraulo, A.B.; Cohen, M.; Liburd, O.E. Visible/near infrared reflectance (VNIR) spectroscopy for detecting twospotted spider mite (Acari: Tetranychidae) damage in strawberries. Environ. Èntomol. 2009, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Crockett, C.; Liburd, O.; Abd-Elrahman, A. Development of image based detection methods for two-spotted spider mite, Tetranychus urticae Koch, on strawberries. In Proceedings of the Entomological Society of America Annual Meeting 2014, Portland, OR, USA, 16–19 November 2014. [Google Scholar]
- Nieuwenhuizen, A.; Kool, J.; Suh, H.; Hemming, J. Automated spider mite damage detection on tomato leaves in greenhouses. Acta Hortic. 2020, 1268, 165–172. [Google Scholar] [CrossRef]
- Uygun, T.; Ozguven, M.M.; Yanar, D. A new approach to monitor and assess the damage caused by two-spotted spider mite. Exp. Appl. Acarol. 2020, 82, 335–346. [Google Scholar] [CrossRef]
- Urbaneja, A.; Catalán, J.; Tena, A.; Jacas, J.A. Plagas y Enfermedades. Tetraníquidos. Araña Roja. In Gestión Integrada de Plagas de Cítricos; Urbaneja, A., Catalán, J., Tena, A., Jacas, J.A., Eds.; IVIA: Moncada, Spain, 2020; Available online: http://gipcitricos.ivia.es/area/plagas-principales/tetraniquidos/arana-roja (accessed on 2 May 2021).
- Quiñones, A.; Martínez-Alcántara, B.; Primo-Millo, E.; Legaz, F. Fertigation: Concept and Application in Citrus. In Advances in Citrus Nutrition; Springer Science and Business Media LLC: Berlin/Heisenberg, Germany, 2012; pp. 281–301. [Google Scholar]
- Futch, S.H.; Tucker, D.P.H. A Guide to Citrus Nutritional Deficiency and Toxicity Identification. University of Florida IFAS Extension Publication HS-797. 2000. Available online: https://edis.ifas.ufl.edu/pdffiles/CH/CH14200.pdf (accessed on 2 May 2021).
- Zekri, M.; Obreza, T. Manganese (Mn) and Zinc (Zn) for Citrus Trees. Publication #SL403 of the Department of Soil and Wa-ter Sciences, UF/IFAS Extension. 2019. Available online: https://edis.ifas.ufl.edu/pdffiles/SS/SS61600.pdf (accessed on 2 May 2021).
- Landgrebe, D. Hyperspectral image data analysis. IEEE Signal Process. Mag. 2002, 19, 17–28. [Google Scholar] [CrossRef]
- Rivera, N.V.; Gómez-Sanchis, J.; Chanona-Pérez, J.; Carrasco, J.J.; Millán-Giraldo, M.; Lorente, D.; Cubero, S.; Blasco, J. Early detection of mechanical damage in mango using NIR hyperspectral images and machine learning. Biosyst. Eng. 2014, 122, 91–98. [Google Scholar] [CrossRef]
- Kumar, T.K. Multicollinearity in Regression Analysis. Rev. Econ. Stat. 1975, 57, 365. [Google Scholar] [CrossRef]
- Hawkins, D.M. The Problem of Overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Amigo, J.M.; Martí, I.; Gowen, A. Hyperspectral Imaging and Chemometrics. In Multivariate Pattern Recognition in Chemometrics, Illustrated by Case Studies; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 28, pp. 343–370. [Google Scholar]
- Lorente, D.; Aleixos, N.; Gomezsanchis, J.; Cubero, S.; García-Navarrete, O.L.; Blasco, J. Recent Advances and Applications of Hyperspectral Imaging for Fruit and Vegetable Quality Assessment. Food Bioprocess Technol. 2012, 5, 1121–1142. [Google Scholar] [CrossRef]
- Vinzi, V.E.; Chin, W.W.; Henseler, J.; Wang, H. Handbook of Partial Least Squares; Springer: Berlin/Heisenberg, Germany, 2010. [Google Scholar]
- Tagliavini, M.; Quiñones, A. Nutrizione e Concimazione. In Glo Agrumi; Tribulato, E., Inglese, P., Eds.; Bayer CroipScience: Milano, Italy, 2012; pp. 183–193. [Google Scholar]
- Diago, M.P.; Tardáguila, J.; Aleixos, N.; Millán, B.; Prats-Montalban, J.M.; Cubero, S.; Blasco, J. Assessment of cluster yield components by image analysis. J. Sci. Food Agric. 2014, 95, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, G.; Cubero, S.; Moltó, E.; Blasco, J. In-line sorting of irregular potatoes by using automated computer-based machine vision system. J. Food Eng. 2012, 112, 60–68. [Google Scholar] [CrossRef]
- Blasco, J.; Cubero, S.; Gómez-Sanchis, J.; Mira, P.; Moltó, E. Development of a machine for the automatic sorting of pomegranate (Punica granatum) arils based on computer vision. J. Food Eng. 2009, 90, 27–34. [Google Scholar] [CrossRef]
- Blasco, J.; Aleixos, N.; Cubero, S.; Gómez-Sanchís, J.; Moltó, E. Automatic sorting of satsuma (Citrus unshiu) segments using computer vision and morphological features. Comput. Electron. Agric. 2009, 66, 1–8. [Google Scholar] [CrossRef]
- Gat, N. Imaging spectroscopy using tunable filters: A review. Wavelet Appl. VII 2000, 4056, 50–65. [Google Scholar] [CrossRef]
- Williams, P.J.; Kucheryavskiy, S. Classification of maize kernels using NIR hyperspectral imaging. Food Chem. 2016, 209, 131–138. [Google Scholar] [CrossRef]
- Brereton, R.G. Applied Chemometrics for Scientists; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Munera, S.; Amigo, J.M.; Aleixos, N.; Talens, P.; Cubero, S.; Blasco, J. Potential of VIS-NIR hyperspectral imaging and chemometric methods to identify similar cultivars of nectarine. Food Control. 2018, 86, 1–10. [Google Scholar] [CrossRef]
- Rinnan, Å.; Berg, F.V.D.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Hastie, T.; Friedman, J.; Tibshirani, R. Model Assessment and Selection. In Linear and Generalized Linear Mixed Models and Their Applications; Springer Science and Business Media LLC: Berlin, Germany, 2001; pp. 193–224. [Google Scholar]
- Kuhn, M.; Johnson, K. Applied Predictive Modeling 26; Springer: New York, NY, USA, 2013. [Google Scholar]
- Cortés, V.; Rodríguez, A.; Blasco, J.; Rey, B.; Besada, C.; Cubero, S.; Salvador, A.; Talens, P.; Aleixos, N. Prediction of the level of astringency in persimmon using visible and near-infrared spectroscopy. J. Food Eng. 2017, 204, 27–37. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Yan, Y.; Zhu, S.-B.; Nie, S.-P.; Li, C.; Wang, Y.-X.; Gong, X.-F. Discrimination of Ganoderma lucidum according to geographical origin with near infrared diffuse reflectance spectroscopy and pattern recognition techniques. Anal. Chim. Acta 2008, 618, 121–130. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Reid, N.J. ES&T Views: Remote Sensing and Forest Damage. Environ. Sci. Technol. 1987, 21, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Moreno, V.; Rodríguez, E. Determinación Experimental de la Firma Espectral de la Vegetación. Una Sencilla Práctica de itroducción a la teleetección. In TELEDETECCIÓN. Avances y Aplicaciones, Proceedings VIII Congreso Nacional de Teledetección, Albacete, Spain, 22–24 September 1999; Diputación de Albacete: Albacete, Spain, 1999; pp. 429–443. [Google Scholar]
- Arnon, D.I. The Light Reactions of Photosynthesis. Proc. Natl. Acad. Sci. USA 1971, 68, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Rock, B.N.; Vogelmann, J.E.; Williams, D.L.; Hoshizaki, T. Remote Detection of Forest Damage. Bioscience 1986, 36, 439–445. [Google Scholar] [CrossRef]
- Roy, P.S. Spectral reflectance characteristics of vegetation and their use in estimating productive potential. Plant Sci. 1989, 99, 59–81. [Google Scholar] [CrossRef]
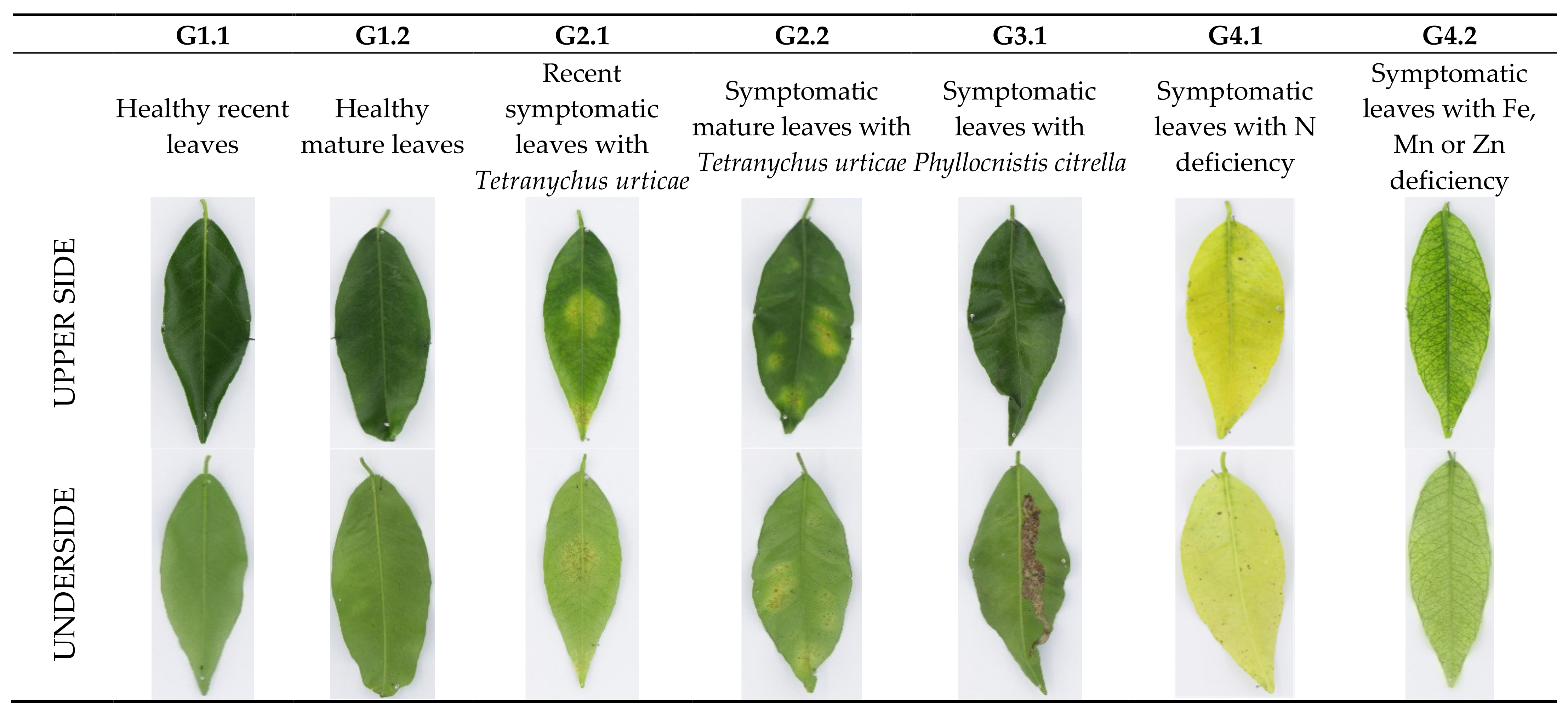

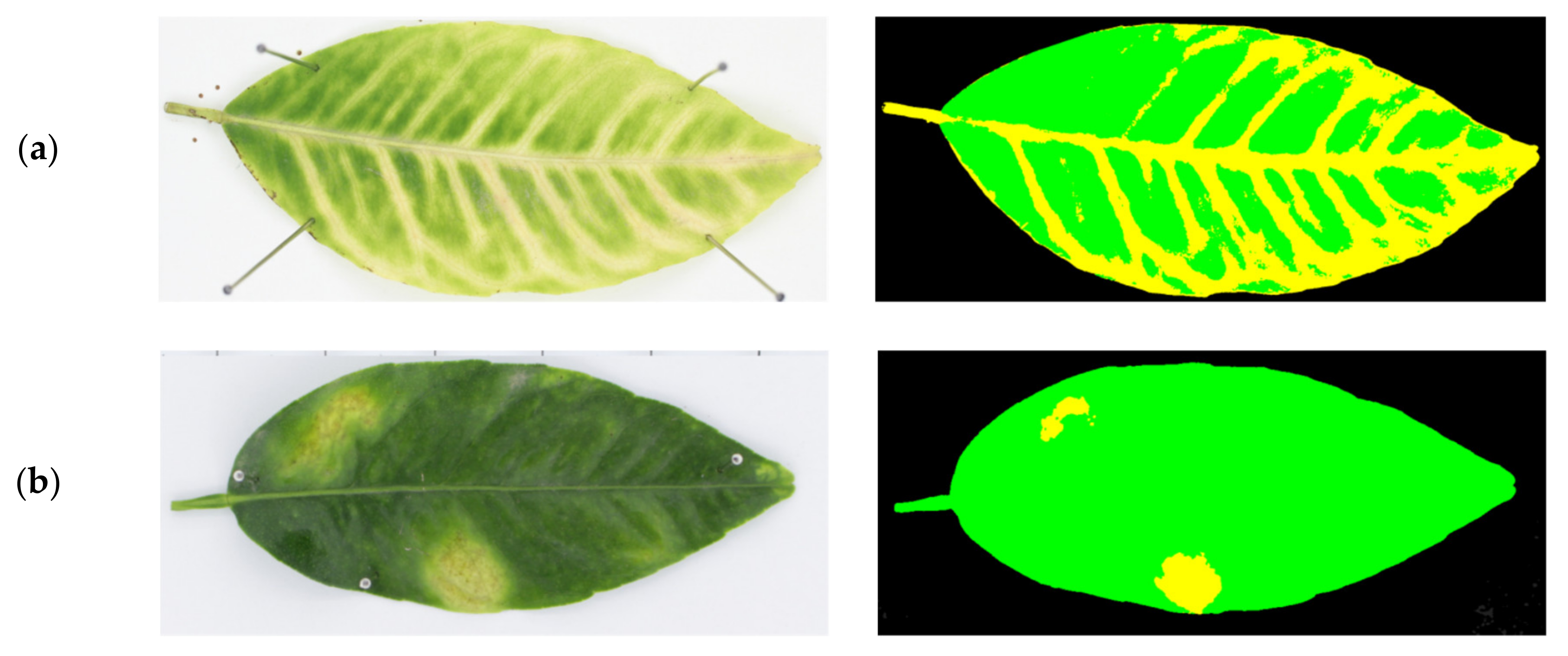
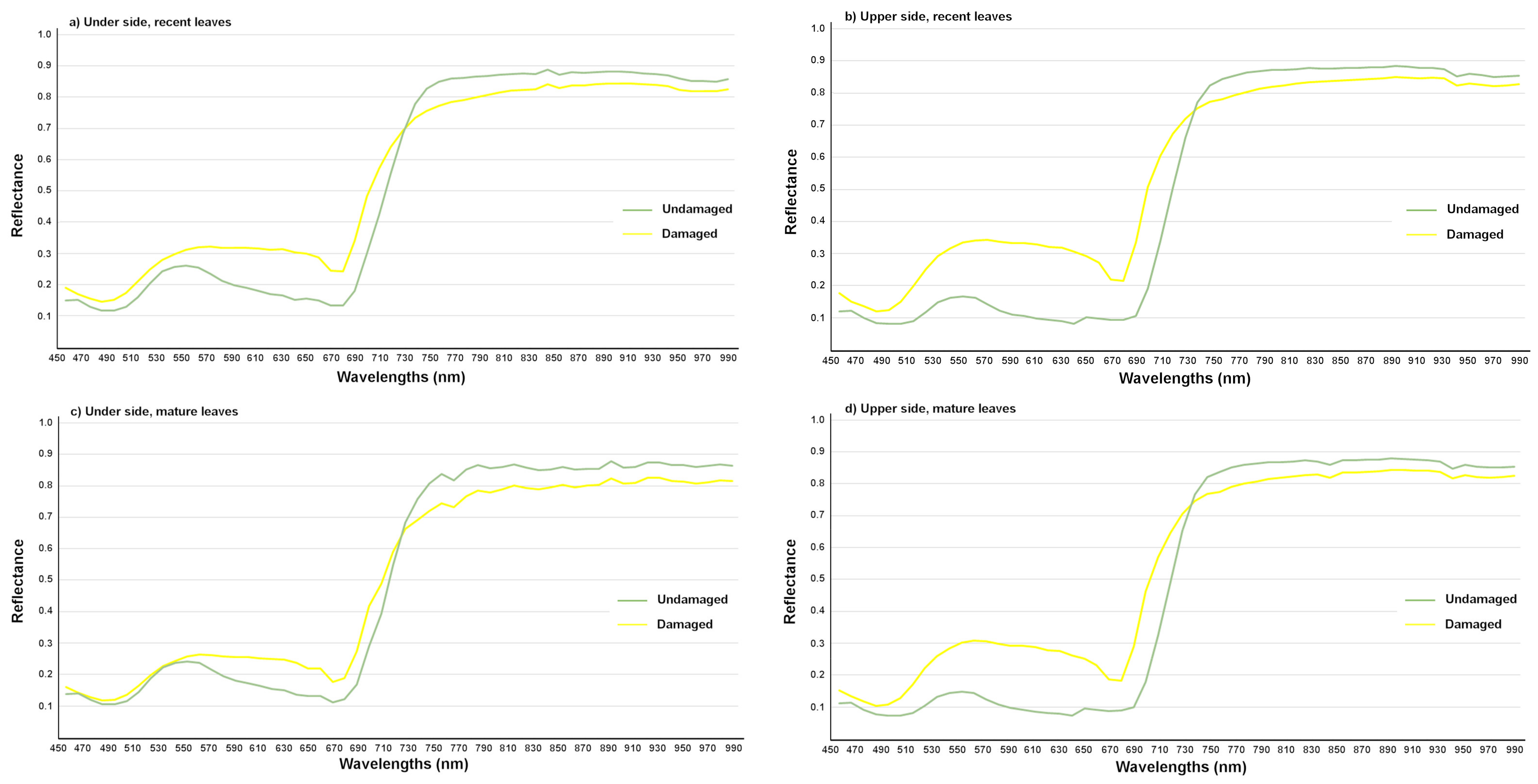
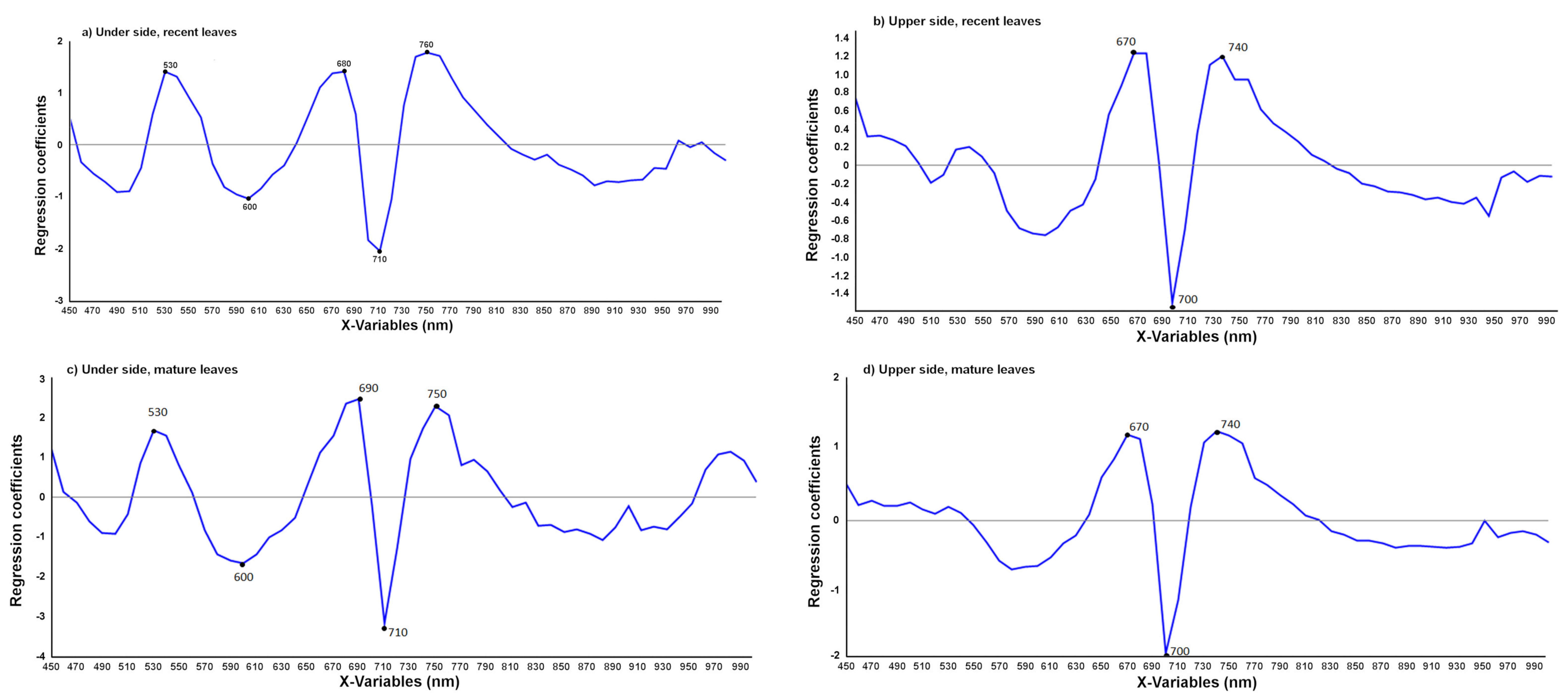

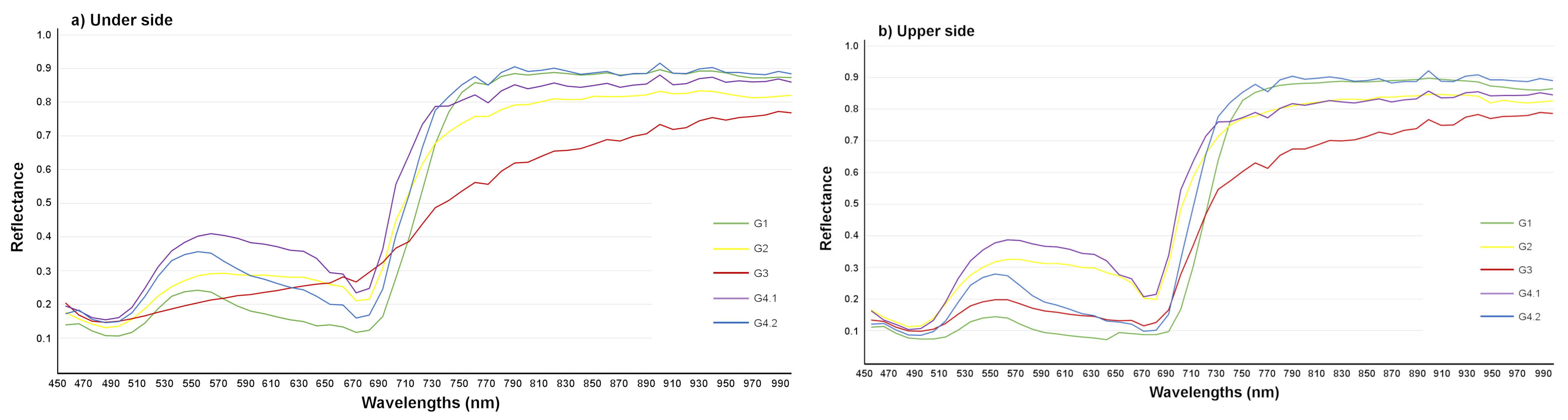

| Name of the Set | Name of the Subset | Leaves | Symptom | Age |
|---|---|---|---|---|
| G1 | G1.1 | 30 | Healthy | Recent |
| G1.2 | 30 | Healthy | Mature | |
| G2 | G2.1 | 30 | Tetranychus urticae | Recent |
| G2.2 | 30 | Tetranychus urticae | Mature | |
| G3 | G3.1 | 10 | Phyllocnistis citrella | Recent and mature |
| G4 | G4.1 | 6 | N deficiency | Recent and mature |
| G4.2 | 6 | Fe, Mn, or Zn deficiency | Recent and mature |
| Subset | Leaves | Number of Hyperspectral Images (Both Sides of the Leaf) | Total Number of ROI |
|---|---|---|---|
| G1.1 | 10 | 20 | 60 |
| G1.2 | 10 | 20 | 60 |
| G2.1 | 30 | 60 | 360 |
| G2.2 | 30 | 60 | 360 |
| G3.1 | 10 | 20 | 60 |
| G4.1 | 6 | 12 | 36 |
| G4.2 | 6 | 12 | 36 |
| TOTAL | 102 | 204 | 972 |
| True T. urticae | Not T. urticae | Statistical Parameters of ANOVA * | ||||
|---|---|---|---|---|---|---|
| Mean | Typical Deviation | Mean | Typical Deviation | F | p-Value | |
| Elongation | 1.539 | 0.272 | 4.230 | 4.596 | 8.161 | 0.0053 |
| Roundness | 0.506 | 0.163 | 0.252 | 0.155 | 44.362 | 0.0000 |
| Individual area | 67,293.94 | 39,350.89 | 224,056.90 | 233,071.55 | 9.476 | 0.0036 |
| Compactness | 34.98 | 17.41 | 83.82 | 75.29 | 15.092 | 0.0002 |
| Perimeter | 3260.06 | 2932.77 | 1848.61 | 2928.42 | 5.699 | 0.0187 |
| Damages per leaf | 1.69 | 0.855 | 9.000 | 6.890 | 11.208 | 0.0032 |
| Total damaged area | 82,321.75 | 35,703.98 | 1,176,298.75 | 517,992.91 | 83.652 | 0.0000 |
| Sets and Subsets | Underside (%) | Upperside (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4.1 | G4.2 | G1 | G2 | G3 | G4.1 | G4.2 | |
| G1 | 100 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 |
| G2 | 5.00 | 91.67 | 3.33 | 0 | 0 | 6.67 | 93.33 | 0 | 0 | 0 |
| G3 | 0 | 0 | 100 | 0 | 0 | 90.0 | 10.00 | 0 | 0 | 0 |
| G4.1 | 100 | 0 | 0 | 0 | 0 | 83.33 | 0 | 0 | 16.67 | 0 |
| G4.2 | 41.67 | 0 | 8.33 | 0 | 50.0 | 50.0 | 0 | 0 | 0 | 50.0 |
| Latent Variables | Class | ||||
|---|---|---|---|---|---|
| Recent | Mature | ||||
| Underside | All wavelengths | 7 | Recent | 100 | 0 |
| mature | 0 | 100 | |||
| Selected wavelengths | 5 | Recent | 100 | 0 | |
| mature | 0 | 100 | |||
| Upper side | All wavelengths | 7 | Recent | 88.89 | 11.11 |
| mature | 3.70 | 96.30 | |||
| Selected wavelengths | 5 | Recent | 86.73 | 13.27 | |
| mature | 1.02 | 98.98 | |||
| Latent Variables | Class (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Set | G1 | G2 | G3 | G4.1 | G4.2 | |||
| Underside | All wavelengths | 7 | G1 | 100 | 0 | 0 | 0 | 0 |
| G2 | 0 | 75.93 | 1.85 | 16.67 | 5.56 | |||
| G3 | 0 | 0 | 100 | 0 | 0 | |||
| G4.1 | 0 | 33.33 | 0 | 66.67 | 0 | |||
| G4.2 | 20 | 80 | 0 | 0 | 0 | |||
| Selected wavelengths | 5 | G1 | 100 | 0 | 0 | 0 | 0 | |
| G2 | 0 | 69.67 | 1.85 | 22.92 | 5.56 | |||
| G3 | 0 | 0 | 100 | 0 | 0 | |||
| G4.1 | 0 | 33.33 | 0 | 66.67 | 0 | |||
| G4.2 | 0 | 100 | 0 | 0 | 0 | |||
| Upper side | All wavelengths | 7 | G1 | 95.83 | 4.17 | 0 | 0 | 0 |
| G2 | 0 | 81.48 | 1.85 | 12.96 | 3.70 | |||
| G3 | 0 | 0 | 100 | 0 | 0 | |||
| G4.1 | 0 | 16.67 | 16.67 | 66.67 | 0 | |||
| G4.2 | 20 | 80 | 0 | 0 | 0 | |||
| Selected wavelengths | 4 | G1 | 95.83 | 4.17 | 0 | 0 | 0 | |
| G2 | 0 | 94.20 | 1.85 | 3.95 | 0 | |||
| G3 | 0 | 0 | 100 | 0 | 0 | |||
| G4.1 | 0 | 33.33 | 0 | 66.67 | 0 | |||
| G4.2 | 0 | 100 | 0 | 0 | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Gonzalez, M.G.; Blasco, J.; Cubero, S.; Chueca, P. Automated Detection of Tetranychus urticae Koch in Citrus Leaves Based on Colour and VIS/NIR Hyperspectral Imaging. Agronomy 2021, 11, 1002. https://doi.org/10.3390/agronomy11051002
Gonzalez-Gonzalez MG, Blasco J, Cubero S, Chueca P. Automated Detection of Tetranychus urticae Koch in Citrus Leaves Based on Colour and VIS/NIR Hyperspectral Imaging. Agronomy. 2021; 11(5):1002. https://doi.org/10.3390/agronomy11051002
Chicago/Turabian StyleGonzalez-Gonzalez, María Gyomar, Jose Blasco, Sergio Cubero, and Patricia Chueca. 2021. "Automated Detection of Tetranychus urticae Koch in Citrus Leaves Based on Colour and VIS/NIR Hyperspectral Imaging" Agronomy 11, no. 5: 1002. https://doi.org/10.3390/agronomy11051002
APA StyleGonzalez-Gonzalez, M. G., Blasco, J., Cubero, S., & Chueca, P. (2021). Automated Detection of Tetranychus urticae Koch in Citrus Leaves Based on Colour and VIS/NIR Hyperspectral Imaging. Agronomy, 11(5), 1002. https://doi.org/10.3390/agronomy11051002






