Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Treatment, and Experimental Design
2.2. Measurement of Plant Growth Parameters
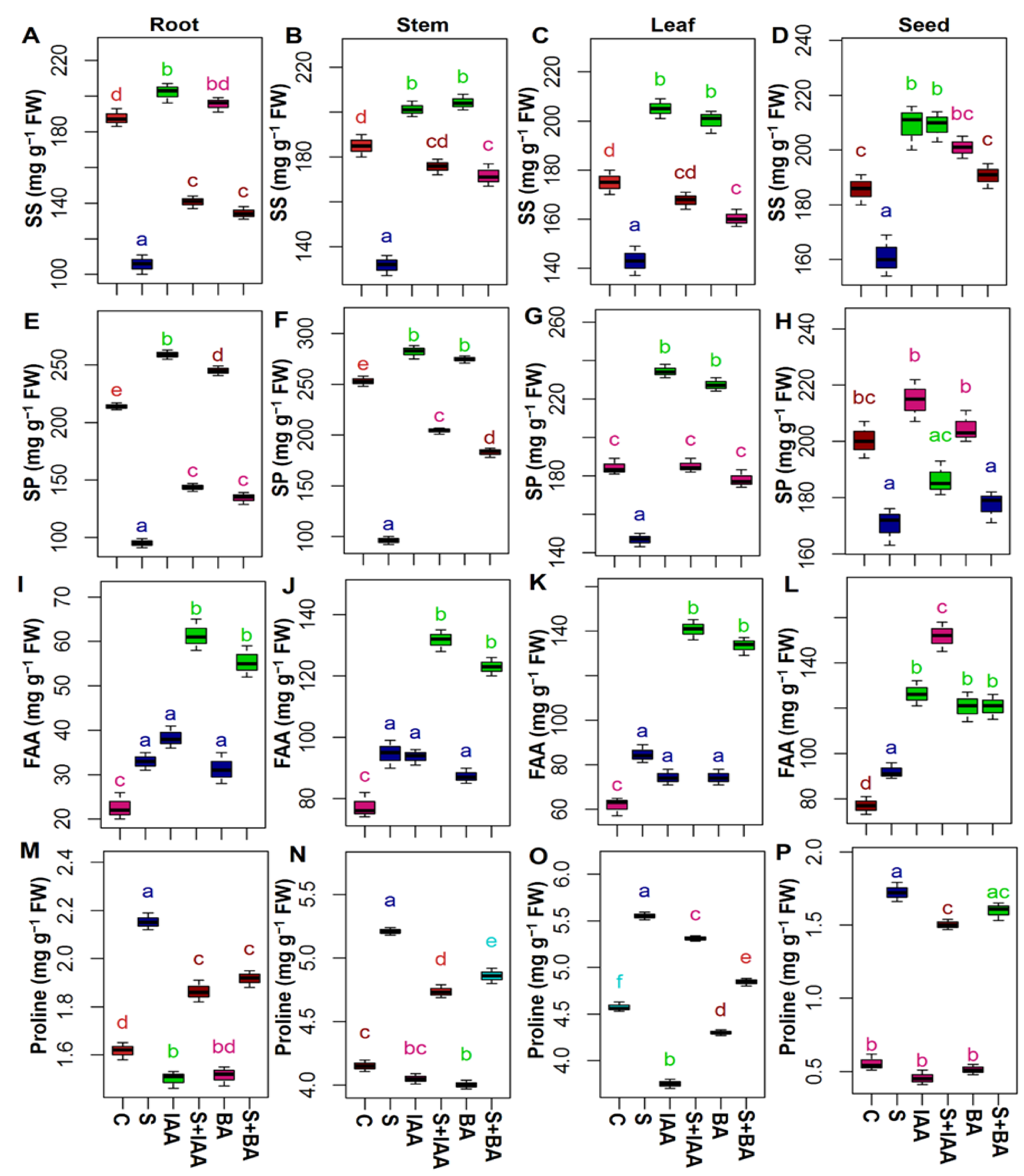
2.3. Extraction and Analysis of Proline, Soluble Sugars, Soluble Proteins, and Free Amino Acid Contents
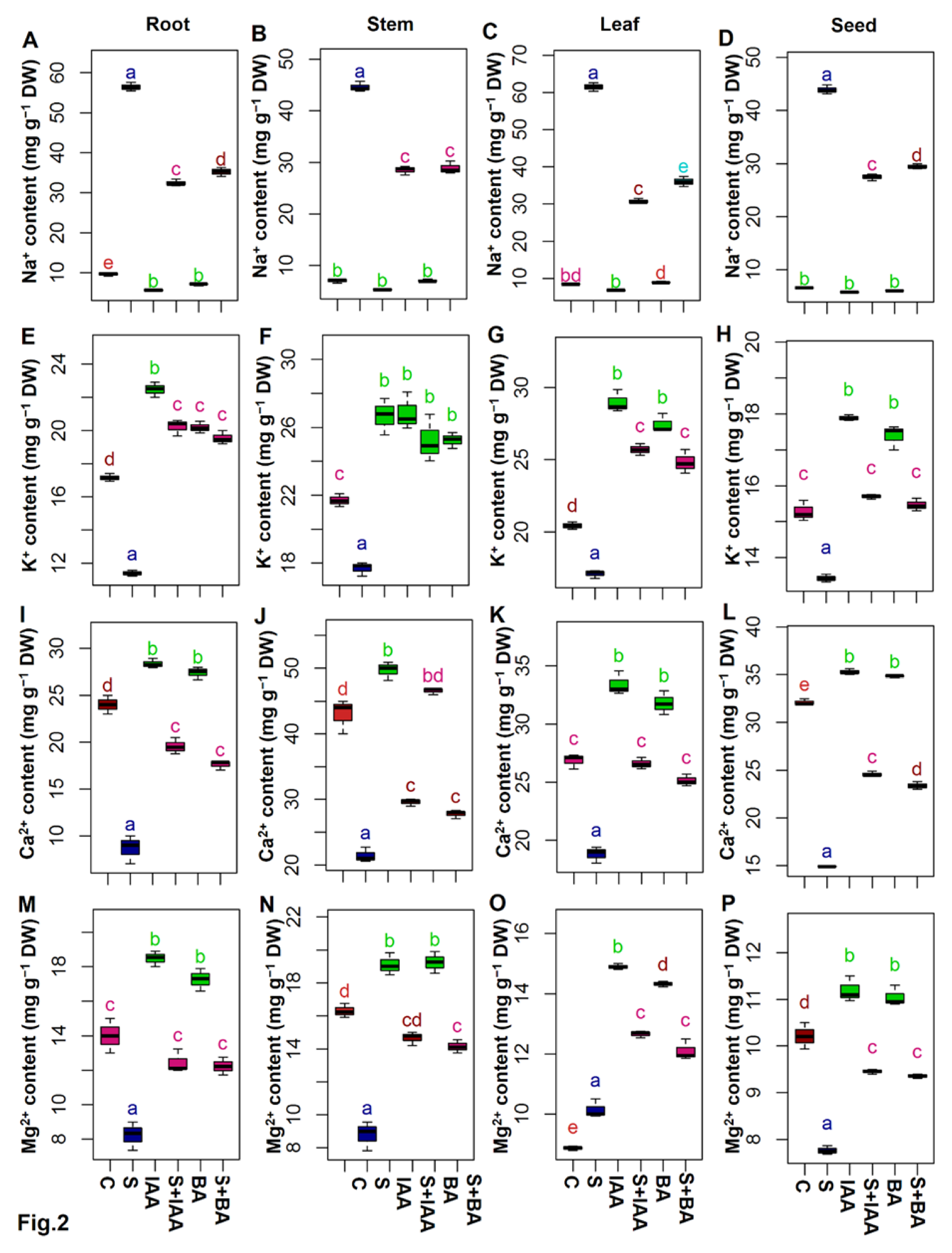
2.4. Estimation of Different Mineral Ion Contents
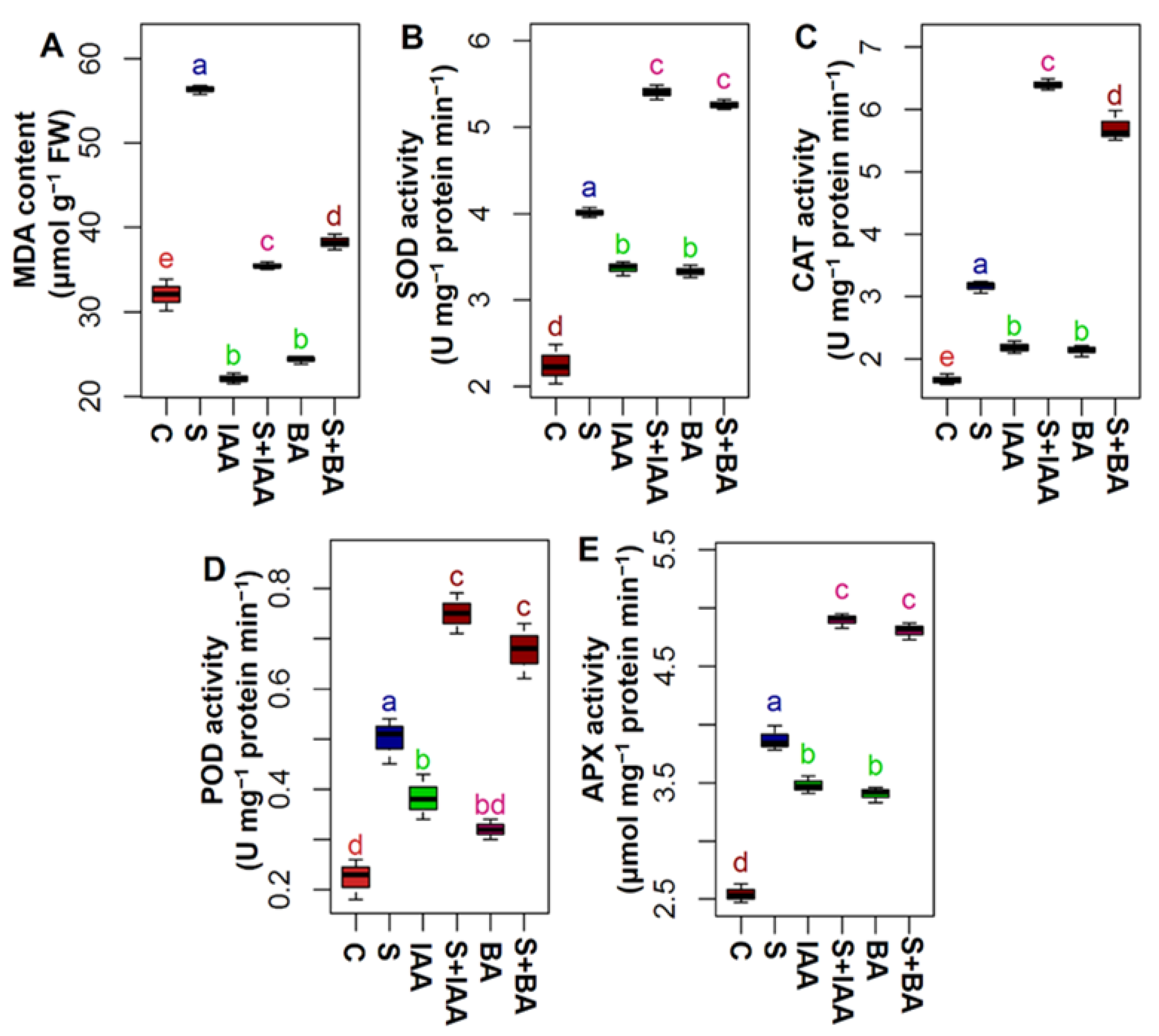
2.5. Measurement of Lipid Peroxidation
2.6. Extraction and Determination of Antioxidant Enzymatic Activity
2.7. Electrophoretic Detection of Protein by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
2.8. Statistical Analysis
3. Results
3.1. Foliar Applications of IAA or BA Improved Plant Growth and Yield Components
3.2. Applications IAA or BA Changed the Accumulation of Osmoprotectants Level
3.3. Exogenous IAA or BA Regulated the Mineral Homeostasis under Salt Stress
3.4. Applications of IAA or BA Prevented Lipid Peroxidation by Enhancing Antioxidant Enzymes
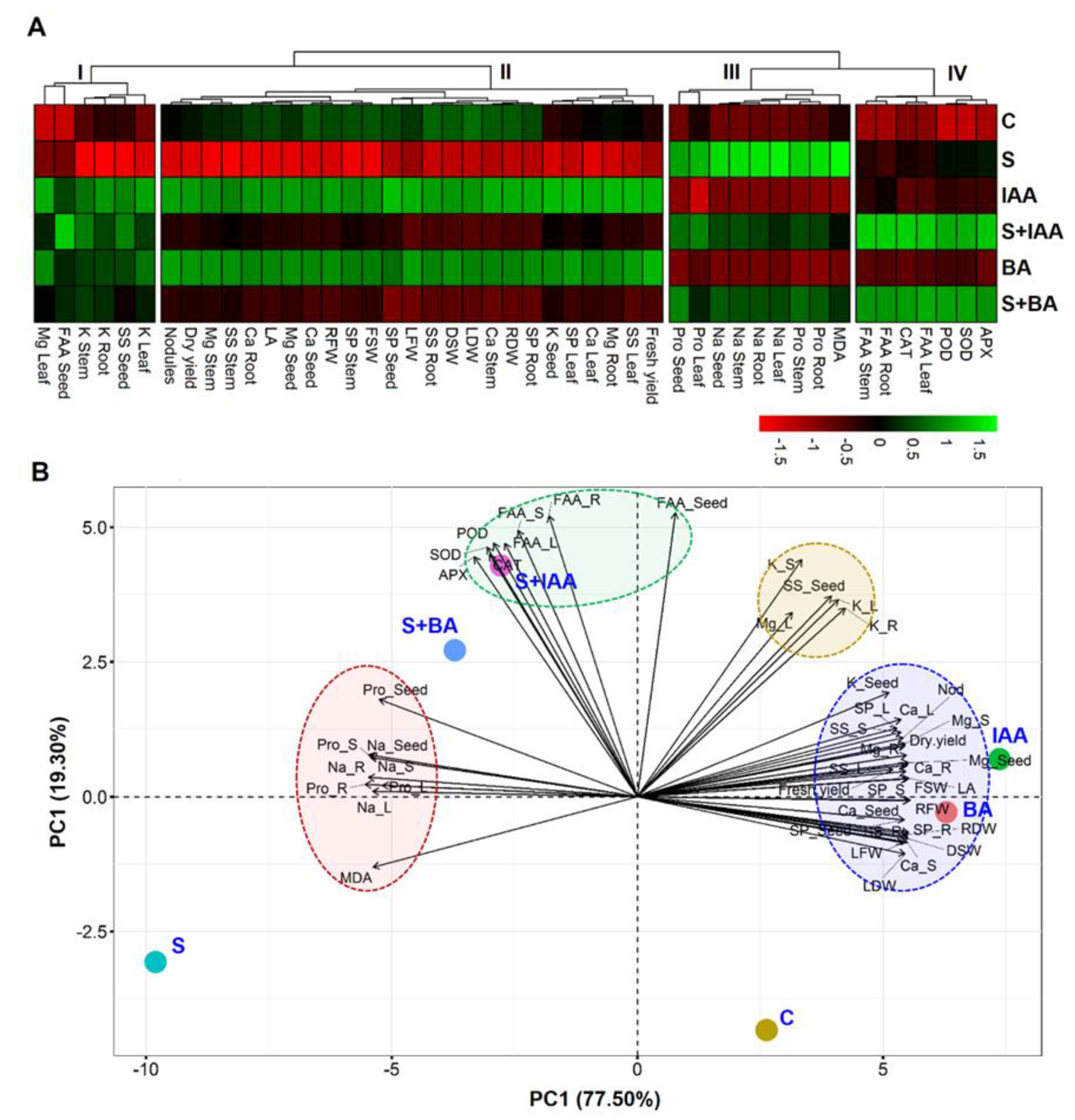
3.5. Visualization and Understanding of Treatment-Variable Relationship through Hierarchical Clustering and PCA
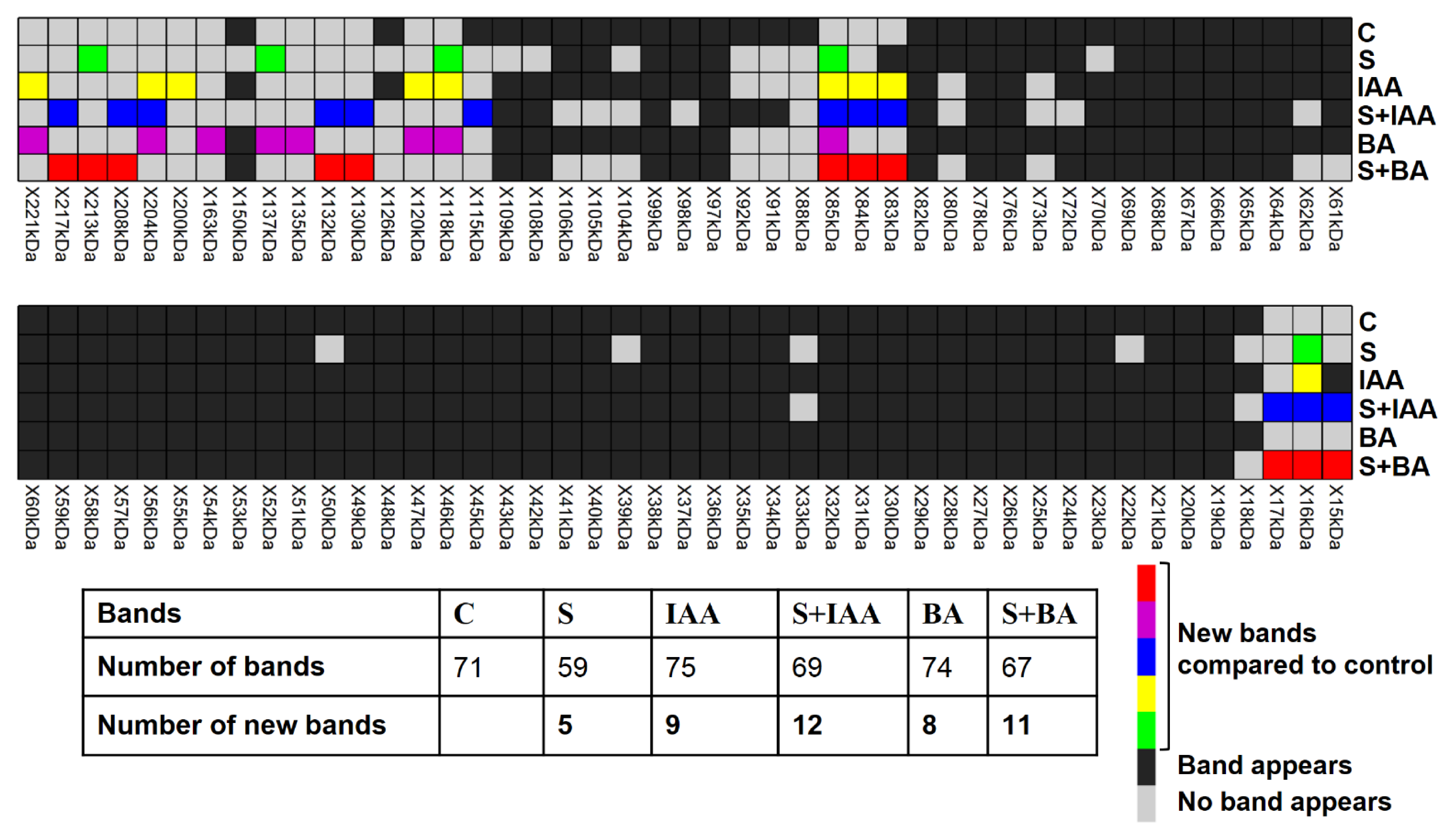
3.6. Foliar Application of IAA or BA Changes Protein Expression Pattern under Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative Stress and Antioxidant Defense Mechanisms in Plants Under Salt Stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–205. ISBN 978-3-030-06117-3. [Google Scholar]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to Mitigate the Salt Stress Effects on Photosynthetic Apparatus and Productivity of Crop Plants. In Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 85–136. ISBN 978-3-319-75671-4. [Google Scholar]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum Lipoferum or Azotobacter Chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Dash, M.; Panda, S.K. Salt Stress Induced Changes in Growth and Enzyme Activities in Germinating Phaseolus Mungo Seeds. Biol. Plant. 2001, 44, 587–589. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Sarwar, G.; Ashraf, M.; Afaf, R.; Sattar, A. Salinity Induced Changes in α-Amylase Activity During Germination and Early Cotton Seedling Growth. Biol. Plant. 2002, 45, 589–591. [Google Scholar] [CrossRef]
- Seckin, B.; Sekmen, A.H.; Türkan, İ. An Enhancing Effect of Exogenous Mannitol on the Antioxidant Enzyme Activities in Roots of Wheat Under Salt Stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.S.R. Effect of Brassinosteroids on Salinity Stress Induced Inhibition of Seed Germination and Seedling Growth of Rice (Oryza Sativa L.). Plant Growth Regul. 2001, 33, 151–153. [Google Scholar] [CrossRef]
- Gregory, P.J.; Ismail, S.; Razaq, I.B.; Wahbi, A. Soil Salinity: Current Status and in Depth Analyses for Sustainable Use; Chapter 2; International Atomic Energy Agency: Vienna, Austria, 2018; pp. 4–11. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.-H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and Salinity: Crosstalk in Crop-Mediated Stress Tolerance Mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Shokr, M.M.B.; Bekheta, M.A. Growth, Root Characteristics, and Leaf Nutrients Accumulation of Four Faba Bean (Vicia Faba L.) Cultivars Differing in Their Broomrape Tolerance and the Soil Properties in Relation to Salinity. Commun. Soil Sci. Plant Anal. 2010, 41, 2713–2728. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; et al. Acetic Acid: A Cost-Effective Agent for Mitigation of Seawater-Induced Salt Toxicity in Mung Bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef] [PubMed]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and Salinity: II. Gas Exchange and Chlorophyll Fluorescence of Sorghum under Salt Stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Aziz, T.; Maqsood, M.A.; Farooq, M.; Abdullah, Y.; Ramzani, P.M.A.; Bilal, H.M. Silicon Nutrition Mitigates Salinity Stress in Maize by Modulating Ion Accumulation, Photosynthesis, and Antioxidants. Photosynthetica 2018, 56, 1047–1057. [Google Scholar] [CrossRef]
- Shah, F.; Wu, W. Soil and Crop Management Strategies to Ensure Higher Crop Productivity within Sustainable Environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Chen, T.; Xing, L.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Regulatory Mechanisms Underlying the Maintenance of Homeostasis in Pyropia Haitanensis under Hypersaline Stress Conditions. Sci. Total Environ. 2019, 662, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst Phytohormones from Planta and PGPR under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone Balance and Abiotic Stress Tolerance in Crop Plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A New Perspective of Phytohormones in Salinity Tolerance: Regulation of Proline Metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Action of Plant Growth Regulators and Salinity on Seed Germination of Ceratoides Lanata. Can. J. Bot. 2004, 82, 37–42. [Google Scholar] [CrossRef]
- Gul, B.; Khan, M.A.; Weber, D.J. Alleviation of Salinity and Dark-Enforced Dormancy in Allenrolfea Occidentalis Seeds under Various Thermoperiods. Aust. J. Bot. 2000, 48, 745–752. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and Plant Responses to Salinity Stress: A Review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Waheed, A.; Hamid, F.; Ahmad, H.; Abbassi, F.; Aslam, S.; Shah, A.; Ahmad, N.; Naheed, Z.; Ali, H.; Khan, N. Effect of Indole Butyric Acid (IBA) on Early Root Formation (Tomato ‘Sahil’Hybrid) Cuttings. J. Mater. Environ. Sci. 2015, 6, 272–279. [Google Scholar]
- Zoubida, B.; Gherroucha, H. Improvement of Salt Tolerance in Durum Wheat (Triticum Durum Desf.) by Auxin and Kenitin Application. Eur. Sci. J. 2017, 13. [Google Scholar] [CrossRef][Green Version]
- Alam, M.; Khan, M.A.; Imtiaz, M.; Khan, M.A.; Naeem, M.; Shah, S.A.; Samiullah; Ahmad, S.H.; Khan, L. Indole-3-Acetic Acid Rescues Plant Growth and Yield of Salinity Stressed Tomato (Lycopersicon Esculentum L.). Gesunde Pflanz. 2020, 72, 87–95. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, A.L.; Okant, A.M. Effect of Foliar Applied Kinetin and Indole Acetic Acid on Maize Plants Grown under Saline Conditions. Turk. J. Agric. For. 2010, 34, 529–538. [Google Scholar]
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research Progress on the Roles of Cytokinin in Plant Response to Stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hwang, I. Cytokinin: Perception, Signal Transduction, and Role in Plant Growth and Development. J. Plant Biol. 2007, 50, 98–108. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.-G. Plant Hormones in Salt Stress Tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Chen, J.; Yang, S.; Zha, D. Alleviation of Exogenous 6-Benzyladenine on Two Genotypes of Eggplant (Solanum Melongena Mill.) Growth under Salt Stress. Protoplasma 2014, 251, 169–176. [Google Scholar] [CrossRef]
- Akter, N.; Islam, M.R.; Karim, M.A.; Hossain, T. Alleviation of Drought Stress in Maize by Exogenous Application of Gibberellic Acid and Cytokinin. J. Crop Sci. Biotechnol. 2014, 17, 41–48. [Google Scholar] [CrossRef]
- Hu, J.; Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Comparative Proteomic Analysis Reveals That Exogenous 6-Benzyladenine (6-BA) Improves the Defense System Activity of Waterlogged Summer Maize. BMC Plant Biol. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Pandey, M.K.; Jha, U.C.; Singh, B.; Singh, I.P.; Datta, D.; Chaturvedi, S.K.; Nadarajan, N.; Varshney, R.K. Genomics-Assisted Breeding in Four Major Pulse Crops of Developing Countries: Present Status and Prospects. Theor. Appl. Genet. 2014, 127, 1263–1291. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Paull, J.; Rengasamy, P.; McDonald, G.K. Comparing Genotypic Variation in Faba Bean (Vicia Faba L.) in Response to Salinity in Hydroponic and Field Experiments. Field Crops Res. 2012, 127, 99–108. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, Tolerance Mechanisms and Management of Salt Stress in Grain Legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.J.; Watson, M.A. Comparative Physiological Studies on the Growth of Field Crops. Ann. Appl. Biol. 1953, 40, 1–37. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fales, F.W. The Assimilation and Degradation of Carbohydrates by Yeast Cells. J. Biol. Chem. 1951, 193, 113–124. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Photometric Nin-Hydrin Method for Use in the Ehromatography of Amino Acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sayed, M.A.; Islam, M.M.; Siddiqui, M.N.; Begum, S.N.; Hossain, M.A. Screening of Rice Landraces (Oryza Sativa L.) for Seedling Stage Salinity Tolerance Using Morpho-Physiological and Molecular Markers. Acta Physiol. Plant. 2018, 40, 70. [Google Scholar] [CrossRef]
- Schwarzenbach, G.; Biedermann, W.; Komplexone, X. Erdalkalikomplexe von o, o’-Dioxyazofarbstoffen. Helv. Chim. Acta 1948, 31, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of Water Stress-induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Scebba, F.; Sebastiani, L.; Vitagliano, C. Protective Enzymes against Activated Oxygen Species in Wheat (Triticum Aestivum L.) Seedlings: Responses to Cold Acclimation. J. Plant Physiol. 1999, 155, 762–768. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Maehly, A.; Chance, B. Catalases and Peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar]
- Chen, G.-X.; Asada, K. Inactivation of Ascorbate Peroxidase by Thiols Requires Hydrogen Peroxide. Plant Cell Physiol. 1992, 33, 117–123. [Google Scholar] [CrossRef]
- Hames, B.D.; Rickwood, D. Gel Electrophoresis of Nucleic Acids. A Practical Approach, 2nd ed.; IRL Press: New York, NY, USA, 1990. [Google Scholar]
- Reed, S.H.; You, Z.; Friedberg, E.C. The Yeast RAD7 and RAD16 Genes Are Required for Postincision Events during Nucleotide Excision Repair: In vitro and in vivo Studies with rad7 and Rad16 Mutants and Purification of a Rad7/Rad16-containing Protein Complex. J. Biol. Chem. 1998, 273, 29481–29488. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Chowdhury, M.B.; Afrin, S.; Burritt, D.J.; Murata, Y.; Hossain, M.A.; Hossain, M.A. Exogenous Glutathione-Mediated Drought Stress Tolerance in Rice (Oryza Sativa L.) Is Associated with Lower Oxidative Damage and Favorable Ionic Homeostasis. Iran J. Sci. Technol. Trans. Sci. 2020, 44, 955–971. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Samineni, S.; Siddique, K.H.M.; Gaur, P.M.; Colmer, T.D. Salt Sensitivity of the Vegetative and Reproductive Stages in Chickpea (Cicer Arietinum L.): Podding Is a Particularly Sensitive Stage. Environ. Exp. Bot. 2011, 71, 260–268. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Metwally, I.M.; Abdelhamid, M.T. Physiological Response of Lupine and Associated Weeds Grown at Salt-Affected Soil to A-tocopherol and Hoeing Treatments. Gesunde Pflanz. 2016, 68, 117–127. [Google Scholar] [CrossRef]
- El-Awadi, M.; Sadak, M.; Dawood, M.; Khater, M.; Elashtokhy, M. Amelioration the Adverse Effects of Salinity Stress by Using γ-Radiation in Faba Bean Plants. Bull. Natl. Res. Cent. 2017, 41, 293–310. [Google Scholar]
- Javid, M.G.; Sorooshzadeh, A.; Sanavy, S.A.M.M.; Allahdadi, I.; Moradi, F. Effects of the Exogenous Application of Auxin and Cytokinin on Carbohydrate Accumulation in Grains of Rice under Salt Stress. Plant Growth Regul. 2011, 65, 305–313. [Google Scholar] [CrossRef]
- Khalid, A.; Aftab, F. Effect of Exogenous Application of IAA and GA 3 on Growth, Protein Content, and Antioxidant Enzymes of Solanum Tuberosum L. Grown in Vitro under Salt Stress. Vitro Cell. Dev. Biol. Plant 2020, 56, 377–389. [Google Scholar] [CrossRef]
- Sá, F.V.d.S.; Brito, M.E.B.; Silva, L.d.A.; Moreira, R.C.L.; Paiva, E.P.d.; Souto, L.S. Exogenous Application of Phytohormones Mitigates the Effect of Salt Stress on Carica Papaya Plants. Rev. Bras. Eng. Agríc. Ambient. 2020, 24, 170–175. [Google Scholar] [CrossRef]
- Muhammad, N.; Manghwar, H.; Quraishi, U.; Chaudhary, H.; Munis, M. IAA (Indole-3-Acetic Acid) Induces Biochemical and Physiological Changes in Wheat under Drought Stress. Philipp. Agric. Sci. 2015, 99. [Google Scholar]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-Acetic Acid Improves Drought Tolerance of White Clover via Activating Auxin, Abscisic Acid and Jasmonic Acid Related Genes and Inhibiting Senescence Genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.-J.; Han, T.-T.; Cai, W.; Fu, Z.-W.; Lu, Y.-T. Salt Stress Reduces Root Meristem Size by Nitric Oxide-Mediated Modulation of Auxin Accumulation and Signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed Enhancement with Cytokinins: Changes in Growth and Grain Yield in Salt Stressed Wheat Plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Huang, B. Cytokinin-Mitigation of Salt-Induced Leaf Senescence in Perennial Ryegrass Involving the Activation of Antioxidant Systems and Ionic Balance. Environ. Exp. Bot. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Fazeli, M.; Naderi, D. Effects of 6-Benzylaminopurine and Salinity Stress on Flowering and Biochemical Characteristics of Winter Jasmine (Jasminum Nudiflorum L.). J. Ornam. Plants 2019, 9, 41–53. [Google Scholar]
- Hayat, S.; Yadav, S.; Wani, D.A.; Irfan, M.; Alyemeni, M.; Ahmad, A. Impact of Sodium Nitroprusside on Nitrate Reductase, Proline Content, and Antioxidant System in Tomato under Salinity Stress. Hortic. Environ. Biotechnol. 2012, 53, 362–367. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in Plants under Abiotic Stresses: New Insights into a Classical Phenomenon. Planta 2020, 251, 1–17. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Evaluation of Proline Functions in Saline Conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Anee, T.I.; Bhuiyan, T.F.; Nahar, K.; Fujita, M. Emerging role of osmolytes in enhancing abiotic stress tolerance in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Hoboken, NJ, USA, 2019; pp. 677–708. [Google Scholar]
- Dawood, M.F.; Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A. Hydrogen Sulfide Priming Can Enhance the Tolerance of Artichoke Seedlings to Individual and Combined Saline-Alkaline and Aniline Stresses. Plant Physiol. Biochem. 2021, 159, 347–362. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus Degradation: Directions of Amino Acid Metabolism during Arabidopsis Abiotic Stress Response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The Role of Amino Acid Metabolism during Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Gadallah, M.A.A. Effects of Indole-3-Acetic Acid and Zinc on the Growth, Osmotic Potential and Soluble Carbon and Nitrogen Components of Soybean Plants Growing under Water Deficit. J. Arid Environ. 2000, 44, 451–467. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M. Kinetin and Indole Acetic Acid Promote Antioxidant Defense System and Reduce Oxidative Stress in Maize (Zea Mays L.) Plants Grown at Boron Toxicity. J. Plant Growth Regul. 2018, 37, 1258–1266. [Google Scholar] [CrossRef]
- Hakim, M.; Juraimi, A.; Hanafi, M.; Ismail, M.; Rafii, M.; Islam, M.; Selamat, A. The Effect of Salinity on Growth, Ion Accumulation and Yield of Rice Varieties. J. Anim. Plant Sci. 2014, 24, 874–885. [Google Scholar]
- Mahmud, S.; Sharmin, S.; Chowdhury, B.L.; Hossain, M.A. Research Article Effect of Salinity and Alleviating Role of Methyl Jasmonate in Some Rice Varieties. Asian J. Plant Sci. 2017, 16, 87–93. [Google Scholar] [CrossRef]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of Salinity on Seed Germination, Growth and Ion Content in Dimorphic Seeds of Salicornia Europaea L.(Chenopodiaceae). Plant Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Guo, W. Chapter 11—Magnesium homeostasis mechanisms and magnesium use efficiency in plants. In Plant Macronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Tran, L.-S.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 197–213. ISBN 978-0-12-811308-0. [Google Scholar]
- Sun, J.; Li, L.; Liu, M.; Wang, M.; Ding, M.; Deng, S.; Lu, C.; Zhou, X.; Shen, X.; Zheng, X.; et al. Hydrogen Peroxide and Nitric Oxide Mediate K+/Na+ Homeostasis and Antioxidant Defense in NaCl-Stressed Callus Cells of Two Contrasting Poplars. Plant Cell Tiss. Organ Cult. 2010, 103, 205–215. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic Acid-Mediated Enhancement of Photosynthesis Attributes and Antioxidant Capacity Contributes to Yield Improvement of Maize Plants Under Salt Stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Guimarães, F.V.A.; de Lacerda, C.F.; Marques, E.C.; de Miranda, M.R.A.; de Abreu, C.E.B.; Prisco, J.T.; Gomes-Filho, E. Calcium Can Moderate Changes on Membrane Structure and Lipid Composition in Cowpea Plants under Salt Stress. Plant Growth Regul. 2011, 65, 55–63. [Google Scholar] [CrossRef]
- Sadak, M.; Elhamid, A.; Mahmoud, M. Glutathione Induced Antioxidant Protection against Salinity Stress in Chickpea (Cicer Arietinum L.) Plant. Egypt. J. Bot 2017, 57, 293–302. [Google Scholar] [CrossRef]
- Latif, M.; Akram, N.A.; Ashraf, M. Regulation of Some Biochemical Attributes in Drought-Stressed Cauliflower (Brassica Oleracea L.) by Seed Pre-Treatment with Ascorbic Acid. J. Hortic. Sci. Biotechnol. 2016, 91, 129–137. [Google Scholar] [CrossRef]
- Akram, N.A.; Iqbal, M.; Muhammad, A.; Ashraf, M.; Al-Qurainy, F.; Shafiq, S. Aminolevulinic Acid and Nitric Oxide Regulate Oxidative Defense and Secondary Metabolisms in Canola (Brassica Napus L.) under Drought Stress. Protoplasma 2018, 255, 163–174. [Google Scholar] [CrossRef]
- Roy, S.; Negrao, S.; Tester, M. Salt Resistant Crop Plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and Antioxidant Activities of Savoury (Satureja Thymbra L.) with Identified Major Terpenes of the Essential Oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Eser, A.; Aydemir, T. The Effect of Kinetin on Wheat Seedlings Exposed to Boron. Plant Physiol. Biochem. 2016, 108, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Shah, S. Kinetin Improves Photosynthetic and Antioxidant Responses of Nigella Sativa to Counteract Salt Stress. Russ. J. Org. Chem. 2011, 58, 454–459. [Google Scholar] [CrossRef]
- Bavei, V.; Shiran, B.; Khodambashi, M.; Ranjbar, A. Protein Electrophoretic Profiles and Physiochemical Indicators of Salinity Tolerance in Sorghum (Sorghum Bicolor L.). Afr. J. Biotechnol. 2011, 10, 2683–2697. [Google Scholar] [CrossRef]
- Parker, R.; Flowers, T.J.; Moore, A.L.; Harpham, N.V.J. An Accurate and Reproducible Method for Proteome Profiling of the Effects of Salt Stress in the Rice Leaf Lamina. J. Exp. Bot. 2006, 57, 1109–1118. [Google Scholar] [CrossRef]
- Younis, M.E.; Hasaneen, M.N.A.; Kazamel, A.M.S. Plant Growth, Metabolism and Adaptation in Relation to Stress Conditions. XXVII. Can Ascorbic Acid Modify the Adverse Effects of NaCl and Mannitol on Amino Acids, Nucleic Acids and Protein Patterns in Vicia Faba Seedlings? Protoplasma 2009, 235, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Polenta, G.A.; Calvete, J.J.; González, C.B. Isolation and Characterization of the Main Small Heat Shock Proteins Induced in Tomato Pericarp by Thermal Treatment. FEBS J. 2007, 274, 6447–6455. [Google Scholar] [CrossRef] [PubMed]
- Sobhanian, N.; Pakniyat, H.; Kordshooli, M.A.; Dorostkar, S.; Aliakbari, M.; Nasiri, Z.F. Electrophresis Study of Wheat (Triticum Aestivum L.) Protein Changes under Salinity Stress. Sci. Res. 2016, 4, 33–36. [Google Scholar] [CrossRef][Green Version]
| Treatment | RFW (g) | RDW (mg) | SFW (g) | SDW (mg) | LFW (g) | LDW (mg) | Fresh Yield (g) | Dry Yield (g) | No. of Nodules | LA (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| C | 2.11 ± 0.02d | 183 ± 1.76e | 3.34 ± 0.02d | 531 ± 1.20d | 3.42 ± 0.03d | 372 ± 3.48d | 2.78 ± 0.04d | 1.70 ± 0.03bc | 2.30 ± 0.02d | 20.90 ± 0.17d |
| S | 1.03 ± 0.01a (−51.2) | 95 ± 3.18a (−48.1) | 2.29 ± 0.02a (−31.4) | 291 ± 2.40a (−45.1) | 2.60 ± 0.03a (−23.9) | 221 ± 2.90a (−40.5) | 1.85 ± 0.04a (−33.4) | 0.94 ± 0.02a (−44.8) | 0.79 ± 0.02a (−65.5) | 14.13 ± 0.17a (−32.3) |
| IAA | 2.41 ± 0.02b (14.2) | 209 ± 2.02b (14.2) | 3.55 ± 0.02b (6.1) | 583 ± 3.18b (9.8) | 3.94 ± 0.02b (14.9) | 392 ± 2.64b (5.3) | 4.38± 0.03b (57.7) | 2.13 ± 0.04b (25.2) | 3.41 ± 0.02b (48.4) | 22.76 ± 0.14b (8.9) |
| S + IAA | 1.64 ± 0.02c (−22.3) | 132 ± 2.33c (−27.9) | 2.95 ± 0.03c (−11.6) | 387 ± 2.33c (−27.0) | 2.85 ± 0.01c (−16.7) | 273 ± 2.51c (−26.6) | 2.53 ± 0.03c (−8.9) | 1.50 ± 0.01c (−11.5) | 2.06 ± 0.03c (−10.4) | 18.43 ± 0.26c (−11.8) |
| BA | 2.35 ± 0.02b (11.7) | 196 ± 3.18d (6.9) | 3.49 ± 0.01b (4.3) | 575 ± 3.18b (8.3) | 3.84 ± 0.02b (12.1) | 381 ± 4.04bd (2.3) | 4.36 ± 0.03b (56.9) | 2.12 ± 0.04b (24.4) | 3.42 ± 0.01b (48.8) | 22.46 ± 0.27b (7.5) |
| S + BA | 1.54 ± 0.02c (−26.8) | 124 ± 2.33c (−32.1) | 2.90 ± 0.02c (−13.1) | 375 ± 3.21c (−29.4) | 2.78 ± 0.03c (−18.7) | 270 ± 2.33c (−27.3) | 2.46 ± 0.03c (−11.3) | 1.54 ± 0.07c (−9.3) | 1.99 ± 0.03c (−13.4) | 18.03 ± 0.20c (−13.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Latef, A.A.H.; Akter, A.; Tahjib-Ul-Arif, M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy 2021, 11, 790. https://doi.org/10.3390/agronomy11040790
Abdel Latef AAH, Akter A, Tahjib-Ul-Arif M. Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy. 2021; 11(4):790. https://doi.org/10.3390/agronomy11040790
Chicago/Turabian StyleAbdel Latef, Arafat Abdel Hamed, Ayasha Akter, and Md. Tahjib-Ul-Arif. 2021. "Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L." Agronomy 11, no. 4: 790. https://doi.org/10.3390/agronomy11040790
APA StyleAbdel Latef, A. A. H., Akter, A., & Tahjib-Ul-Arif, M. (2021). Foliar Application of Auxin or Cytokinin Can Confer Salinity Stress Tolerance in Vicia faba L. Agronomy, 11(4), 790. https://doi.org/10.3390/agronomy11040790








