Effect of White Clover (Trifolium repens L.) Undersowing Cultivation and Nitrogen Fertilization on Weed Infestation, Biomass Yield and Its Component, Content and Uptake of Macroelements of Willow (Salix viminalis L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Soil Conditions
- The soil reaction (KCl) by the potentiometric method;
- Magnesium by Schachtschabel method [28].
2.3. Weather Conditions
2.4. Material and Management Practices
2.5. Measurement
2.6. Chemical Analysis
- Crude ash—determined by burning of 2 g plant material at 600 °C in an electric furnace. After a loss on ignition, the crucibles were placed in a desiccator to cool down and then weighed to the nearest 0.001 g.
- The Kjeldahl method using the Kjeltec apparatus was applied for total nitrogen determination [33].
- Potassium and calcium concentrations were determined by flame photometry using Flapho 4 (Carl Zeiss Jena—GDR).
- Phosphorus concentration was determined by a colorimetric method with vanadium and molybdenum reagents using Spekol 10—Carl Zeiss (Jena—GDR).
- Magnesium concentration was determined by a colorimetric method with yellow titanium using Spekol 10 (Carl Zeiss Jena—GDR).
2.7. Statistical Analysis
3. Results and Discussion
3.1. Weed Assessments
3.2. Plant Mortality Assessments
3.3. Morphological Traits
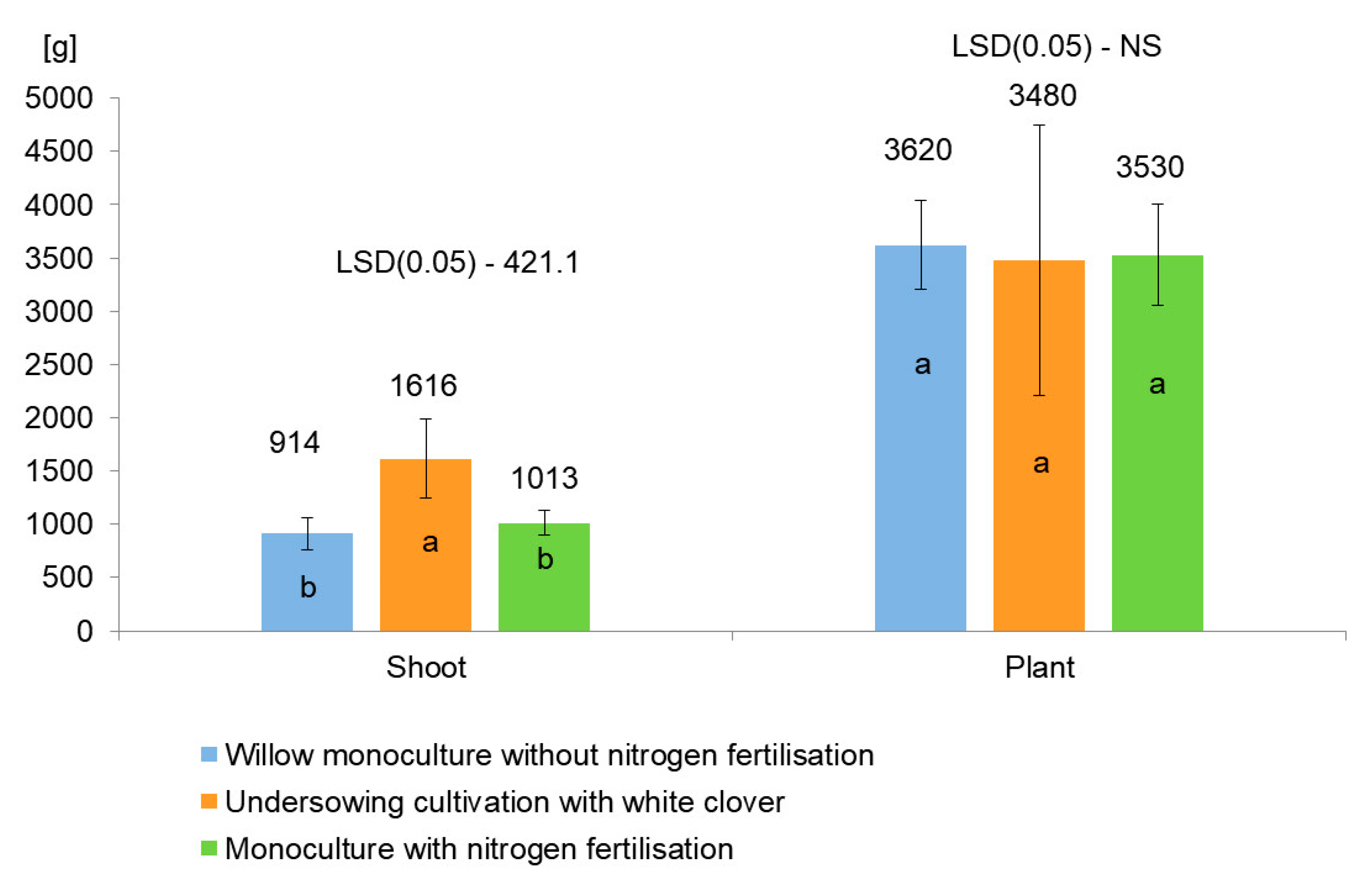
3.4. Yield of Fresh and Dry Mass of Willow
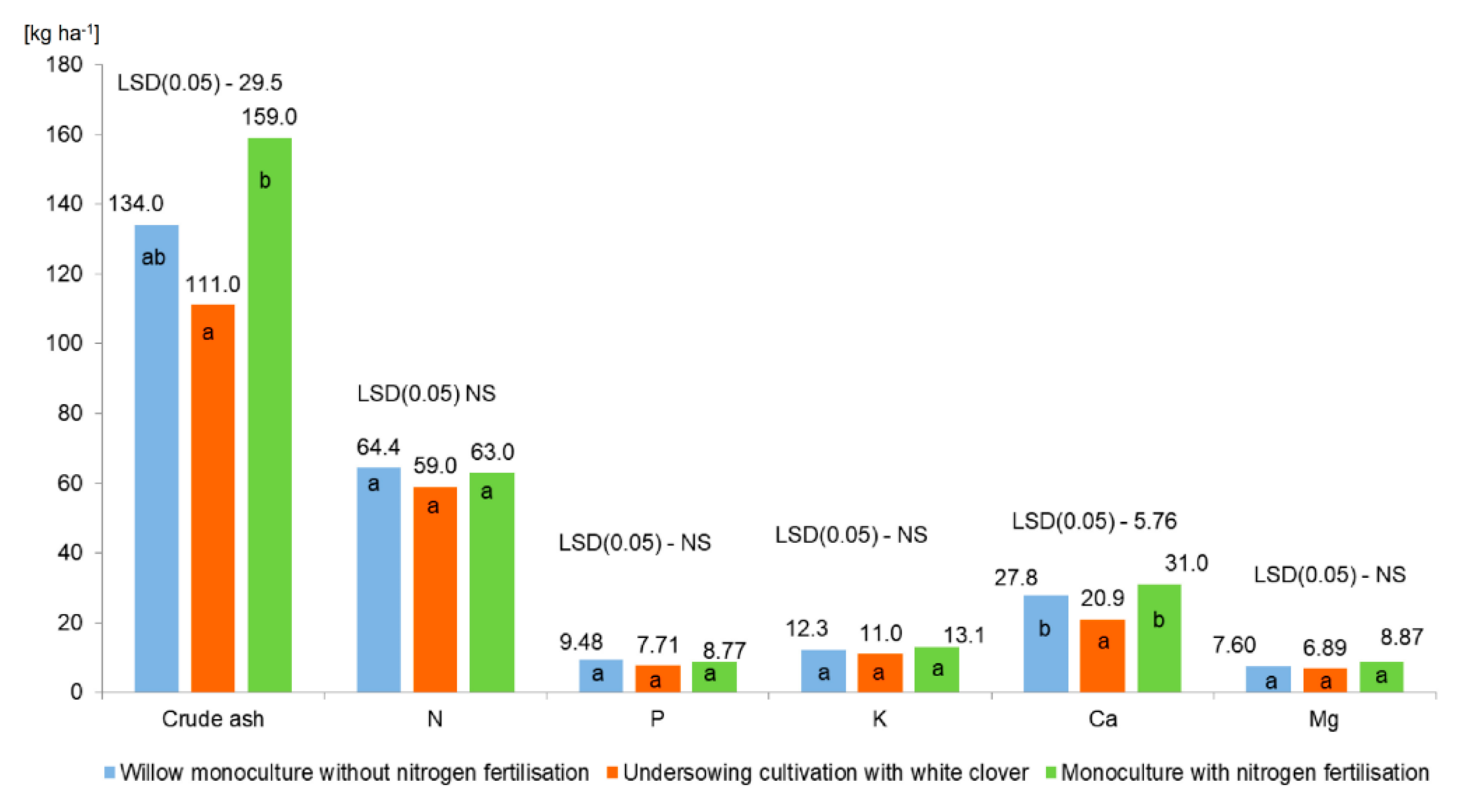
3.5. Chemical Composition and Uptake of Macronutrients in Willow Plants
4. Conclusions
Funding
Conflicts of Interest
References
- Keoleian, G.A.; Volk, T.A. Renewable Energy from Willow Biomass Crops: Life Cycle Energy, Environmental and Economic Performance. CRC Crit. Rev. Plant Sci. 2005, 24, 385–406. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Hastings, A.; Mos, M.; McCalmont, J.P.; Ashman, C.; Awty-Carroll, D.; Cerazy, J.; Chiang, Y.-C.; Cosentino, S.; Cracroft-Eley, W.; et al. Progress in upscaling Miscanthus biomass production for the European bio- economy with seed based hybrids. GCB Bioenergy 2017. [Google Scholar] [CrossRef]
- Hansen, E.M.; Christensen, B.T.; Jensen, L.S.; Kristensen, K. Carbon sequestration in soil beneath long-term Miscanthus plantations as determined by 13C abundance. Biomass Bioenergy 2004, 26, 97–105. [Google Scholar] [CrossRef]
- Caputo, J.; Balogh, S.B.; Volk, T.A.; Johnson, L.; Puettmann, M.; Lippke, B.; Oneil, E. Incorporating Uncertainty into a Life Cycle Assessment (LCA) Model of Short-Rotation Willow Biomass (Salix spp.) Crops. Bioenergy Res. 2014, 7, 48–59. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Breuer, J.; Jones, M.B. Carbon mitigation by the energy crop, Miscanthus. Glob. Chang. Biol. 2007, 13, 2296–2307. [Google Scholar] [CrossRef]
- McLaughlin, S.; Walsh, M. Evaluating environmental consequences of producing herbaceous crops for bioenergy. Biomass Bioenergy 1998, 14, 317–324. [Google Scholar] [CrossRef]
- Stolarski, M.; Szczukowski, S.; Tworkowski, J.; Klasa, A. Productivity of seven clones of willow coppice in annual and quadrennial cutting cycles. Biomass Bioenergy 2008, 32, 1227–1234. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Szczukowski, S.; Tworkowski, J.; Załuski, D.; Bieniek, A.; Gołaszewski, J. Effect of Increased Soil Fertility on the Yield and Energy Value of Short-Rotation Woody Crops. Bioenergy Res. 2015, 8, 1136–1147. [Google Scholar] [CrossRef]
- Verwijst, T. Willows: An underestimated resource for environment and society. For. Chron. 2001, 77, 281–285. [Google Scholar] [CrossRef]
- Serapiglia, M.J.; Cameron, K.D.; Stipanovic, A.J.; Abrahamson, L.P.; Volk, T.A.; Smart, L.B. Yield and Woody Biomass Traits of Novel Shrub Willow Hybrids at Two Contrasting Sites. Bioenergy Res. 2013, 6, 533–546. [Google Scholar] [CrossRef]
- Eckersten, H.; Noroha-Sannervik, A.; Torssell, B.; Nyman, P. (Eds.) Modelling Radiation Use, Water and Nitrogen in Willow Forest; Report from the Department of Crop Production Ecology (VPE); Swedish University of Agricultural Sciences (SLU): Uppsala, Sweden, 2006; ISBN 91-576-6898-1-21. [Google Scholar]
- Heilman, P.; Norby, R.J. Nutrient cycling and fertility management in temperate short rotation forest systems. Biomass Bioenergy 1998, 14, 361–370. [Google Scholar] [CrossRef]
- Malik, R.K.; Green, T.H.; Brown, G.F.; Mays, D. Use of cover crops in short rotation hardwood plantations to control erosion. Biomass Bioenergy 2000, 18, 479–487. [Google Scholar] [CrossRef]
- Mann, L.; Tolbert, V. Soil Sustainability in Renewable Biomass Plantings. AMBIO J. Hum. Environ. 2000, 29, 492–498. [Google Scholar] [CrossRef]
- Kort, J.; Collins, M.; Ditsch, D. A review of soil erosion potential associated with biomass crops. Biomass Bioenergy 1998, 14, 351–359. [Google Scholar] [CrossRef]
- Andrews, M.; Scholefield, D.; Abberton, M.T.; McKenzie, B.A.; Hodge, S.; Raven, J.A. Use of white clover as an alternative to nitrogen fertiliser for dairy pastures in nitrate vulnerable zones in the UK: Productivity, environmental impact and economic considerations. Ann. Appl. Biol. 2007, 151, 11–23. [Google Scholar] [CrossRef]
- Balasus, A.; Bischoff, W.-A.; Schwarz, A.; Scholz, V.; Kern, J. Nitrogen fluxes during the initial stage of willows and poplars in short-rotation coppices. J. Plant Nutr. Soil Sci. 2012, 175, 729–738. [Google Scholar] [CrossRef]
- Mc Laughlin, R.A.; Pope, P.E.; Hansen, E.A. Nitrogen Fertilization and Ground Cover in a Hybrid Poplar Plantation: Effects on Nitrate Leaching. J. Environ. Qual. 1985, 14, 241–245. [Google Scholar] [CrossRef]
- Fabio, E.S.; Smart, L.B. Effects of nitrogen fertilization in shrub willow short rotation coppice production—A quantitative review. GCB Bioenergy 2018, 10, 548–564. [Google Scholar] [CrossRef]
- Densmore, R.V.; Neiland, B.J.; Zasada, J.C.; Masters, M.A. Planting Willow for Moose Habitat Restoration on the North Slope of Alaska, U.S.A. Arct. Alp. Res. 1987, 19, 537–543. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Drew, A.P.; Volk, T.A. The effect of common Dutch white clover (Trifolium repens L.), as a green manure, on biomass production, allometric growth and foliar nitrogen of two willow clones. Biomass Bioenergy 2005, 29, 22–31. [Google Scholar] [CrossRef]
- Helios, W. Effect of white clover (Trifolium repens L.) intercropping system on weed infestation of willow (Salix viminalis L.). Prog. Plant Prot./Postępy Ochr. Roślin 2013, 53, 303–309. [Google Scholar]
- Smil, V. Global Population and the Nitrogen Cycle. Sci. Am. 1997, 277, 76–81. [Google Scholar] [CrossRef]
- Malik, R. Biomass production of short-rotation bioenergy hardwood plantations affected by cover crops. Biomass Bioenergy 2001, 21, 21–33. [Google Scholar] [CrossRef]
- Cogliastro, A.; Gagnon, D.; Coderre, D.; Bhéreur, P. Response of seven hardwood tree species to herbicide, rototilling, and legume cover at two southern Quebec plantation sites. Can. J. For. Res. 1990, 20, 1172–1182. [Google Scholar] [CrossRef]
- Egner, H. Bestimmung der Kalibedürftigkeit des Bodens auf Chemischen Wege; Bodenkunde und Pflanzenbau: Berlin, Germany, 1940. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Utersuchungen über die chemische Bodenanalyse als Grudlage für die Beurteilung des Nährstoff-zunstandes der Boden. II. K. Lantbrhogsk. Annlr. 1960, 20, 199–216. [Google Scholar]
- Schachtschabel, P. Das pflanzenverfügbare Magnesium des Boden und seine Bestimmung. Z. Pflanzenernaehr. Dueng. Bodenk. 1954, 67, 9–23. [Google Scholar] [CrossRef]
- McCracken, A.; Dawson, W.; Bowden, G. Yield responses of willow (Salix) grown in mixtures in short rotation coppice (SRC). Biomass Bioenergy 2001, 21, 311–319. [Google Scholar] [CrossRef]
- Kim, H.-G.; Song, H.-J.; Jeong, M.-J.; Seo, Y.-L.; Yang, J.-K.; Yoo, S.-B.; Choi, M.-S. Bioethanol production by enzymatic saccharification of Salix viminalis var. gigantea biomass. For. Sci. Technol. 2014, 10, 67–72. [Google Scholar] [CrossRef]
- Jama-Rodzeńska, A.; Bocianowski, J.; Nowak, W.; Ciszek, D.; Nowosad, K. The influence of communal sewage sludge on the content of macroelements in the stem of selected clones of willow (Salix viminalis L.). Ecol. Eng. 2016, 87, 212–217. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R. Two extremely different crops, Salix and Sida, as sources of renewable bioenergy. Biomass Bioenergy 2012, 36, 234–240. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Fresenius Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Analysis of Variance for Orthogonal Sets. The awa programme. In Description of Statistical Programmes Elaborated at the Institute of Information Technology; Bartkowiak, A., Ed.; The University of Wrocław: Wrocław, Poland, 1978. [Google Scholar]
- Albertsson, J.; Hansson, D.; Bertholdsson, N.-O.; Åhman, I.; Kempenaar, C. Site-related set-back by weeds on the establishment of 12 biomass willow clones. Weed Res. 2014, 54, 398–407. [Google Scholar] [CrossRef]
- Welc, M.; Lundkvist, A.; Verwijst, T. Effects of Cutting Phenology (Non-dormant Versus Dormant) on Early Growth Performance of Three Willow Clones Grown Under Different Weed Treatments and Planting Dates. Bioenergy Res. 2017, 10, 1094–1104. [Google Scholar] [CrossRef]
- Albertsson, J.; Verwijst, T.; Hansson, D.; Bertholdsson, N.-O.; Åhman, I. Effects of competition between short-rotation willow and weeds on performance of different clones and associated weed flora during the first harvest cycle. Biomass Bioenergy 2014, 70, 364–372. [Google Scholar] [CrossRef]
- Edelfeldt, S.; Lundkvist, A.; Forkman, J.; Verwijst, T. Establishment and Early Growth of Willow at Different Levels of Weed Competition and Nitrogen Fertilization. Bioenergy Res. 2016, 9, 763–772. [Google Scholar] [CrossRef]
- Adiele, J.G.; Volk, T.A. Developing live cover crop systems for willow (Salix spp.) bioenergy crop. Sky J. Soil Sci. Environ. Manag. 2013, 2, 84–100. [Google Scholar]
- McCracken, A.R.; Walsh, L.; Moore, P.J.; Lynch, M.; Cowan, P.; Dawson, M.; Watson, S. Yield of willow (Salix spp.) grown in short rotation coppice mixtures in a long-term trial. Ann. Appl. Biol. 2011, 159, 229–243. [Google Scholar] [CrossRef]
- Hangs, R.D.; Stevenson, F.C.; Schoenau, J.J.; Van Rees, K.C.J. Measuring Harvestable Biomass in Short-Rotation Willow Bioenergy Plantations Using Light Attenuation. Bioenergy Res. 2013, 6, 83–90. [Google Scholar] [CrossRef]
- Brown, K.R.; van den Driessche, R. Growth and nutrition of hybrid poplars over 3 years after fertilization at planting. Can. J. For. Res. 2002, 32, 226–232. [Google Scholar] [CrossRef]
- Perttu, K.L. Environmental and hygienic aspects of willow coppice in Sweden. Biomass Bioenergy 1999, 16, 291–297. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.; Szczukowski, S. Willow biomass obtained from different soils as a feedstock for energy. Ind. Crops Prod. 2015, 75, 114–121. [Google Scholar] [CrossRef]
- Mirck, J.; Schroeder, W. Composition, Stand Structure, and Biomass Estimates of “Willow Rings” on the Canadian Prairies. Bioenergy Res. 2013, 6, 864–876. [Google Scholar] [CrossRef]
- Mitchell, C.P. New cultural treatments and yield optimisation. Biomass Bioenergy 1995, 9, 11–34. [Google Scholar] [CrossRef]
- Aylott, M.J.; Casella, E.; Tubby, I.; Street, N.R.; Smith, P.; Taylor, G. Yield and spatial supply of bioenergy poplar and willow short-rotation coppice in the UK. New Phytol. 2008, 178, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Szczukowski, S.; Stolarski, M.; Tworkowski, J.; Przyborowski, J.; Klasa, A. Productivity of willow coppice plants grown in short rotations. Plant Soil Environ. 2011, 51, 423–430. [Google Scholar] [CrossRef]
- Szczukowski, S.; Tworkowski, J.; Klasa, A.; Stolarski, M. Productivity and chemical composition of wood tissues of short rotation willow coppice cultivated on arable land. Plant Soil Environ. 2011, 48, 413–417. [Google Scholar] [CrossRef]
- Heller, M.C.; Keoleian, G.A.; Volk, T.A. Life cycle assessment of a willow bioenergy cropping system. Biomass Bioenergy 2003, 25, 147–165. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Gołąb-Bogacz, I.; Helios, W.; Kotecki, A.; Kozak, M.; Jama-Rodzeńska, A. Content and Uptake of Ash and Selected Nutrients (K, Ca, S) with Biomass of Miscanthus × giganteus Depending on Nitrogen Fertilization. Agriculture 2021, 11, 76. [Google Scholar] [CrossRef]
- Schroeder, W.; Kort, J.; Savoie, P.; Preto, F. Biomass Harvest from Natural Willow Rings around Prairie Wetlands. Bioenergy Res. 2009, 2, 99–105. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. High biomass yield achieved by Salix clones in SRIC following two 3-year coppice rotations on abandoned farmland in southern Quebec, Canada. Biomass Bioenergy 2003, 25, 135–146. [Google Scholar] [CrossRef]
- Sevel, L.; Ingerslev, M.; Nord-Larsen, T.; Jørgensen, U.; Holm, P.E.; Schelde, K.; Raulund-Rasmussen, K. Fertilization of SRC Willow, II: Leaching and Element Balances. Bioenergy Res. 2014, 7, 338–352. [Google Scholar] [CrossRef]
- Scholz, V. The growth productivity, and environmental impact of the cultivation of energy crops on sandy soil in Germany. Biomass Bioenergy 2002, 23, 81–92. [Google Scholar] [CrossRef]

| Years | pH | Content of Assimilable Forms (mg kg−1) | ||
|---|---|---|---|---|
| (1 M KCl) | P | K | Mg | |
| 2013 | 7.45 | 159 | 154 | 36.0 |
| 2014 | 7.85 | 140 | 160 | 38.9 |
| 2015 | 7.80 | 162 | 153 | 30.8 |
| 2016 | 7.64 | 178 | 186 | 36.4 |
| 2017 | 7.75 | 170 | 193 | 34.8 |
| Mean | 7.70 | 162 | 169 | 35.4 |
| SD | 0.159 | 14.250 | 18.89 | 2.963 |
| Months | Years | Standard Deviation 2013–2017 | Mean Temperature (30-Year Monthly) | ||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | |||
| January | −1.6 | 0.0 | 2.3 | −1.2 | −3.4 | 2.11 | −0.4 |
| February | 0.1 | 3.7 | 1.5 | 3.8 | 0.9 | 1.67 | 0.6 |
| March | −0.9 | 7.0 | 5.4 | 4.3 | 6.8 | 3.22 | 3.8 |
| April | 9.2 | 10.6 | 8.9 | 8.7 | 7.9 | 0.99 | 8.9 |
| May | 14.6 | 13.3 | 13.5 | 15.3 | 14.2 | 0.82 | 14.4 |
| June | 17.7 | 16.6 | 16.6 | 18.6 | 18.5 | 0.98 | 17.3 |
| July | 20.5 | 21.2 | 20.3 | 19.5 | 19.0 | 0.86 | 19.6 |
| August | 19.0 | 17.3 | 22.7 | 17.9 | 19.4 | 2.10 | 18.6 |
| September | 12.9 | 15.5 | 15.1 | 16.4 | 13.3 | 1.49 | 13.7 |
| October | 10.8 | 10.7 | 8.4 | 8.5 | 12.0 | 1.57 | 9.1 |
| November | 5.6 | 6.6 | 6.2 | 3.4 | 5.5 | 1.24 | 4.3 |
| December | 3.0 | 2.3 | 5.4 | 1.2 | 2.9 | 1.54 | 0.6 |
| Mean | 9.2 | 10.4 | 10.5 | 9.7 | 9.8 | 0.54 | 9.2 |
| Months | Years | Standard Deviation 2013–2017 | Sums of Precipitation (30-Year Monthly Average) | ||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | |||
| January | 51.3 | 35.8 | 46.0 | 33.4 | 16.9 | 13.26 | 34.9 |
| February | 29.5 | 1.2 | 15.6 | 56.2 | 24.2 | 20.30 | 27.9 |
| March | 43.0 | 40.1 | 39.5 | 55.9 | 31.1 | 8.98 | 38.2 |
| April | 42.7 | 55.2 | 15.8 | 46.4 | 57 | 16.55 | 33.6 |
| May | 135.9 | 101.4 | 21.0 | 5.3 | 24.1 | 57.55 | 54.1 |
| June | 171.7 | 40.2 | 73.3 | 44.6 | 52.5 | 54.74 | 67.4 |
| July | 36.3 | 52.9 | 55.6 | 114.3 | 112.2 | 36.36 | 78.9 |
| August | 68.2 | 75.0 | 5.6 | 27.1 | 43.6 | 28.75 | 65.3 |
| September | 105.8 | 72.2 | 23.2 | 44.7 | 65.7 | 30.99 | 44.9 |
| October | 7.8 | 59.4 | 20.0 | 83.8 | 71.4 | 33.01 | 33.7 |
| November | 25.8 | 15.5 | 52.5 | 36.3 | 28.4 | 13.80 | 36.6 |
| December | 13.0 | 17.5 | 24.0 | 36.1 | 29.6 | 9.23 | 36.3 |
| Sum | 731.0 | 566.4 | 392.1 | 584.1 | 556.6 | 120.29 | 551.8 |
| Years | Cultivation Willow | Number of Weeds (pcs m−2) | Dry Weight of Weeds (g m−2) |
|---|---|---|---|
| A | 21 e ± 4.4 | 384 ab ± 270.8 | |
| 2013 | B | 13 abc ± 1.2 | 23 a ± 22.6 |
| C | 19 de ± 1.6 | 698 b ± 391.2 | |
| A | 18 cde ± 4.0 | 244 a ± 77.2 | |
| 2014 | B | 11 a ± 0.6 | 201 a ± 71.6 |
| C | 17 bcde ± 2.4 | 325 a ± 133.4 | |
| A | 16 abcde ± 2.8 | 255 a ± 41.2 | |
| 2015 | B | 12 ab ± 0.4 | 30 a ± 7.8 |
| C | 13 abc ± 1.8 | 279 a ± 94.8 | |
| A | 15 abcd ± 0.8 | 223 a ± 7.0 | |
| 2016 | B | 12 ab ± 0.6 | 168 a ± 22.6 |
| C | 15 abcd ± 1.0 | 216 a ± 18.6 | |
| A | 13 abc ± 0.8 | 207 a ± 197.8 | |
| 2017 | B | 12 ab ± 0.6 | 252 a ± 74.0 |
| C | 16 abcde ± 1.6 | 180 a ± 91.0 | |
| LSD (α = 0.05) | 2.7 | 189.4 | |
| Mean for Cultivation System and Years | |||
| A | 17 b ± 3.9 | 262 b ± 152.1 | |
| B | 12 a ± 0.9 | 135 a ± 104.2 | |
| C | 16 b ± 2.6 | 339 b ± 257.3 | |
| LSD (α = 0.05) | 1.2 | 84.7 | |
| 2013 | 18 b ± 4.3 | 368 b ± 380.7 | |
| 2014 | 15 ab ± 4.1 | 257 ab ± 103.7 | |
| 2015 | 14 a ± 2.5 | 188 a ± 129.1 | |
| 2016 | 14 a ± 1.7 | 202 ab ± 30.0 | |
| 2017 | 14 a ± 2.0 | 213 ab ± 124.0 | |
| LSD (α = 0.05) | 1.6 | 109.3 | |
| Years | Cultivation System | Number of Plants (pcs m−2) | Plant Mortality * | Number of Shoots | ||
|---|---|---|---|---|---|---|
| a | b | (pcs m−2) | (pcs plant−1) | |||
| A | 1.52 a ± 0.077 | 8.98 | 2.93 a ± 0.752 | 4.98 ab ± 0.720 | 3.27 abc ± 0.342 | |
| 2013 | B | 1.48 ab ± 0.077 | 11.38 | 3.31 a ± 0.735 | 3.22 bcdef ± 0.552 | 2.19 bc ± 0.421 |
| C | 1.44 ± 0.161 | 13.92 | 3.22 a ± 2.169 | 4.67 abc ± 0.889 | 3.23 abc ± 0.284 | |
| A | 1.50 a ± 0.065 | 1.27 | 0.56 a ± 1.125 | 4.88 abc ± 0.745 | 3.25 abc ± 0.473 | |
| 2014 | B | 1.46 ab ± 0.046 | 1.27 | 0.56 a ± 1.125 | 3.03 cdef ± 0.660 | 2.08 bc ± 0.454 |
| C | 1.44 ab ± 0.161 | 0.0 | 0.00 a ± 0.000 | 4.55 abcd ± 0.757 | 3.16 abc ± 0.181 | |
| A | 1.36 ab ± 0.126 | 9.39 | 2.16 a ± 2.505 | 5.16 a ± 1.415 | 3.86 a ± 1.298 | |
| 2015 | B | 1.29 ab ± 0.146 | 11.7 | 2.91 a ± 2.067 | 2.70 def ± 0.890 | 2.08 bc ± 0.600 |
| C | 1.38 ab ± 0.052 | 3.74 | 0.97 a ± 1.935 | 4.26 abcde ± 0.190 | 3.10 abc ± 0.223 | |
| A | 1.36 ab ± 0.126 | 0.00 | 0.00 a ± 0.000 | 4.97 ab ± 0.832 | 3.65 a ± 0.417 | |
| 2016 | B | 1.25 ab ± 0.181 | 3.36 | 1.29 a ± 1.497 | 2.38 ef ± 0.471 | 1.91 c ± 0.296 |
| C | 1.35 ab ± 0.045 | 1.59 | 0.63 a ± 1.260 | 4.11 abcdef ± 0.538 | 3.05 abc ± 0.449 | |
| A | 1.29 ab ± 0.221 | 5.00 | 1.12 a ± 2.235 | 5.12 a ± 0.734 | 4.06 a ± 0.987 | |
| 2017 | B | 1.13 b ± 0.336 | 11.65 | 2.36 a ± 2.848 | 2.36 f ± 0.762 | 2.15 bc ± 0.490 |
| C | 1.27 ab ± 0.077 | 6.00 | 1.73 a ± 2.001 | 4.42 abcd ± 0.262 | 3.48 ab ± 0.159 | |
| LSD (α = 0.05) | NS | - | NS | NS | NS | |
| Mean for Cultivation System and Years | ||||||
| A | 1.40 a ± 0.151 | 4.93 | 1.35 a ± 1.804 | 5.02 a ± 0.831 | 3.62 a ± 0.781 | |
| B | 1.32 a ± 0.215 | 7.86 | 2.09 a ± 1.921 | 2.74 c ± 0.703 | 2.08 c ± 0.423 | |
| C | 1.37 a ± 0.18 | 5.05 | 1.31 a ± 1.873 | 4.40 b ± 0566 | 3.20 b ± 0.293 | |
| LSD (α = 0.05) | NS | - | NS | 0.454 | 0.329 | |
| 2013 | 1.48 a ± 0.107 | 11.43 | 3.15 b ± 1.271 | 4.29 a ± 1.0377 | 2.89 a ± 0.612 | |
| 2014 | 1.47 a ± 0.98 | 0.84 | 0.37 a ± 0.876 | 4.15 a ± 1.067 | 2.83 a ± 0.661 | |
| 2015 | 1.34 ab ± 0111 | 8.26 | 2.01 ab ± 2.143 | 4.04 a ± 1.379 | 3.01 a ± 1.073 | |
| 2016 | 1.32 ab ± 0.128 | 1.65 | 0.64 a ± 1.161 | 3.82 a ± 1.261 | 2.87 a ± 0.834 | |
| 2017 | 1.23 b ± 0.227 | 7.55 | 1.74 ab ± 2.224 | 3.97 a ± 1.352 | 3.23 a ± 1.018 | |
| LSD (α = 0.05) | 0.120 | - | 1.316 | NS | NS | |
| Years | Cultivation System | Height of Plants at the End of Vegetation (cm) | Yearly Increase in the Height of Plants (cm) | Diameter of Shoots After the End of Vegetation (mm) | Yearly Increase in Shoot Diameter (mm) |
|---|---|---|---|---|---|
| A | 80 f ± 3.5 | 80 a ± 3.5 | 10.0 e ± 0.22 | 10.0 a ± 0.22 | |
| 2013 | B | 75 f ± 10.3 | 75 a ± 10.3 | 9.8 e ± 0.14 | 9.8 a ± 0.14 |
| C | 83 f ± 6.8 | 83 a ± 6.8 | 10.2 e ± 0.14 | 10.2 a ± 0.14 | |
| A | 212 e ± 42.6 | 132 a ± 45.7 | 17.5 de ± 2.38 | 7.5 a ± 2.25 | |
| 2014 | B | 215 e ± 24.2 | 141 a ± 31.4 | 17.4 de ± 5.29 | 7.6 a ± 5.24 |
| C | 237 e ± 20.9 | 154 a ± 16.8 | 20.0 cde ± 1.93 | 9.7 a ± 2.05 | |
| A | 287 de ± 39.9 | 76 a ± 22.5 | 26.2 bcd ± 2.87 | 8.7 a ± 0.83 | |
| 2015 | B | 300 de ± 50.8 | 85 a ± 37.8 | 24.3 bcd ± 3.73 | 6.9 a ± 2.27 |
| C | 332 cd ± 12.4 | 95 a ± 13.0 | 26.9 bcd ± 3.89 | 7.0 a ± 2.26 | |
| A | 365 bcd ± 56.0 | 77 a ± 78.8 | 30.0 abc ± 4.04 | 3.8 a ± 2.39 | |
| 2016 | B | 415 bc ± 80.6 | 115 a ± 35.1 | 33.0 ab ± 6.89 | 8.7 a ± 6.49 |
| C | 440 ab ± 21.6 | 108 a ± 14.0 | 34.8 ab ± 7.34 | 7.8 a ± 10.41 | |
| A | 510 a ± 30.7 | 145 a ± 57.8 | 40.4 a ± 6.20 | 10.4 a ± 6.67 | |
| 2017 | B | 522 a ± 21.8 | 107 a ± 82.6 | 42.0 a ± 10.25 | 9.1 a ± 6.02 |
| C | 526 a ± 20.0 | 86 a ± 36.9 | 42.0 a ± 5.13 | 7.1 a ± 2.95 | |
| LSD (α = 0.05) | NS | NS | NS | NS | |
| Mean for Cultivation System and Years | |||||
| A | 291 b ± 152.1 | 102 a ± 53.7 | 24.8 a ± 11.2 | 8.1 a ± 3.83 | |
| B | 305 ab ± 164.1 | 104 a ± 47.3 | 25.3 a ± 12.89 | 8.4 a ± 4.32 | |
| C | 324 a ± 159.7 | 105 a ± 31.8 | 26.8 a ± 12.06 | 8.4 a ± 4.67 | |
| LSD (α = 0.05) | 22.9 | NS | NS | NS | |
| 2013 | 79 e ± 7.7 | 79 b ± 7.68 | 10.0 e ± 0.23 | 10.0 a ± 0.23 | |
| 2014 | 221 d ± 30.2 | 142 a ± 31.6 | 18.3 d ± 3.43 | 8.3 a ± 3.35 | |
| 2015 | 307 c ± 39.6 | 85 b ± 25.4 | 25.8 c ± 3.40 | 7.5 a ± 1.94 | |
| 2016 | 407 b ± 61.9 | 100 ab ± 48.7 | 32.6 b ± 6.03 | 6.8 a ± 6.90 | |
| 2017 | 519 a ± 23.4 | 113 ab ± 61.6 | 41.5 a ± 6.85 | 8.9 a ± 5.13 | |
| LSD (α = 0.05) | 29.6 | 34.5 | 3.98 | NS | |
| Cultivation System | Mean | Standard Deviation | Correlation Coefficients * | ||
|---|---|---|---|---|---|
| Dry Mass of the Main Shoot | Diameter of Shoots | Height of Plants | |||
| Dry mass of the main shoot | 1187 g | 605 | 1 | ||
| Diameter of shoots | 39.5 mm | 8.99 | 0.90 | 1 | |
| Height of plants | 510 cm | 100 | 0.74 | 0.75 | 1 |
| Cultivation System | Mean | Standard Deviation | Correlation Coefficients | ||
|---|---|---|---|---|---|
| Dry Mass of the Main Shoot | Diameter of Shoots | Height of Plants | |||
| Willow monoculture without nitrogen fertilization | |||||
| Dry mass of the main shoot | 908 g | 545 | 1 | ||
| Diameter of shoots | 34.3 mm | 6.7 | 0.98 | 1 | |
| Height of plants | 452 cm | 72.7 | 0.81 | 0.82 | 1 |
| Undersowing cultivation with white clover | |||||
| Dry mass of the main shoot | 1385 g | 729 | 1 | ||
| Diameter of shoots | 41.8 mm | 9.6 | 0.93 | 1 | |
| Height of plants | 556 cm | 123.7 | 0.71 | 0.69 | 1 |
| Monoculture with nitrogen fertilization | |||||
| Dry mass of the main shoot | 1269 g | 468 | 1 | ||
| Diameter of shoots | 42.6 mm | 8.89 | 0.81 | 1 | |
| Height of plants | 552 cm | 76.3 | NS * | 0.71 | 1 |
| Cultivation System | Dry Mass Yield (Mg ha−1) | Fresh Mass Yield (Mg ha−1) | ||
|---|---|---|---|---|
| Wood | Phloem | Total | ||
| Willow monoculture without nitrogen fertilization | 36.8 a ± 4.02 | 9.4 a ± 1.06 | 46.2 a ± 5.08 | 91.2 a ± 7.12 |
| Undersowing cultivation with white clover | 29.0 a ± 4.19 | 7.3 a ± 1.04 | 36.3 a ± 5.23 | 71.1 b ± 1.39 |
| Monoculture with nitrogen fertilization | 35.7 a ± 3.54 | 9.0 a ± 0.88 | 44.7 a ± 4.42 | 90.5 a ± 6.33 |
| LSD (α = 0.05) | NS | NS | NS | 9.02 |
| Cultivation System | Crude Ash | N | P | K | Ca |
|---|---|---|---|---|---|
| g kg−1 | |||||
| Willow Wood | |||||
| Willow monoculture without nitrogen fertilization | 7.2 | 3.9 | 0.7 | 0.8 | 1.4 |
| Undersowing cultivation with white clover | 7.3 | 5.1 | 0.9 | 1.0 | 1.5 |
| Monoculture with nitrogen fertilization | 10.2 | 3.8 | 0.7 | 0.9 | 1.9 |
| Willow Phloem | |||||
| Willow monoculture without nitrogen fertilization | 43.1 | 19.0 | 2.3 | 3.4 | 9.3 |
| Undersowing cultivation with white clover | 47.5 | 20.2 | 1.7 | 3.6 | 8.4 |
| Monoculture with nitrogen fertilization | 47.9 | 20.0 | 2.1 | 3.7 | 9.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helios, W. Effect of White Clover (Trifolium repens L.) Undersowing Cultivation and Nitrogen Fertilization on Weed Infestation, Biomass Yield and Its Component, Content and Uptake of Macroelements of Willow (Salix viminalis L.). Agronomy 2021, 11, 786. https://doi.org/10.3390/agronomy11040786
Helios W. Effect of White Clover (Trifolium repens L.) Undersowing Cultivation and Nitrogen Fertilization on Weed Infestation, Biomass Yield and Its Component, Content and Uptake of Macroelements of Willow (Salix viminalis L.). Agronomy. 2021; 11(4):786. https://doi.org/10.3390/agronomy11040786
Chicago/Turabian StyleHelios, Waldemar. 2021. "Effect of White Clover (Trifolium repens L.) Undersowing Cultivation and Nitrogen Fertilization on Weed Infestation, Biomass Yield and Its Component, Content and Uptake of Macroelements of Willow (Salix viminalis L.)" Agronomy 11, no. 4: 786. https://doi.org/10.3390/agronomy11040786
APA StyleHelios, W. (2021). Effect of White Clover (Trifolium repens L.) Undersowing Cultivation and Nitrogen Fertilization on Weed Infestation, Biomass Yield and Its Component, Content and Uptake of Macroelements of Willow (Salix viminalis L.). Agronomy, 11(4), 786. https://doi.org/10.3390/agronomy11040786






