1. Introduction
Increasing climate warming and advances in soybean breeding have allowed cultivation of this species in East-Central Europe, including Poland. Soybean (
Glycine max (L.) Merr.), one of the most important legumes in the world, is capable of fixing atmospheric nitrogen [
1,
2,
3]. Following crop yields are higher in subsequent years despite lack of artificial fertilizer application [
4]. Chen et al. [
5] and Chen and Wiatrak [
6] claim that soybean is a significant nitrogen source for maize grown as a following crop. In order to obtain high production and economic effects, modern intensive agriculture heavily relies on mineral nitrogen fertilizer application. However, excessive amounts of mineral nitrogen forms in soil pose a threat to the natural environment as nitrogen may leach to groundwater and be released to the atmosphere. Effective soil nitrogen management which is safe for the environment is a key element of sustainable and organic agriculture. In this respect, inclusion of leguminous plants in the crop rotation seems to provide a safe nitrogen source. These plants establish specific symbiotic relationships with soil-borne bacteria. Atmospheric nitrogen is fixed by these microorganisms and such a process in called biological nitrogen fixation (BNF) [
7,
8]. In nature, BNF occurs with the participation of microbes (bacteria) which are prokaryotic organisms. Seed inoculation with
Rhizobium increases NPK uptake (by as much as 39%) in soybean stems and enhances soil fertility [
9].
That having been said, recent years have seen increasing interest in biological methods of improving yield performance and quality, and soybean plants are more and more frequently introduced into crop rotation [
10,
11]. This, however, can often be restricted by climate and agrotechnological conditions [
12,
13,
14,
15,
16]. Sowing date and density as well as fertilization and cultivation practices have the greatest effect on yield performance [
17,
18,
19,
20,
21,
22,
23]. When the number of plants per 1 m
2 is low and there is no competition among plants, the yield is proportional to stand density [
24]. Increased density results in increased competition and a declining rate of yield increase. Lima et al. [
25] demonstrated that, with such sowing, seed yield increased by 8.6% compared with conventional planting. If growers have access to information on improved soybean cultivars, agrotechnology and benefits of soybean cultivation, they are more likely to meet the demand on the market and producers’ expectations [
26,
27].
The objective of the research reported here was to determine the amount of nitrogen fixed from the atmosphere and taken up from mineral fertilizer and soil reserves by soybean cv. Abelina grown at three densities (per 1 m2) under central European conditions. Moreover, an attempt was made to determine what amount of nitrogen taken up from the individual sources was removed from the field with seed yield and that was introduced to the soil with post-harvest residues and will be the source of this macronutrient for the following plants.
Research hypotheses assumed that, regardless of sowing density, the atmosphere will be the predominant nitrogen source for soybean cv. Abelina, and the quantity of nitrogen taken up by this crop plant from different sources will vary according to sowing density and environmental conditions.
2. Materials and Methods
2.1. Description of the Experiment
A field experiment was carried out at Łączka, eastern Poland, (N52°15′, E21°95′) in 2017–2018. It was arranged as a randomized block design with three replicates. The experimental factor was the seeding density of soybean cv. Abelina. The cultivar is included in the List of Cultivars Recommended for Cultivation in Poland (Lista Odmian Zalecanych). It is a second early variety 000++ characterized by a rapidly closing canopy, high attached lowest pods and high fat and protein contents. The following densities were used: A
1—50 seeds, A
2—70 seeds and A
3—90 seeds per 1 m
2. The seeds which are supplied by the company are ready for sowing as they are chemically treated and coated with the nodulating bacteria
Bradyrhizobium japonicum. The trial was established on soil classified as Haplic Luvisol according to the World Reference Base for Soil Resources [
28]. The soil was consisting of 69.4% sand, 28.2% silt, and 2.4% clay. Selected properties of this soil are given in
Table 1. The soil had an average phosphorus content, organic carbon content and total nitrogen content, a high potassium content and a low magnesium content of plant-available forms.
The research involved isotope dilution analysis which included mineral fertilizers enriched with the isotope
15N (5%) and simultaneous cultivation of control plant-maize (
Zea mays L.)—unable to form symbiotic relations with nodulating bacteria. Soybean was grown in micro-plots whose area was 1 m
2 and which were established in plots with an appropriate between-row spacing. Both soybean and maize were fertilized with ammonium sulphate (NH
4)
2SO
4 applied at the rate corresponding to 30 kg N∙ha
−1 (3 g N∙m
−2) introduced into the soil. P and K rates were based on soil availability of these nutrients (
Table 1) and they amounted to 30 kg P and 90 kg K per 1 ha. Each year, soybean was preceded by maize and it was grown in a conventional soil cultivations system.
Both soybean and maize were sown by hand on 4 and 5 May at the between-row spacing of 22 cm and the depth of 4 cm. Maize sowing density was 75 seeds per 1 m2. No herbicides were applied to either soybean or maize, weeds being removed by hand. The whole plants of maize and soybean were harvested manually in late September at the full maturity stage (BBCH99) by digging them out of the depth of 0.25 m. Next, each plant was separated into roots, above-ground post-harvest residues and seeds. The post-harvest residues included all the above-ground parts of soybean or maize plants, excluding seeds. Then, the harvested mass of each group was weighed and sampled to obtain representative samples.
2.2. Weather Conditions
During the soybean growing season, the crop’s thermal and precipitation requirements differ according to a stage of soybean development. From seeding to full emergence, soybean requires high temperatures and precipitation. There are two critical periods during soybean development, both characterized by increased thermal requirements. The first one, from sowing to full emergence, may be extended to as many as 45 days when its temperatures are too low (lower than 10–15 °C) [
29]. In terms of precipitation, the stages of sprouting, flowering and pod fill seem to be critical periods [
30,
31]. The precipitation and thermal conditions during the study period are presented in
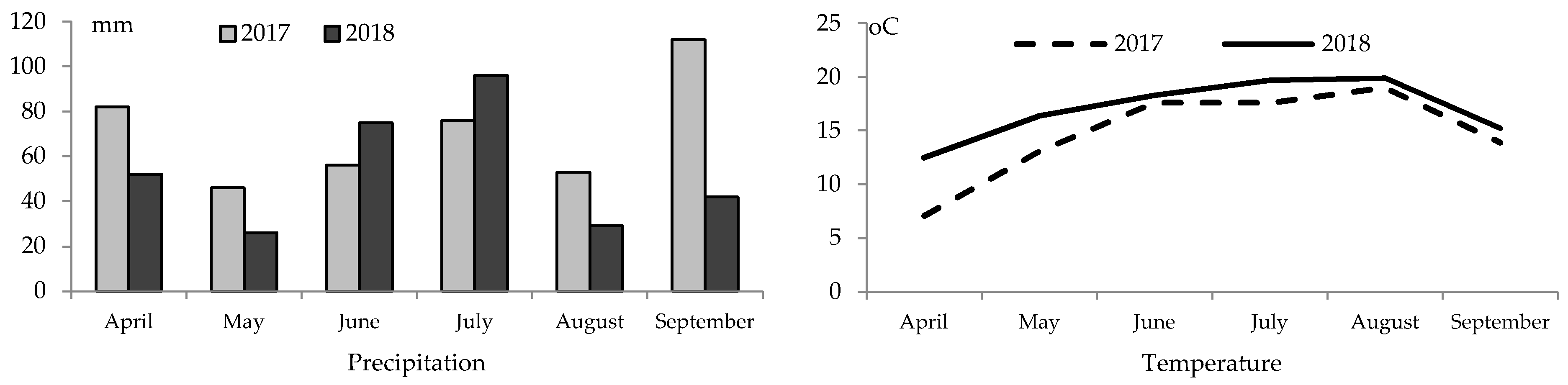
Figure 1.
At the beginning of 2017, values of the average monthly air temperature were lower but precipitation sums were higher. Low rainfall in May 2018 contributed to poorer plant germination and emergence whereas low temperature from seeding to full emergence. In 2017, the temperature in May of 13 °C and precipitation of 45 mm extended the germination period with a marked number of seeds germinating successfully.
Consequently, when improved thermal conditions followed, they had no significant impact on yield performance. In both the study years, August turned out to be the warmest month of soybean growing season with respective average temperatures of 19.0 and 19.9 °C. The highest precipitation sum was recorded in September (112 mm) 2017, and July (96 mm) 2018. In contrast, the lowest precipitation sums in 2017 and 2018 were recorded in May and August, respectively 46 and 26 mm, and 53 and 29 mm. In 2017 and 2018, May, June and August were dry. Very dry May in 2018 contributed to delayed germination and emergence, the status of plants being slightly enhanced by improved water status in July and August. Most reports mention higher temperatures at the initial stage of soybean development as a factor which contributed to earlier flowering [
32,
33]. A very high water demand which, when not met, results in the greatest yield losses coincides with the stage of pod fill [
34,
35,
36].
2.3. Laboratory Analyses
The following were determined in all samples of soybean and maize:
Dry matter content—70 °C
Total nitrogen content—Kjeldahl method
Enrichment with the 15N isotope—after wet mineralization by the Kiejdahl method and distillation to an acid solution (5% HCl), on the NOI-6e emission spectrometer (Leipzig, Germany).
2.4. Calculations
The percentage of nitrogen derived from different sources: from the atmosphere—
Ndfa, from mineral fertilizer—
NdfF and from soil—
Ndfs in soybean was calculated using the formulas given by Azam and Farooq [
37] and Kalembasa et al. [
38]:
- (a)
the percentage of nitrogen derived from the atmosphere, % Ndfa:
at % 15Nlegumefx—15N isotope excess in soybean,
at % 15Nnolegumenfx—15N isotope excess in the control plant-maize,
- (b)
the percentage of nitrogen derived from the fertilizer, % Ndff:
at % 15Nlegumefx—15N isotope excess in soybean,
at % 15Nfertilizer—15N isotope excess of fertilizer,
- (c)
the percentage of nitrogen derived from soil, % Ndfs:
% Ndfa—the percentage of nitrogen derived from the atmosphere,
% Ndff—the percentage of nitrogen derived from the fertilizer
The nitrogen pool conventionally called “from soil”—Ndfs—includes all the remaining sources, excluding the atmosphere and mineral fertilizer.
- (d)
Total nitrogen accumulation (uptake) in soybean, Nup:
- (e)
The amount of nitrogen uptake from the atmosphere, Ndfa
- (f)
The amount of nitrogen uptake from the fertilizer, Ndff
Ndff—the amount of nitrogen uptake from the fertilizer,
Nup—total nitrogen accumulation (uptake) in soybean,
%Ndff—% of nitrogen derived from the fertilizer
- (g)
The amount of nitrogen uptake from the soil, Ndfs
2.5. Statistical Analyses
The results of the experiments were analysed by two-way analysis of variance (ANOVA) with interaction according to the following model:
where:
yijl—the value of the examined characteristic
m—population average;
ai—the effect of the i-th level of factor A (sowing density)
bj—the effect of the j-th level of factor B (Year);
abij—the effect of the interaction of factor A and B (sowing density x year);
eijl—the random error (numbers)
The significance of sources of variation was checked with the Fisher-Snedecor test, and mean values were separated with the Tukey’s test at the significance level of p < 0.05. For these calculations the Statistica 13 PL (StatSoft, Tulsa, OK, USA) and were used.
4. Discussion
As possibilities of soybean cultivation under European conditions are improving, changing consumer awareness, research into this crop plant seems to be reasonable from the practical point of view as well. An increase in non-GMO soybean demand in the European Union (EU) has been observed. More and more countries are considering cultivation of non-GMO cultivars on a larger scale. Cultivated varieties should be certified as free of any genetic modifications. Availability of information on improved soybean cultivars and their agrotechnology as well as benefits associated with its cultivation will allow farmers to meet the market demand and expectations of producers. Additionally, soybean growing may produce benefits resulting from a limited application of mineral fertilizers which increase yields but also cause environmental degradation [
39]. From the point of view of agriculture, soybean is a good component in a rotation which, due to its ability to symbiotically fix atmospheric nitrogen, improves soil fertility [
40,
41]. Plant parts remaining after soybean harvest may be a nitrogen source for other crop plants following in rotation [
1,
4,
42] so it is important to determine the amount of nitrogen they contain.
In the experiment reported here, the total biomass amount of soybean cv. Abelina was affected by sowing density and growing conditions. In 2017, the weather conditions during the soybean growing season contributed to a higher mass of roots, post-harvest residues and seeds, compared with 2018. In this, a significant role was played by higher precipitation sums in June and July 2017 as they positively affected the growth of all plant parts. Literature reports mention precipitation and thermal conditions as one of factors hindering soybean development and yielding [
43]. According to Newark [
44] and El Kheir et al. [
45], insufficient precipitation may reduce plant height by about 30 to as much as 70%. Yield levels are influenced by disease occurrence and severity, applied agrotechnology, fertilisation levels and cultivar selection [
12,
13,
14,
24,
46,
47,
48]. It is well known that warm and wet weather increases soybean infection by some fungi, in particular
Phomopsis longicolla [
49].
The present research demonstrated that the total biomass amount was lower at the sowing density of 50 seeds∙m
−2 compared with the density of 70 or 90 seeds∙m
−2. Kozak et al. [
50] reported that, regardless of cultivar, the highest seed and post-harvest residue yields are produced at the sowing density of 100 seeds∙m
−2. Number of plants per unit area is another factor influencing morphological characteristics. Increased sowing density is associated with higher, more slender soybean plants whose bottom pods are set higher on the stem [
51,
52,
53,
54], they have fewer internodes, pods, seeds per plant, seeds per pod, and their 1000 seed weight is lower [
20]. Egli [
24] claims that when sowing density is low and there is no competition among plants, the yield is proportional to the density. Increased stand density contributes to growing competition and is followed by a declining yield increase rate. Research has confirmed that stand density has a marked impact on the intensity and quality of solar radiation intercepted by soybean plants [
54,
55,
56]. Duncan [
57] has suggested that light intercepted during and after seed set was the major yield determinant.
Soybean takes up nitrogen from three alternative sources: biological nitrogen fixation (BNF), absorption from soil and fertilizer. The share of each N source depends on environmental conditions, agrotechnology and genetic factors. In the case of soybean, the largest percentage is associated with atmospheric nitrogen (BNF). Herridge et al. [
58], using just seven sets of data, calculated legume (symbiotic) N
2 fixation in the range 3–90 kg N∙ha
−1∙year
−1 and free-living (non-symbiotic) N
2 fixation at 3–30 kg N∙ha
−1∙year
−1. The present work demonstrated that, in both study years, the amount of nitrogen accumulated by the whole plant biomass was the lowest in plants sown at the density of 50 seeds∙m
−2. Nitrogen uptake was the highest for plant seeds and the lowest for roots. The quantity of nitrogen derived from the atmosphere by different plant parts and the whole biomass was also affected by sowing density and was the largest at the density of 70 and 90 seeds∙m
−2. Similar results were obtained by Kihara et al. [
59] who recorded a higher
15N content in seeds vs. roots. Trawczyński [
60] claims that higher amounts of nitrogen obtained during symbiotic fixation of N
2 are stored in above-ground parts and seeds of legumes compared with quantities supplied to the soil with post-harvest residues. Single-stage harvest only involves removal of seeds from the field whereas the remaining straw and other parts are treated as post-harvest residues and are incorporated to the soil.
In the current work, both the quantity of BNF-related nitrogen and its percentage share were affected by weather conditions. In the slightly dry and warmer 2018, an accumulation of this type of nitrogen in the whole biomass of soybean plants exceeded 37.5 kg N·ha
−1, which was less than in 2017 (79.5 N·ha
−1). Apart from inoculation of leguminous plants with appropriate
Rhizobium strain, an effective course of the process of symbiotic nitrogen fixation is contingent on favourable weather and soil conditions during the growing season [
61,
62]. According to Korsak-Adamowicz et al. [
63], drought and high temperature as the factors which may adversely affect nodulating bacteria-crop plant symbiosis. Meteorological conditions were a significant factor of a model developed by Collino et al. [
64] and used for predicting the amount of nitrogen biologically fixed by soybean plants. Water shortages in soil have been reported by Wysokiński et al. [
65] and Divito and Sadras [
66] as a factor reducing the percentage share and quantity of nitrogen biologically reduced by legumes.
5. Conclusions
The research reported here demonstrated that the atmosphere and soil reserves were major nitrogen sources for soybean. The share of nitrogen derived from the atmosphere, soil reserves and mineral fertilizer and taken up by the total plant biomass was 46.28, 45.52 and 8.2%, respectively. The whole biomass accumulated 58.10, 52.85 and 9.70 kg N∙ha−1 (5.81, 5.28, 0.87 g N∙m−2) from the respective sources. Nitrogen uptake from the atmosphere was affected by sowing density and study years. In more favourable conditions (2017) and the sowing density of 70 and 90 seeds∙m−2, both individual plant parts and the whole biomass derived more nitrogen from the atmosphere. Sowing density affected the amount of nitrogen taken up from fertilizer by roots. More soil-derived nitrogen was taken up by the roots of plants sown at the lowest density.
In the year characterised by more favourable precipitation and thermal conditions (2017), soybean took up more nitrogen from all the sources compared with 2018. An average of 95.24 kg N∙ha−1 (9.24 g N∙m−2) was removed from the field with seeds, it being 46.17, 42.20 and 6.68 kg N∙ha−1 for an uptake from the atmosphere, soil reserves and mineral fertilizer, respectively. An incorporation into soil of residues and roots provided over 25 kg N∙kg−1 associated with all the sources (atmosphere, fertilizer and soil reserves).
As there is a constant increase in the area planted to soybean, research into atmospheric nitrogen fixation by soybean plants is gaining more and more importance.
The amount of biologically fixed nitrogen is strongly affected by weather patterns and agrotechnology. Thus, further research is substantiated based on precise modelling including conditions of plant growth and development.