Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Sites
2.2. Experimental Design
2.3. Soil and Plant Sampling
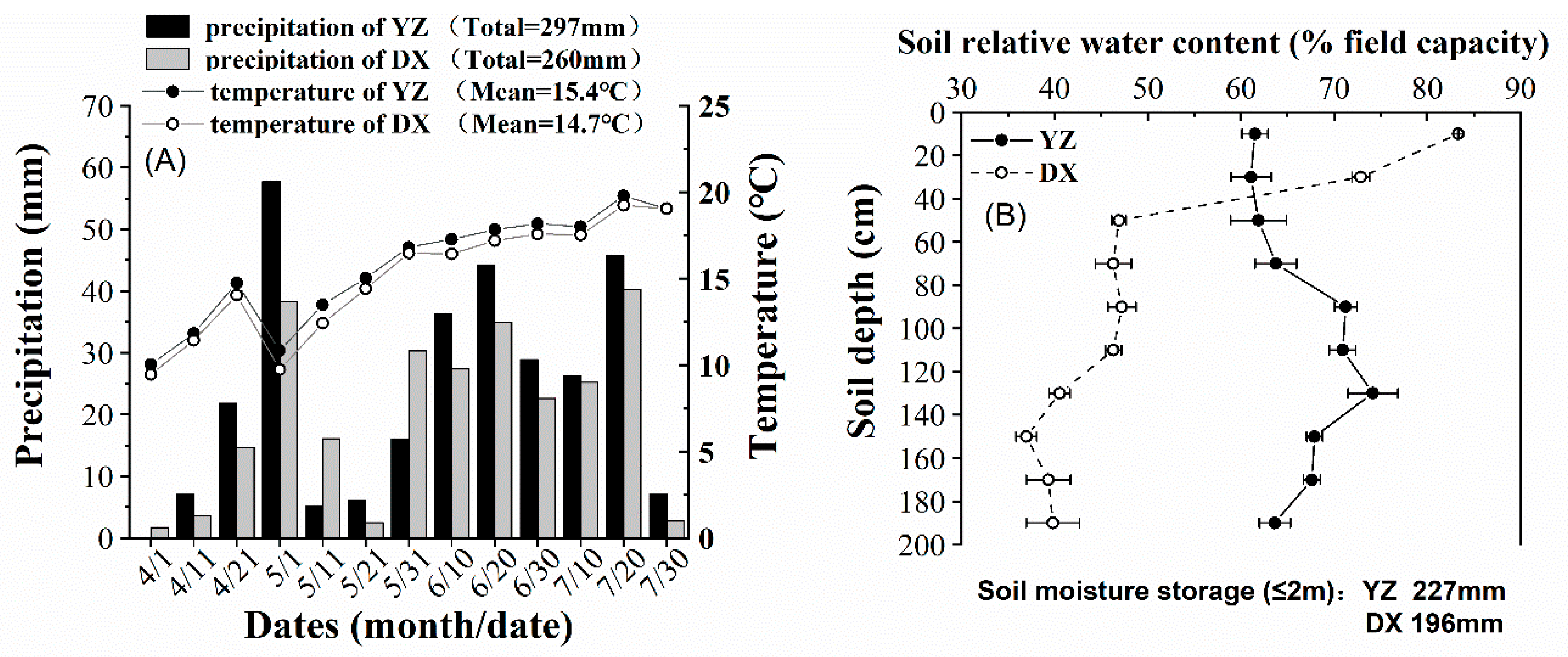
2.3.1. Field Capacity and Soil Bulk Density
2.3.2. Soil Water Content at Sowing, Anthesis, and Maturity
2.3.3. Phenological Observations
2.3.4. Aboveground Biomass and Pre-Anthesis Biomass Mobilization
2.3.5. Yield and Aboveground Biomass at Maturity
2.4. Statistical Analysis
3. Results
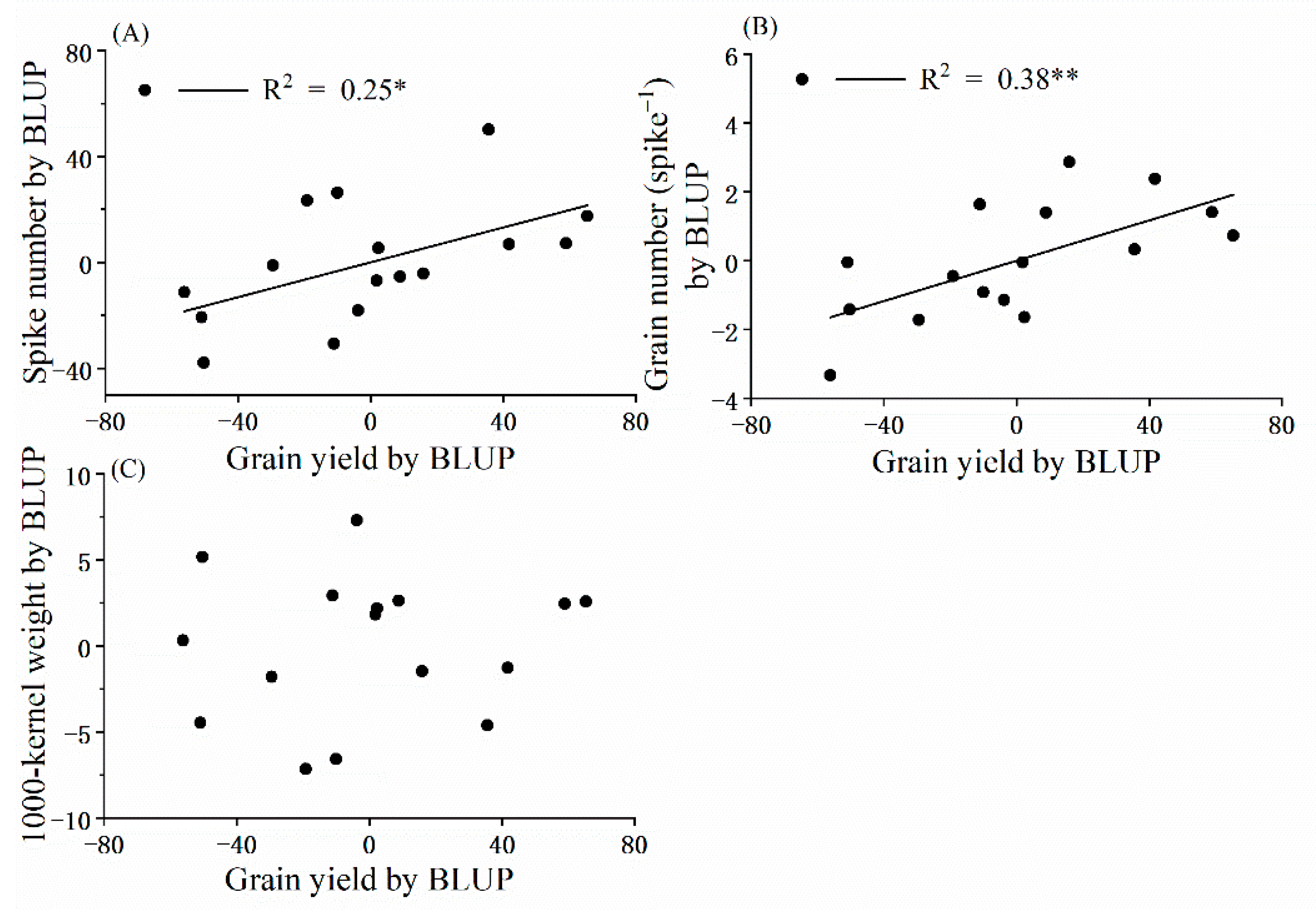
3.1. Yield and Its Components
3.2. Phenology
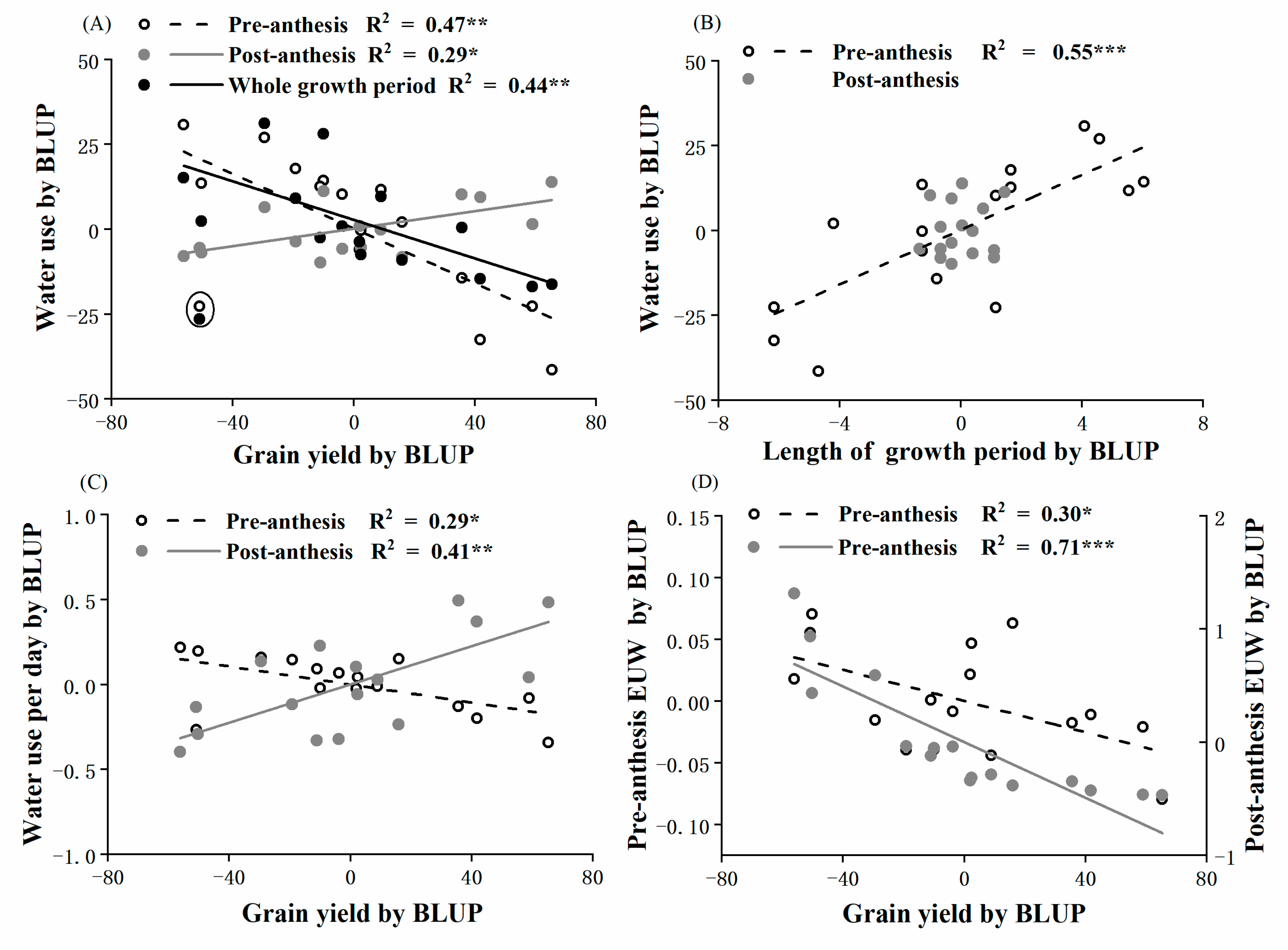
3.3. Water Use
3.4. Soil Water Content at Anthesis
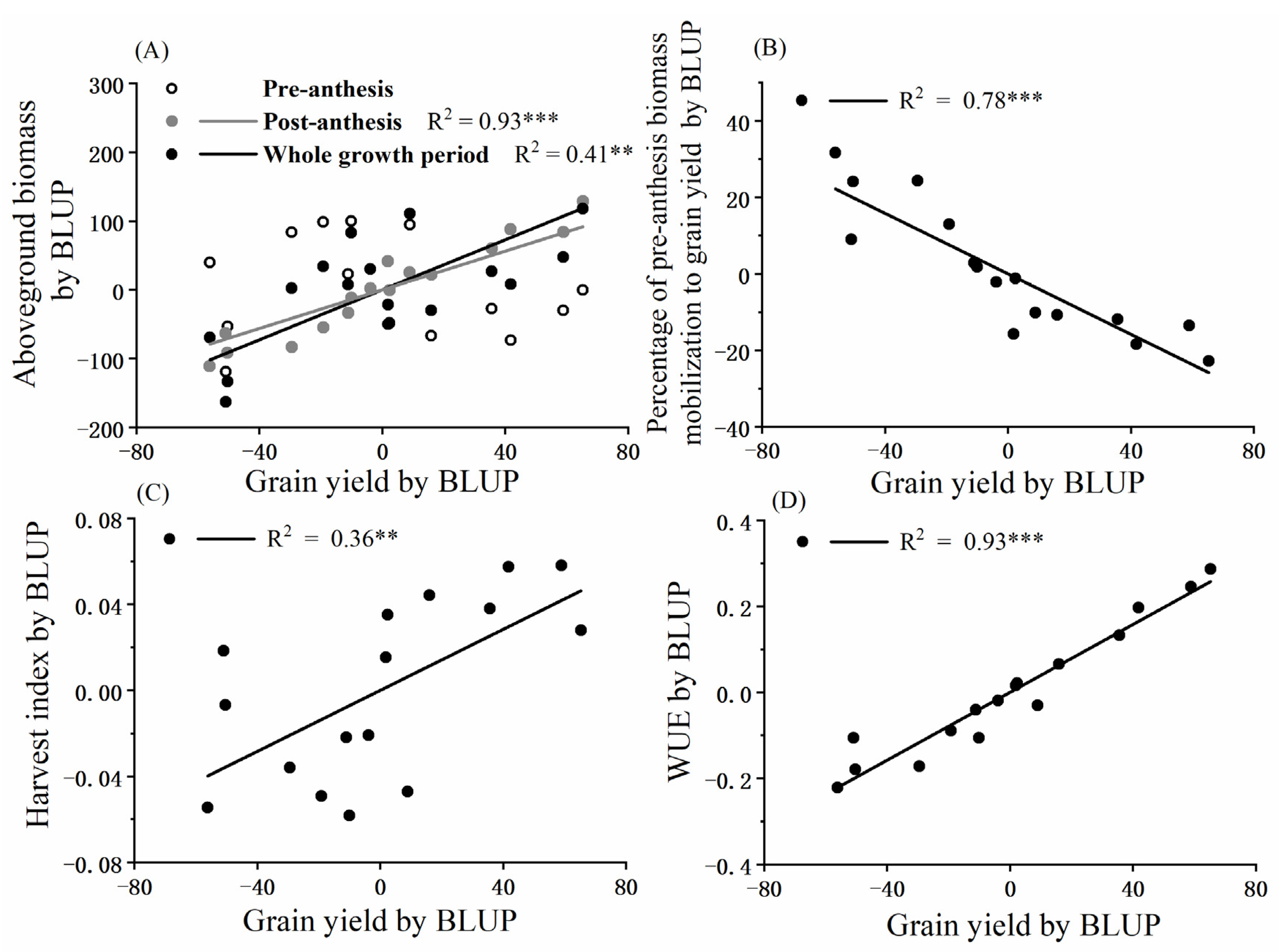
3.5. Aboveground Biomass, Harvest Index and Water Use Efficiency
4. Discussion
4.1. Changes in Agronomic Traits Related to Grain Yield Increase
4.2. Changes in Growing Periods
4.3. Post-Anthesis Growth and 1000-Kernel Weight
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trethowan, R.M.; Pfeiffer, W.H. Challenges and future strategies in breeding wheat for adaptation to drought stressed environments: A CIMMYT wheat program perspective. In Molecular Approaches for the Genetic Improvement of Cereals for Stable Production in Water-Limited Environments; Ribaut, J.-M., Poland, D., Eds.; CIMMYT: Mexico City, Mexico, 2000; pp. 21–25. [Google Scholar]
- Aisawi, K.A.B.; Reynolds, M.P.; Singh, R.P.; Foulkes, M.J. The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009. Crop. Sci. 2015, 55, 1749–1764. [Google Scholar] [CrossRef]
- Xiao, Y.G.; Qian, Z.G.; Wu, K.; Liu, J.J.; Xia, X.C.; Ji, W.Q.; He, Z.H. Genetic gains in grain yield and physiological traits of winter wheat in Shandong Province, China, from 1969 to 2006. Crop Sci. 2012, 52, 44–56. [Google Scholar] [CrossRef]
- Austin, R.; Bingham, J.; Blackwell, R.; Evans, L.; Ford, M.; Morgan, C.; Taylor, M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. J. Agric. Sci. 1980, 94, 675–689. [Google Scholar] [CrossRef]
- Brancourt-Hulmel, M.; Doussinault, G.; Lecomte, C.; Bérard, P.; Trottet, M. Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci. 2003, 43, 37–45. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, H.Z.; Cai, S.B.; He, Z.H.; Zhang, X.K.; Xia, X.C.; Zhang, G.S. Genetic improvement of grain yield and associated traits in the southern China winter wheat region: 1949 to 2000. Euphytica 2007, 157, 465–473. [Google Scholar] [CrossRef]
- Morgounov, A.; Zykin, V.; Belan, I.; Roseeva, L.; Zelenskiy, Y.; Gomez-Becerra, H.F.; Budak, H.; Bekes, F. Genetic gains for grain yield in high latitude spring wheat grown in Western Siberia in 1900–2008. Field Crop. Res. 2010, 117, 101–112. [Google Scholar] [CrossRef]
- Sadras, V.O.; Slafer, G.A. Environmental modulation of yield components in cereals: Heritabilities reveal a hierarchy of phenotypic plasticities. Field Crop. Res. 2012, 127, 215–224. [Google Scholar] [CrossRef]
- Abdolshahi, R.; Nazari, M.; Safarian, A.; Sadathossini, T.S.; Salarpour, M.; Amiri, H. Integrated selection criteria for drought tolerance in wheat (Triticum aestivum L.) breeding programs using discriminant analysis. Field Crop. Res. 2015, 174, 20–29. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Dolores Serret, M. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Aziz, M.M.; Palta, J.A.; Siddique, K.H.M.; Sadras, V.O. Five decades of selection for yield reduced root length density and increased nitrogen uptake per unit root length in Australian wheat varieties. Plant Soil 2016, 413, 181–192. [Google Scholar] [CrossRef]
- Foulkes, M.; Snape, J.; Shearman, V.; Reynolds, M.; Gaju, O.; Sylvester-Bradley, R. Genetic progress in yield potential in wheat: Recent advances and future prospects. J. Agric. Sci. 2007, 145, 17–29. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crop. Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P.; Slafer, G.A.; Satorre, E.H.; Slafer, G.A. Genetic gains in wheat yield and associated physiological changes during the twentieth century. Wheat Ecol. Physiol. Yield Determ. 1999, 61, 351–377. [Google Scholar]
- Hall, A.J.; Richards, R.A. Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crop. Res. 2013, 143, 18–33. [Google Scholar] [CrossRef]
- Zheng, T.C.; Zhang, X.K.; Yin, G.H.; Wang, L.N.; Han, Y.L.; Chen, L.; Huang, F.; Tang, J.W.; Xia, X.C.; He, Z.H. Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crop. Res. 2011, 122, 225–233. [Google Scholar] [CrossRef]
- Perry, M.W.; D’Antuono, M.F. Yield improvement and associated characteristics of some Australian spring wheat cultivars introduced between 1860 and 1982. Aust. J. Agric. Res. 1989, 40, 457–472. [Google Scholar]
- Richards, R.A. Defining selection criteria to improve yield under drought. Plant Growth Regul. 1996, 20, 157–166. [Google Scholar] [CrossRef]
- Kobata, T.; Koç, M.; Barutçular, C.; Tanno, K.-I.; Inagaki, M. Harvest index is a critical factor influencing the grain yield of diverse wheat species under rain-fed conditions in the Mediterranean zone of southeastern Turkey and northern Syria. Plant Prod. Sci. 2018, 21, 71–82. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; Appendino, M.L.; Lombardo, L.A.; Vanzetti, L.S.; Helguera, M.; Miralles, D.J. Variability of duration of pre-anthesis phases as a strategy for increasing wheat grain yield. Field Crops Res. 2011, 124, 408–416. [Google Scholar] [CrossRef]
- Sadras, V.O.; Lawson, C. Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop Pasture Sci. 2011, 62, 533–549. [Google Scholar] [CrossRef]
- Shearman, V.; Sylvester-Bradley, R.; Scott, R.; Foulkes, M. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar]
- Pampana, S.; Mariotti, M.; Ercoli, L.; Masoni, A. Remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by genotype and environment. Ital. J. Agron. 2011, 2, 303–314. [Google Scholar] [CrossRef]
- Regmi, K.C.; Yogendra, K.; Farias, J.G.; Li, L.; Kandel, R.; Yadav, U.P.; Sha, S.; Trittermann, C.; Short, L.; George, J.; et al. Improved yield and photosynthate partitioning in AVP1 expressing wheat (Triticum aestivum) plants. Front. Plant Sci. 2020, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Wang, H.; Dong, H.; Qi, X.; Zhao, M.; Fang, Y.; Gao, C.; Hu, L. Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crop. Res. 2016, 199, 117–128. [Google Scholar] [CrossRef]
- Peixouto, L.S.; Nunes, J.A.R.; Furtado, D.F. Factor analysis applied to the G+GE matrix via REML/BLUP for multi-environment data. Crop Breed. Appl. Biotechnol. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Resende, M.D.V.d. Software Selegen-REML/BLUP: A useful tool for plant breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Piepho, H.P.; Möhring, J.; Melchinger, A.E.; Büchse, A. BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 2007, 161, 209–228. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A. Nitrogen and dry matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. Agron. J. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- Du, Y.-L.; Xi, Y.; Cui, T.; Anten, N.P.R.; Weiner, J.; Li, X.; Turner, N.C.; Zhao, Y.-M.; Li, F.-M. Yield components, reproductive allometry and the tradeoff between grain yield and yield stability in dryland spring wheat. Field Crop. Res. 2020, 257, 107930. [Google Scholar] [CrossRef]
- Fischer, R.A. Understanding the physiological basis of yield potential in wheat. J. Agric. Sci. 2007, 145, 99–113. [Google Scholar] [CrossRef]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Field Crop. Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Duggan, B.L.; Domitruk, D.R.; Fowler, D.B. Yield component variation in winter wheat grown under drought stress. Can. J. Plant Sci. 2000, 80, 739–745. [Google Scholar] [CrossRef]
- Tian, Z.; Jing, Q.; Dai, T.; Jiang, D.; Cao, W. Effects of genetic improvements on grain yield and agronomic traits of winter wheat in the Yangtze River Basin of China. Field Crop. Res. 2011, 124, 417–425. [Google Scholar] [CrossRef]
- Zhou, B.; Sanz-Sáez, Á.; Elazab, A.; Shen, T.; Sánchez-Bragado, R.; Bort, J.; Serret, M.D.; Araus, J.L. Physiological traits contributed to the recent increase in yield potential of winter wheat from Henan Province, China. J. Integr. Plant Biol. 2014, 56, 492–504. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Sun, H.; Pei, D.; Wang, Y. Dry matter, harvest index, grain yield and water use efficiency as affected by water supply in winter wheat. Irrig. Sci. 2008, 27, 1–10. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H. Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiol. 1999, 121, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Pheloung, P.C.; Siddique, K.H.M. Contribution of stem dry-matter to grain-yield in wheat cultivars. Aust. J. Plant Physiol. 1991, 18, 53–64. [Google Scholar] [CrossRef]
- Hossain, A.B.S.; Sears, R.G.; Cox, T.S.; Paulsen, G.M. Desiccation tolerance and its relationship to assimilate partitioning in winter-wheat. Crop Sci. 1990, 30, 622–627. [Google Scholar] [CrossRef]
- Blum, A.; Golan, G.; Mayer, J.; Sinmena, B.; Shpiler, L.; Burra, J. The drought response of landraces of wheat from the northern Negev Desert in Israel. Euphytica 1989, 43, 87–96. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Zhang, S.; Palta, J.A.; Deng, X. The proportion of superior grains and the sink strength are the main yield contributors in modern winter wheat varieties grown in the Loess Plateau of China. Agronomy 2019, 9, 612. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Sun, H.; Wang, Y.; Shao, L. Water use efficiency and associated traits in winter wheat cultivars in the North China Plain. Agric. Water Manag. 2010, 97, 1117–1125. [Google Scholar] [CrossRef]
- Li, F.M.; Liu, X.L.; Li, S.Q. Effects of early soil water distribution on the dry matter partition between roots and shoots of winter wheat. Agric. Water Manag. 2001, 49, 163–171. [Google Scholar] [CrossRef]
- Simane, B.; Peacock, J.M.; Struik, P.C. Differences in developmental plasticity and growth rate among drought-resistant and susceptible cultivars of durum wheat (Triticum turgidum L. var. durum). Plant Soil 1993, 157, 155–166. [Google Scholar] [CrossRef]
- Turner, N.C. Further progress in crop water relations. Adv. Agron. 1996, 58, 293–338. [Google Scholar]
- Álvaro, F.; Isidro, J.; Villegas, D.; García del Moral, L.F.; Royo, C. Breeding effects on grain filling, biomass partitioning, and remobilization in Mediterranean durum wheat. Agron. J. 2008, 100, 361–370. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P.; Jalal-Kamali, M.R.; Moussa, M.; Feltaous, Y.; Tahir, I.S.A.; Barma, N.; Vargas, M.; Mannes, Y.; Baum, M. The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crop. Res. 2012, 128, 129–136. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Bort, J.; Steduto, P.; Villegas, D.; Royo, C. Breeding cereals for Mediterranean conditions: Ecophysiological clues for biotechnology application. Ann. Appl. Biol. 2003, 142, 129–141. [Google Scholar] [CrossRef]
- Álvaro, F.; Royo, C.; García del Moral, L.F.; Villegas, D. Grain filling and dry matter translocation responses to source-sink modifications in a historical series of durum wheat. Crop Sci. 2008, 48, 1523–1531. [Google Scholar] [CrossRef]
- Li, F.R.; Zhao, S.L.; Geballe, G.T. Water use patterns and agronomic performance for some cropping systems with and without fallow crops in a semi-arid environment of northwest China. Agric. Ecosyst. Environ. 2000, 79, 129–142. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, J.; Wang, R.; Wang, H.; Qiang, Y.U. Determining agricultural drought for spring wheat with statistical models in a semi-arid climate. J. Agric. Meteorol. 2018, 74, 162–172. [Google Scholar] [CrossRef]
- Austin, R.B.; Ford, M.A.; Morgan, C.L. Genetic improvement in the yield of winter wheat: A further evaluation. J. Agric. Sci. 1989, 112, 295–301. [Google Scholar] [CrossRef]
- Loss, S.P.; Kirby, E.J.M.; Siddique, K.H.M.; Perry, M.W. Grain growth and development of old and modern Australian wheats. Field Crop. Res. 1989, 21, 131–146. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. N. Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Metzger, D.D.; Czaplewski, S.J.; Rasmusson, D.C. Grain-Filling duration and yield in sring barley.1. Crop Sci. 1984, 24, 1101–1105. [Google Scholar] [CrossRef]
- Sadras, V.O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crop. Res. 2007, 100, 125–138. [Google Scholar] [CrossRef]
- Ugarte, C.; Calderini, D.F.; Slafer, G.A. Grain weight and grain number responsiveness to pre-anthesis temperature in wheat, barley and triticale. Field Crop. Res. 2007, 100, 240–248. [Google Scholar] [CrossRef]


| Traits | Genotype (G) | Environment (E) | G × E |
|---|---|---|---|
| Spike number (m−2) | 46.61 *** | 16.48 *** | 65.92 ** |
| Grain number (spike−1) | 3.63 *** | 1.28 *** | 5.13 *** |
| 1000-kernel weight (g) | 2.03 *** | 0.72 *** | 2.86 *** |
| Water use (L) | 23.09 *** | 8.16 *** | 32.65 ns |
| WUE (g L−1) | 0.14 *** | 0.05 ns | 0.20 ns |
| Harvest index | 0.035 *** | 0.012 ** | 0.049 ** |
| Pre-anthesis aboveground biomass accumulation (g m−2) | 53.12 *** | 18.78 *** | 75.12 ** |
| Post-anthesis aboveground biomass accumulation (g m−2) | 46.59 *** | 16.47 * | 65.89 ** |
| Total aboveground biomass (g m−2) | 81.73 *** | 28.90 *** | 115.58 ns |
| Grain yield (g m−2) | 38.82 *** | 13.73 *** | 54.90 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Xi, Y.; Zhu, Y.-H.; Chai, N.; Zhang, X.-T.; Jin, Y.; Turner, N.C.; Li, F.-M. Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China. Agronomy 2021, 11, 663. https://doi.org/10.3390/agronomy11040663
Feng T, Xi Y, Zhu Y-H, Chai N, Zhang X-T, Jin Y, Turner NC, Li F-M. Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China. Agronomy. 2021; 11(4):663. https://doi.org/10.3390/agronomy11040663
Chicago/Turabian StyleFeng, Tao, Yue Xi, Yong-He Zhu, Ning Chai, Xin-Tan Zhang, Yi Jin, Neil C. Turner, and Feng-Min Li. 2021. "Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China" Agronomy 11, no. 4: 663. https://doi.org/10.3390/agronomy11040663
APA StyleFeng, T., Xi, Y., Zhu, Y.-H., Chai, N., Zhang, X.-T., Jin, Y., Turner, N. C., & Li, F.-M. (2021). Reduced Vegetative Growth Increases Grain Yield in Spring Wheat Genotypes in the Dryland Farming Region of North-West China. Agronomy, 11(4), 663. https://doi.org/10.3390/agronomy11040663







