Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Treatments and Experimental Design
2.3. Variables Recorded
2.3.1. Evaluation of Plant Symptoms
2.3.2. Plant Growth
2.3.3. Chlorophyll Index (SPAD)
2.3.4. Biomass
2.3.5. Mineral Contents in Leaves and Roots
2.4. Data Analysis
3. Results
3.1. Plant Symptoms
3.2. Plant Growth
3.3. Chlorophyll Index (SPAD)
3.4. Biomass
3.5. Concentration of Salt Ions (Chloride and Sodium) in Leaves and Roots
3.6. Macronutrient Concentration in Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 3 March 2021).
- Puigdefábregas, J.; Mendizabal, T. Perspectives on desertification: Western Mediterranean. J. Arid Environ. 1998, 39, 209–224. [Google Scholar] [CrossRef]
- Safriel, U.N. Status of desertification in the Mediterranean region. In Water Scarcity, Land Degradation and Desertification in the Mediterranean Region; Pedrazzini, J.L.R.S.D.B., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 33–73. ISBN 978-90-481-2526-5. [Google Scholar]
- López-Bermúdez, F. Soil erosion by water on the desertification of a semi-arid Mediterranean fluvial basin: The Segura basin, Spain. Agric. Ecosyst. Environ. 1990, 33, 129–145. [Google Scholar] [CrossRef]
- Ruiz, I.; Almagro, M.; García de Jalón, S.; Solà, M.d.M.; Sanz, M.J. Assessment of sustainable land management practices in Mediterranean rural regions. J. Environ. Manag. 2020, 276. [Google Scholar] [CrossRef]
- Vanwalleghem, T.; Gómez, J.A.; Infante Amate, J.; González de Molina, M.; Vanderlinden, K.; Guzmán, G.; Laguna, A.; Giráldez, J.V. Impact of historical land use and soil management change on soil erosion and agricultural sustainability during the Anthropocene. Anthropocene 2017, 17, 13–29. [Google Scholar] [CrossRef]
- CABI. 2021. Available online: Ttps://www.cabi.org/isc/datasheet/16567 (accessed on 1 February 2021).
- EPPO. 2021. Available online: https://gd.eppo.int/ (accessed on 1 February 2021).
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Le Houerou, H.N. An agro bioclimatic classification of arid and semiarid lands in the isoclimatic mediterranean zones. Arid L. Res. Manag. 2004, 18, 301–346. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Carvajal, M.; Sanchez-Pina, M.A.; Martínez, V.; Cerdá, A. Salinity resistance of Citrus seedlings in relation to hydraulic conductance, plasma membrane ATPase and anatomy of the roots. J. Plant Physiol. 2000, 156, 724–730. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Jifon, J.L.; Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to Na’ and Cl- accumulation in ‘Sunburst’ mandarin grafted on different rootstocks. Plant Sci. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Simpson, C.R.; Nelson, S.D.; Melgar, J.C.; Jifon, J.; Schuster, G.; Volder, A. Effects of salinity on physiological parameters of grafted and ungrafted citrus trees. Sci. Hortic. (Amsterdam) 2015, 197, 483–489. [Google Scholar] [CrossRef]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Shalhevet, J.; Bielorai, H. Effect of irrigation regime and water salinity on grapefruit quality. J. Am. Soc. Hortic. Sci. 1979, 104, 356–359. [Google Scholar]
- Murkute, A.A.; Sharma, S.; Singh, S.K. Citrus in terms of soil and water salinity: A review. J. Sci. Ind. Res. (India) 2005, 64, 393–402. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.J. Chloride toxicity in citrus. Irrig. Sci. 1985, 6, 63–71. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: I. Response of growth, water relations, and ion accumulation to NaCl salinity. Crop Sci. 2004, 44, 797–805. [Google Scholar] [CrossRef]
- Adams, S.N.; Ac-pangan, W.O.; Rossi, L. Effects of soil salinity on citrus rootstock ‘US-942′ physiology and anatomy. HortScience 2019, 54, 787–792. [Google Scholar] [CrossRef]
- Pérez-Tornero, O.; Tallón, C.I.; Porras, I.; Navarro, J.M. Physiological and growth changes in micropropagated Citrus macrophylla explants due to salinity. J. Plant Physiol. 2009, 166, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Luro, F.; Costantino, G.; Ollitrault, P.; Morillon, R. Physiological analysis of salt stress behaviour of citrus species and genera: Low chloride accumulation as an indicator of salt tolerance. S. Afr. J. Bot. 2012, 81, 103–112. [Google Scholar] [CrossRef]
- Romero-Aranda, R.; Moya, J.L.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talon, M. Physiological and anatomical disturbances induced by chloride salts in sensitive and tolerant citrus: Beneficial and detrimental effects of cations. Plant Cell Environ. 1998, 21, 1243–1253. [Google Scholar] [CrossRef]
- Levy, Y.; Shalhevet, J.; Lifshitz, J. The effect of salinity on citrus rootstocks and scions. Proc. Int. Soc. Citric. 1992, 1, 391–396. [Google Scholar]
- Raga, V.; Bernet, G.P.; Carbonell, E.A.; Asin, M.J. Inheritance of rootstock effects and their association with salt tolerance candidate genes in a progeny derived from ‘Volkamer’ lemon. J. Am. Soc. Hortic. Sci. 2014, 139, 518–528. [Google Scholar] [CrossRef]
- Legua, P.; Forner, J.B.; Hernández, F.; Forner-Giner, M.A. Physicochemical properties of orange juice from ten rootstocks using multivariate analysis. Sci. Hortic. (Amsterdam) 2013, 160, 268–273. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the α subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, K.; Hoa, N.V.; Bang, D.V.; Tuan, D.H.; Dien, L.Q. Limited efficacy of guava interplanting on citrus greening disease: Effectiveness of protection against disease invasion breaks down after one year. Crop Prot. 2012, 34, 119–126. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fuentes, L.M.; Urias-López, M.A.; López-Arroyo, J.I.; Gómez-Jaimes, R.; Bautista-Martínez, N. Control químico de Diaphorina citri Kuwayama (Hemíptera: Psyllidae) en lima persa Citrus latifolia Tanaka. Rev. Mex. Ciencias Agrícolas 2012, 3, 427–439. [Google Scholar] [CrossRef][Green Version]
- Garnier, M. Transmission of the organism associated with citrus greening disease from sweet orange to periwinkle by dodder. Phytopathology 1983, 73, 1358. [Google Scholar] [CrossRef]
- Andrade, M.; Li, J.; Wang, N. Candidatus Liberibacter asiaticus: Virulence traits and control strategie. Trop. Plant Pathol. 2020, 1–13. [Google Scholar] [CrossRef]
- Food Safety—European Commission. Available online: https://ec.europa.eu/food/plant/pesticides/approval_active_substances_en (accessed on 2 December 2020).
- Albrecht, U.; Bowman, K.D. Reciprocal influences of rootstock and scion citrus cultivars challenged with Ca. Liberibacter asiaticus. Sci. Hortic. (Amsterdam) 2019, 254, 133–142. [Google Scholar] [CrossRef]
- Ajene, I.J.; Khamis, F.; Mohammed, S.; Rasowo, B.; Ombura, F.L.; Pietersen, G.; van Asch, B.; Ekesi, S. First report of field population of Trioza erytreae carrying the Huanglongbing associated pathogen Candidatus Liberibacter asiaticus in Ethiopia. Plant Dis. 2019, 103, 1766. [Google Scholar] [CrossRef]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Is the presence of Trioza erytreae, vector of huanglongbing disease, endangering the Mediterranean citrus industry? Survey of its population density and geographical spread over the last years. J. Plant Pathol. 2018, 100, 567–574. [Google Scholar] [CrossRef]
- Arenas-Arenas, F.J.; Duran-Vila, N.; Quinto, J.; Hervalejo, Á. Geographic spread and inter-annual evolution of populations of Trioza erytreae in the Iberian Peninsula. J. Plant Pathol. 2019, 101, 1151–1157. [Google Scholar] [CrossRef]
- Cocuzza, G.E.M.; Alberto, U.; Hernández-Suárez, E.; Siverio, F.; Di Silvestro, S.; Tena, A.; Carmelo, R. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest. Sci. 2017, 90, 1–17. [Google Scholar] [CrossRef]
- Stover, E.; Hall, D.G.; Grosser, J.; Gruber, B.; Moore, G.A. Huanglongbing-related responses of ‘Valencia’ sweet orange on eight citrus rootstocks during greenhouse trials. Horttechnology 2019, 8, 776–782. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Simón-Grao, S.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Mattson, N.S.; Garcia-Sanchez, F. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. (Amsterdam) 2019, 250, 1–11. [Google Scholar] [CrossRef]
- Bowman, K.D.; Joubert, J. Citrus Rootstocks; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128121634. [Google Scholar]
- Silva, M.C.; Sousa, A.R.O.; Cruz, E.S.; Schlichting, A.F.; Filho, W.S.S.; Gesteira, A.S.; Filho, M.A.C.; Costa, M.G.C. Phenotyping of new hybrid citrus rootstocks under water deficit reveals conserved and novel physiological attributes of drought tolerance. Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Alcaide, A.; Primo-Millo, E.; Forner, J.B. Performance of ‘Navelina’ orange on 14 rootstocks in Northern Valencia (Spain). Sci. Hortic. (Amsterdam) 2003, 98, 223–232. [Google Scholar] [CrossRef]
- Florida Citrus Rootstock Selection Guide, 4th ed. 2020. Available online: https://crec.ifas.ufl.edu/extension/citrus_rootstock/tables.html (accessed on 21 December 2020).
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. (Amsterdam) 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Bowman, K.D.; Mccollum, G.; Albrecht, U. Scientia Horticulturae Performance of ‘Valencia’ orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by huanglongbing. Sci. Hortic. (Amsterdam) 2016, 201, 355–361. [Google Scholar] [CrossRef]
- Bowman, K.D.; Faulkner, L.; Kesinger, M. Field performance and nursery characteristics. Hortscience 2016, 51, 1208–1214. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Bandaranayake, W. Salinity tolerance of “Hamlin” orange trees on the hybrid rootstocks US-897 and x639 is greater than of trees on cleopatra mandarin. Proc. Fla. State Hort. Soc. Proc. Fla. State Hort. Soc. 2012, 125, 56–60. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; Smithsonian Institution Annual Report; Smithsonian Institution: Washington, DC, USA, 1938; pp. 1884–1949. [Google Scholar]
- Campbell, C.L.; Madden, L.V. (Eds.) Temporal analysis of epidemics I: Descriptions and comparisons of disease progress curve. In Introduction to Plant Disease Epidemiology; Wiley: New York, NY, USA, 1990; pp. 161–202. [Google Scholar]
- Vincent, J.M. Distortion of fungal hyphæ in the presence of certain inhibitors. Nature 1947, 159, 850. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Storey, R.; Walker, R.R. Citrus and salinity. Sci. Hortic. (Amsterdam) 1998, 78, 39–81. [Google Scholar] [CrossRef]
- Grattan, S.R.; Díaz, F.J.; Pedrero, F.; Vivaldi, G.A. Assessing the suitability of saline wastewaters for irrigation of Citrus spp.: Emphasis on boron and specific-ion interactions. Agric. Water Manag. 2015, 157, 48–58. [Google Scholar] [CrossRef]
- William, S.; Castle, K.D.; Bowman, J.W.; Grosser, S.H.; Futch, J.H.G. Florida Citrus Rootstock Selection Guide. Univ. Fla. Coop. Ext. Publ. 2016, SP-248, 1–4. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Pérez-Tornero, O. Improved salt-tolerance in Citrus macrophylla mutant rootstocks. Sci. Hortic. (Amsterdam). 2020, 259, 108815. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Syvertsen, J.P.; Martínez, V.; Melgar, J.C. Salinity tolerance of “Valencia” orange trees on rootstocks with contrasting salt tolerance is not improved by moderate shade. J. Exp. Bot. 2006, 57, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Forner-Giner, M.A.; Primo-Millo, E.; Forner, J.B. Performance of Forner-Alcaide 5 and Forner-Alcaide 13, hybrids of Cleopatra mandarin x Poncirus trifoliata, as Salinity-Tolerant Citrus Rootstocks. Impreso 2009, 63, 72–80. [Google Scholar]
- Bandaranayake, W. Salinity tolerance of cleopatra mandarin seedlings and two of its trifoliata hybrids, US-897 and x639. Proc. Fla. State Hortic. Soc. 2011, 124, 47–51. [Google Scholar]
- Bañuls, J.; Primo-Millo, E. Effects of chloride and sodium on gas exchange parameters and water relations of Citrus plants. Physiol. Plant. 1992, 86, 115–123. [Google Scholar] [CrossRef]
- Walker, R.R.; Torokfalvy, E.; Grieve, A.M.; Prior, L.D. Water relations and ion concentrations of leaves on salt-stressed citrus plants. Aust. J. Plant Physiol. 1983, 10, 265–277. [Google Scholar] [CrossRef]
- Grosser, J.; Omar, A.; Gmitter, J.; Syvertsen, J.P. Salinity tolerance of ‘Valencia’ Orange trees on allotetraploid rootstocks. Proc. Fla. State Hort. Soc. 2012, 120, 50–55. [Google Scholar]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. (Amsterdam) 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Pestana, M.; De Varennes, A.; Abadía, J.; Faria, E.A. Differential tolerance to iron deficiency of citrus rootstocks grown in nutrient solution. Sci. Hortic. (Amsterdam) 2005, 104, 25–36. [Google Scholar] [CrossRef]
- Fadli, A.; Tofail, I.; Agronomique, R. Screening of six citrus rootstocks for salt tolerance at emergence and early seedling stages. Int. J. Recent Sci. 2015, 6, 7672–7678. [Google Scholar]
- Zekri, M. Salinity and calcium effects on emergence, growth and mineral composition of seedlings of eight citrus rootstocks. J. Hortic. Sci. 1993, 68, 53–62. [Google Scholar] [CrossRef]
- Ruiz, D.; Martinez, V.; Cerda, A. Citrus response to salinity. Tree Physiol. 1997, 17, 141–150. [Google Scholar] [CrossRef]
- Chen, C.Z.; Lv, X.F.; Li, J.Y.; Yi, H.Y.; Gong, J.M. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 2012, 159, 1582–1590. [Google Scholar] [CrossRef]
- Shelke, D.B.; Nikalje, G.C.; Nikam, T.D.; Maheshwari, P.; Punita, D.L.; Rao, K.R.S.S.; Suprasanna, P. Chloride (Cl−) Uptake, Transport, and Regulation in Plant Salt. Biol. Biotechnol. 2019, 241–268. [Google Scholar] [CrossRef]
- Cerdá, A.; Fernandez, F.G.; Caro, M.; Guillen, M.G. Growth and mineral composition of two lemon varieties irrigated with saline waters. Agrochimica 1979, 23, 387–398. [Google Scholar]
- Obreza, T.T.; Morgan, K.T. Nutrition of Florida citrus trees. EDIS 2008, 2008. Available online: https://journals.flvc.org/edis/article/view/117225 (accessed on 5 February 2021).
- Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Quiñones, A.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Comparative expression of candidate genes involved in sodium transport and compartmentation in citrus. Environ. Exp. Bot. 2015, 111, 52–62. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Ruiz, M.; Quiñones, A.; Martínez-Alcántara, B.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martínez-Cuenca, M.R. Effects of salinity on diploid (2x) and doubled diploid (4x) Citrus macrophylla genotypes. Sci. Hortic. (Amsterdam) 2016, 207, 33–40. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Pérez-Tornero, O. Mutants of Citrus macrophylla rootstock obtained by gamma radiation improve salt resistance through toxic ion exclusion. Plant Physiol. Biochem. 2020, 155, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nieves, M.; Martinez, V.; Cerda, A.; Guillen, M.G. Yield and mineral composition of ‘Verna’ lemon trees as affected by salinity and rootstock combination. J. Hortic. Sci. 1990, 65, 359–366. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Legaz, F.; Primo-Millo, E.; Forner, J. Nutritional responses of citrus rootstocks to salinity: Performance of the new hybrids forner-alcaide 5 and forner-alcaide 13. J. Plant Nutr. 2011, 34, 1437–1452. [Google Scholar] [CrossRef]
- Tozlu, I.; Moore, G.A.; Guy, C.L. Effects of increasing NaCl concentration on stem elongation, dry mass production, and macro- and micro-nutrient accumulation in Poncirus trifoliata. Aust. J. Plant Physiol. 2000, 27, 35–42. [Google Scholar] [CrossRef]
- Paudel, I.; Bar-Tal, A.; Raveh, E.; Bernstein, N.; Cohen, S. Tolerance of citrus rootstocks to poor water quality is improved by root zone aeration via selective uptake of ions, higher photosynthesis and carbon storage. Sci. Hortic. (Amsterdam) 2019, 251, 9–19. [Google Scholar] [CrossRef]

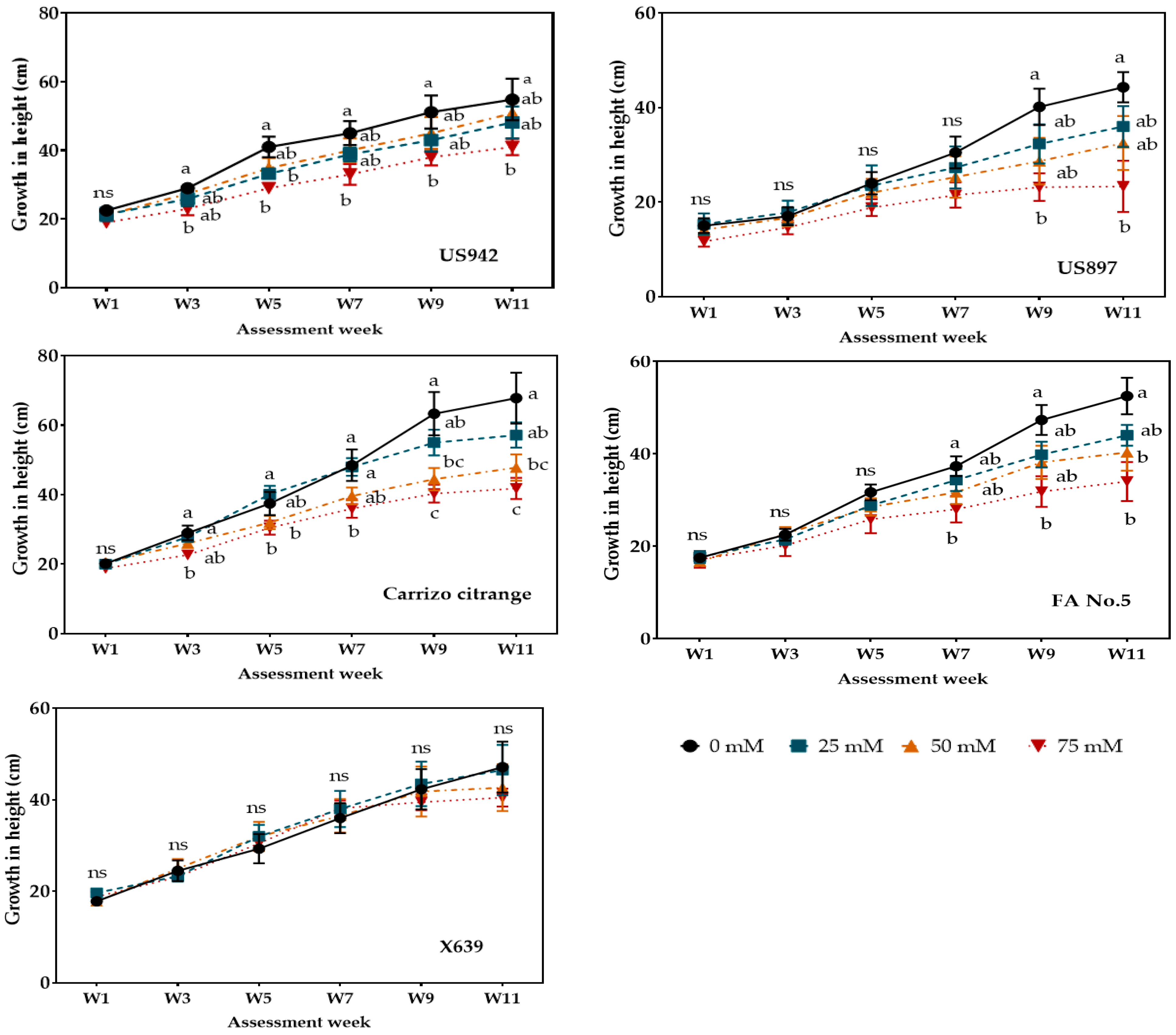
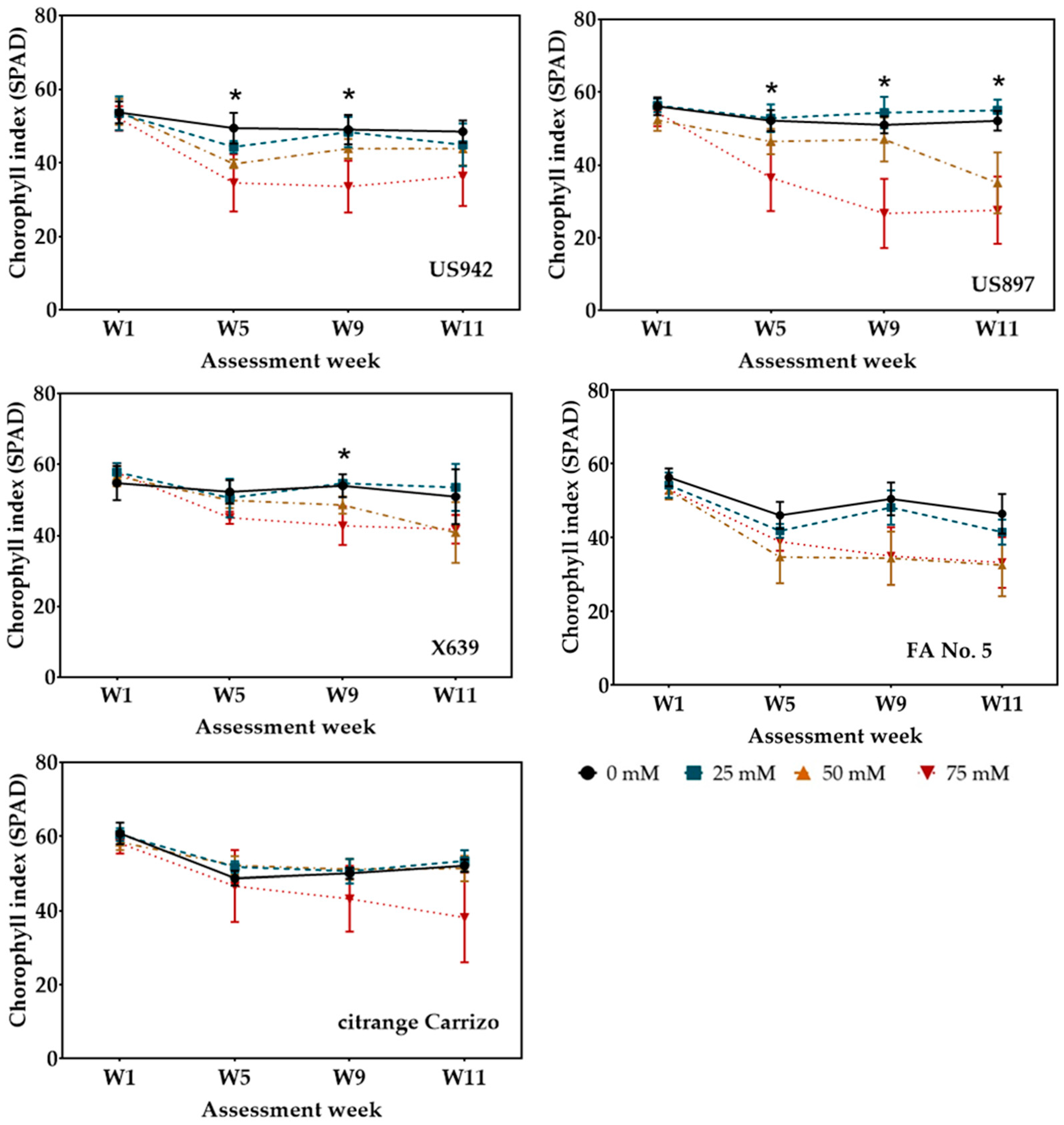
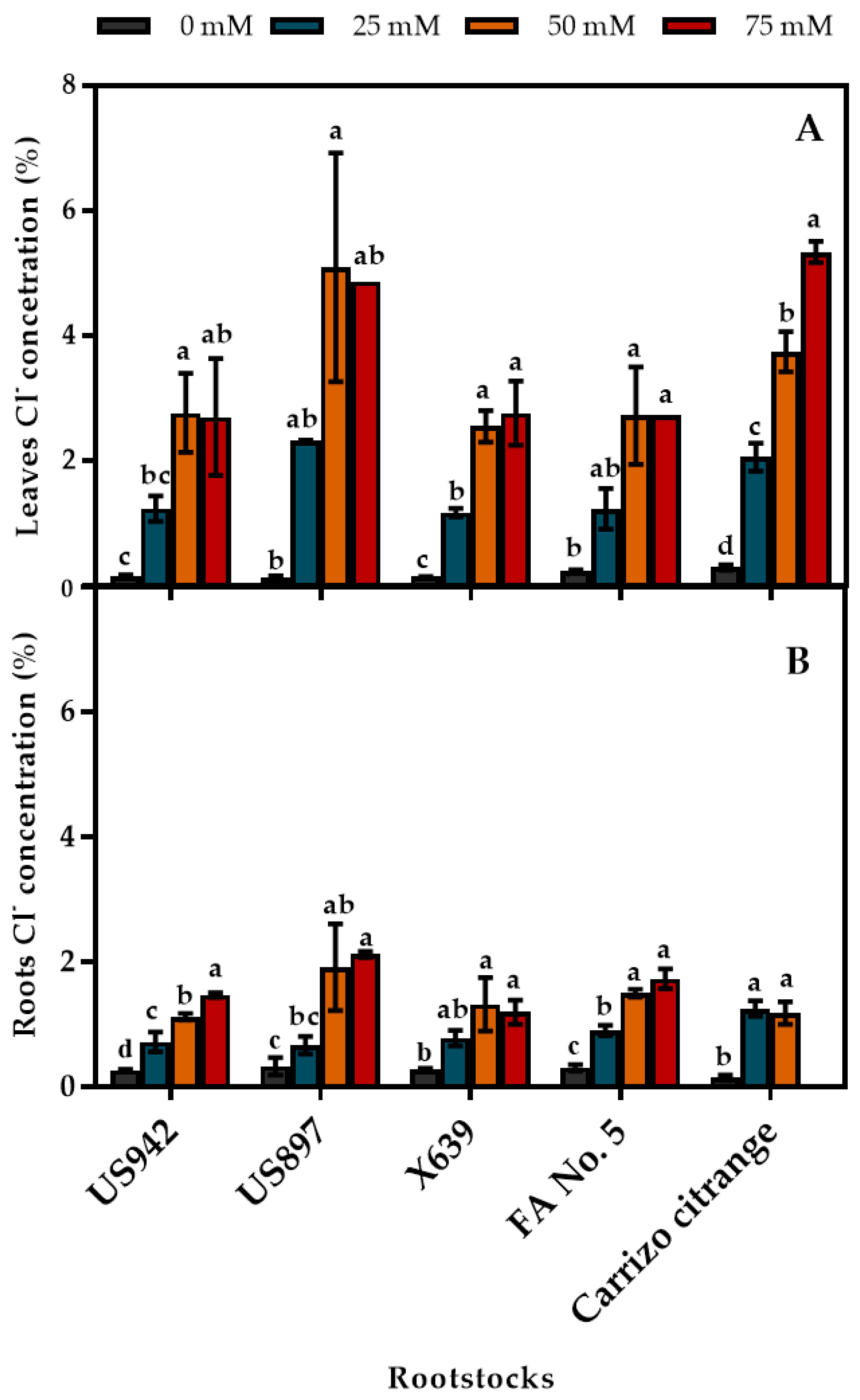
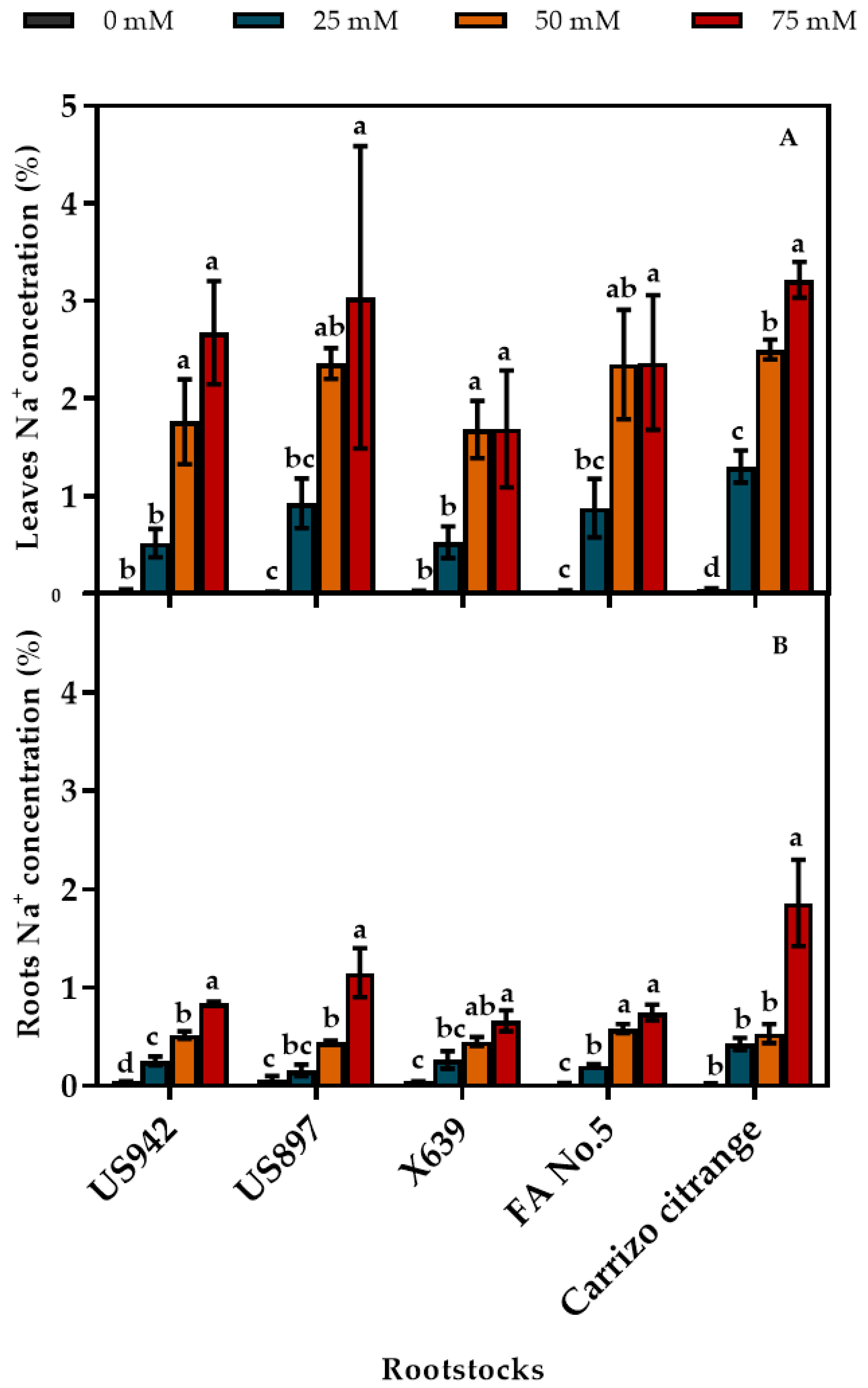
| Salinity Treatments (mM) | |||||
|---|---|---|---|---|---|
| Rootstock | 0 | 25 | 50 | 75 | |
| SAUDPC ± SE | US942 | 0 i | 0.47 ± 0.07 h | 0.69 ± 0.10 efg | 1.01 ± 0.13 bcd |
| US897 | 0 i | 0.24 ± 0.04 hi | 0.79 ± 0.06 def | 1.31 ± 0.14 a | |
| X639 | 0 i | 0.26 ± 0.06 h | 0.66 ± 0.14 fg | 0.74 ± 0.07 efg | |
| FA No. 5 | 0 i | 0.23 ± 0.06 hi | 0.92 ± 0.10 cde | 1.25 ± 0.19 ab | |
| Carrizo citrange | 0 i | 0.25 ± 0.06 h | 0.58 ± 0.10 gh | 1.07 ± 0.18 abc | |
| Mortality (%) | US942 | 0 | 0 | 0 | 25 |
| US897 | 0 | 0 | 12.50 | 37.50 | |
| X639 | 0 | 0 | 12.50 | 0 | |
| FA No. 5 | 0 | 0 | 12.50 | 12.50 | |
| Carrizo citrange | 0 | 0 | 0 | 12.50 | |
| NaCl | Rootstock | FWR ± SE | FWS ± SE | FWL ± SE | DWR ± SE | DWS ± SE | DWL ± SE |
|---|---|---|---|---|---|---|---|
| 25 mM | US942 | 28.06 ± 11.05 ab | 41.09 ± 9.91 ns | 24.35 ± 9.86 ns | 25.98 ± 10.13 ab | 41.85 ± 7.94 ns | 20.67 ± 8.12 ns |
| US897 | 27.12 ± 9.47 ab | 34.94 ± 11.23 ns | 22.14 ± 11.67 ns | 7.95 ± 7.95 b | 28.00 ± 13.14 ns | 6.25 ± 6.25 ns | |
| X639 | 15.04 ± 6.26 b | 23.21 ± 7.36 ns | 18.31 ± 8.07 ns | 41.23 ± 5.38 a | 28.18 ± 7.59 ns | 12.50 ± 6.55 ns | |
| FA No. 5 | 16.96 ± 5.61 b | 23.57 ± 7.76 ns | 25.00 ± 8.33 ns | 29.39 ± 11.97 ab | 31.84 ± 10.28 ns | 25.38 ± 12.15 ns | |
| Carrizo citrange | 46.84 ± 4.96 a | 40.85 ± 7.78 ns | 8.09 ± 6.57 ns | 33.92 ± 5.64 a | 20.41 ± 9.33 ns | 11.31 ± 5.90 ns | |
| 50 mM | US942 | 48.67 ± 4.04 ns | 56.06 ± 6.99 ns | 31.76 ± 7.19 ab | 41.67 ± 5.76 b | 59.51 ± 5.91 ns | 37.50 ± 0.07 b |
| US897 | 62.57 ± 12.26 ns | 52.24 ± 12.23 ns | 51.43 ± 12.56 a | 49.24 ± 12.63 ab | 54.00 ± 12.69 ns | 45.31 ± 0.13 b | |
| X639 | 50.48 ± 10.71 ns | 45.13 ± 11.56 ns | 39.79 ± 13.6 ab | 67.86 ± 7.88 a | 57.45 ± 9.42 ns | 38.10 ± 0.15 b | |
| FA No. 5 | 53.97 ± 7.30 ns | 51.43 ± 8.33 ns | 61.90 ± 11.76 a | 73.65 ± 8.75 a | 68.42 ± 4.43 ns | 78.46 ± 0.08 a | |
| Carrizo citrange | 69.04 ± 3.51 ns | 64.15 ± 4.05 ns | 14.46 ± 6.00 b | 64.34 ± 6.91 ab | 57.19 ± 6.38 ns | 32.9 4± 0.11 b | |
| 75 mM | US942 | 64.36 ± 7.48 ab | 77.10 ± 4.90 a | 68.24 ± 9.39 a | 55.88 ± 10.99 ns | 78.26 ± 5.90 ns | 66.35 ± 09.39 a |
| US897 | 67.91 ± 10.64 ab | 76.60 ± 4.45 a | 84.29 ± 4.55 a | 57.58 ± 8.26 ns | 67.00 ± 7.47 ns | 71.88 ± 10.76 a | |
| X639 | 56.51 ± 4.83 ab | 56.49 ± 4.43 b | 39.61 ± 5.32 b | 70.78 ± 6.01 ns | 63.04 ± 4.26 ns | 36.31 ± 6.36 b | |
| FA No. 5 | 53.09 ± 10.35 b | 55.43 ± 9.63 b | 64.29 ± 10.86 a | 62.84 ± 5.91 ns | 63.68 ± 6.98 ns | 68.46 ± 7.30 a | |
| Carrizo citrange | 77.17 ± 3.38 a | 74.04 ±2.41 a | 27.94 ± 8.16 b | 71.33 ± 2.35 ns | 69.42 ± 3.52 ns | 50.79 ± 8.20 ab |
| Macronutrients Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|
| Rootstock | NaCl (mM) | N ± SE | P ± SE | K ± SE | Ca ± SE | Mg ± SE | S ± SE |
| US942 | 0 | 3.74 ± 0.10 ns | 0.15 ± 0.02 b | 2.14 ±0.05 ns | 2.11 ± 0.11 a | 0.18 ± 0.00 a | 0.26 ± 0.02 ns |
| 25 | 4.06 ± 0.10 ns | 0.19 ± 0.00 ab | 2.32 ±0.13 ns | 1.51 ± 0.07 b | 0.14 ± 0.02 b | 0.23 ± 0.02 ns | |
| 50 | 3.37 ± 0.43 ns | 0.23 ± 0.02 a | 2.21 ± 0.06 ns | 1.53 ± 0.08 b | 0.14 ± 0.01 b | 0.31 ± 0.04 ns | |
| 75 | 3.71 ± 0.23 ns | 0.21 ± 0.01 a | 2.19 ± 0.07 ns | 1.47 ± 0.17 b | 0.16 ± 0.02 ab | 0.31 ± 0.02 ns | |
| US897 | 0 | 3.28 ± 0.19 ns | 0.18 ± 0.01 ns | 1.90 ± 0.08 ab | 2.23 ± 0.10 a | 0.17 ± 0.00 a | 0.25 ± 0.01 ns |
| 25 | 3.54 ± 0.16 ns | 0.22 ± 0.00 ns | 2.04 ± 0.06 a | 1.75 ± 0.09 b | 0.17 ± 0.02 a | 0.24 ± 0.01 ns | |
| 50 | 3.55 ± 0.01 ns | 0.22 ± 0.00 ns | 2.03 ± 0.03 a | 1.41 ± 0.03 c | 0.15 ± 0.01 ab | 0.25 ± 0.00 ns | |
| 75 | 3.46 ± 0.08 ns | 0.21 ± 0.08 ns | 1.45 ± 0.46 b | 0.87 ± 0.09 d | 0.11 ± 0.02 b | 0.21 ± 0.06 ns | |
| X639 | 0 | 3.55 ± 0.07 ns | 0.13 ± 0.02 b | 1.93 ± 0.07 b | 2.09 ± 0.02 a | 0.22 ± 0.03 a | 0.23 ± 0.02 ns |
| 25 | 3.46 ± 0.33 ns | 0.20 ± 0.01 a | 2.33 ± 0.12 ab | 1.73 ± 0.08 b | 0.15 ± 0.01 b | 0.24 ± 0.02 ns | |
| 50 | 3.32 ± 0.18 ns | 0.19 ± 0.01 a | 2.72 ± 0.14 a | 1.69 ± 0.05 b | 0.18 ± 0.01 ab | 0.26 ± 0.00 ns | |
| 75 | 3.29 ± 0.04 ns | 0.21 ± 0.01 a | 2.18 ± 0.15 b | 1.40 ± 0.09 c | 0.16 ± 0.02 b | 0.25 ± 0.01 ns | |
| FA No. 5 | 0 | 3.05 ± 0.09 ns | 0.17 ± 0.01 b | 1.57 ± 0.03 b | 1.97 ± 0.08 a | 0.20 ± 0.01 ns | 0.20 ± 0.02 b |
| 25 | 3.39 ± 0.08 ns | 0.21 ± 0.01 a | 2.08 ± 0.04 a | 1.75 ± 0.07 ab | 0.16 ± 0.01 ns | 0.23 ± 0.00 ab | |
| 50 | 3.17 ± 0.39 ns | 0.20 ± 0.02 ab | 1.82 ± 0.38 ab | 1.38 ± 0.12 b | 0.11 ± 0.02 ns | 0.24 ± 0.03 ab | |
| 75 | 3.09 ± 0.17 ns | 0.22 ± 0.01 a | 1.82 ± 0.14 ab | 1.40 ± 0.27 b | 0.15 ± 0.04 ns | 0.25 ± 0.01 a | |
| Carrizo citrange | 0 | 3.98 ± 0.23 ns | 0.19 ± 0.01 c | 1.56 ± 0.06 c | 2.49 ± 0.17 ns | 0.21 ± 0.01 a | 0.21 ± 0.01 b |
| 25 | 4.02 ± 0.23 ns | 0.24 ± 0.01 b | 1.80 ± 0.04 b | 2.12 ± 0.08 ns | 0.14 ± 0.01 b | 0.29 ± 0.01 a | |
| 50 | 4.02 ± 0.07 ns | 0.24 ± 0.01 b | 2.35 ± 0.07 a | 2.36 ± 0.08 ns | 0.16 ± 0.02 b | 0.31 ± 0.02 a | |
| 75 | 3.39 ± 0.08 ns | 0.28 ± 0.01 a | 1.77 ± 0.12 bc | NA | 0.17 ± 0.01 ab | 0.27 ± 0.02 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Durán, L.; Hervalejo, A.; Calero-Velázquez, R.; Arjona-López, J.M.; Arenas-Arenas, F.J. Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks. Agronomy 2021, 11, 653. https://doi.org/10.3390/agronomy11040653
Aparicio-Durán L, Hervalejo A, Calero-Velázquez R, Arjona-López JM, Arenas-Arenas FJ. Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks. Agronomy. 2021; 11(4):653. https://doi.org/10.3390/agronomy11040653
Chicago/Turabian StyleAparicio-Durán, Lidia, Aurea Hervalejo, Rocío Calero-Velázquez, Juan M. Arjona-López, and Francisco J. Arenas-Arenas. 2021. "Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks" Agronomy 11, no. 4: 653. https://doi.org/10.3390/agronomy11040653
APA StyleAparicio-Durán, L., Hervalejo, A., Calero-Velázquez, R., Arjona-López, J. M., & Arenas-Arenas, F. J. (2021). Salinity Effect on Plant Physiological and Nutritional Parameters of New Huanglongbing Disease-Tolerant Citrus Rootstocks. Agronomy, 11(4), 653. https://doi.org/10.3390/agronomy11040653







