Management to Promote Flowering Understoreys Benefits Natural Enemy Diversity, Aphid Suppression and Income in an Agroforestry System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
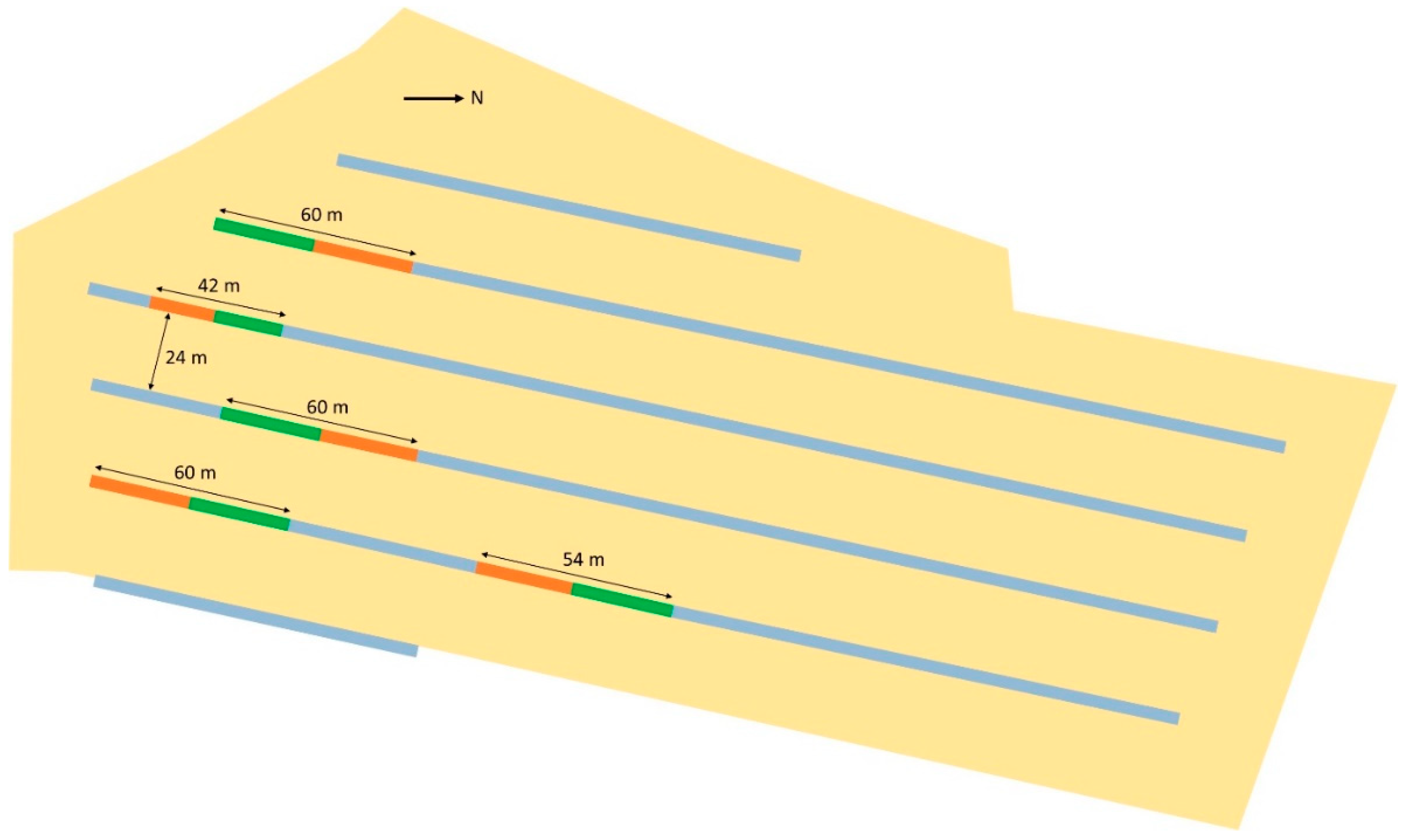
2.2. Experimental Design
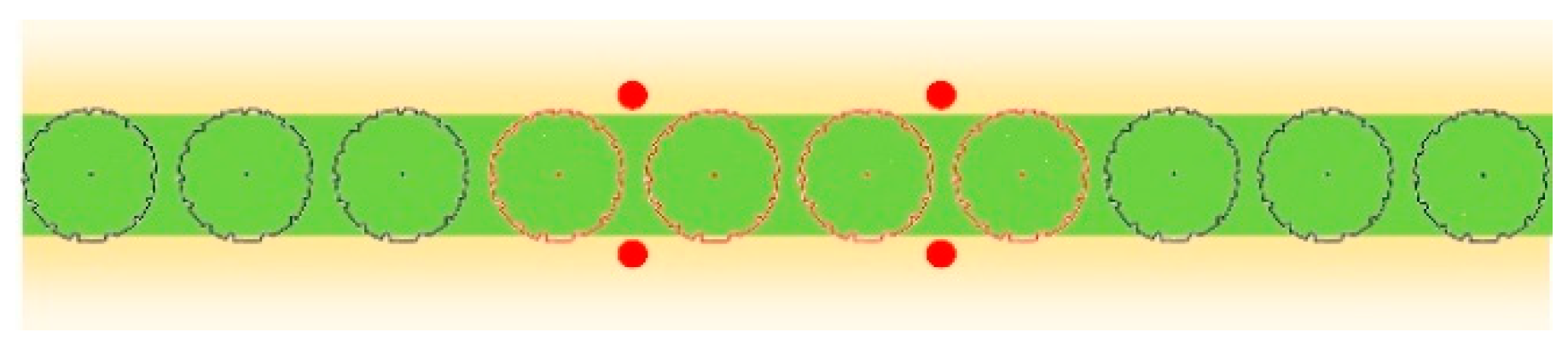
2.3. Sampling Techniques
2.4. Analysis
3. Results
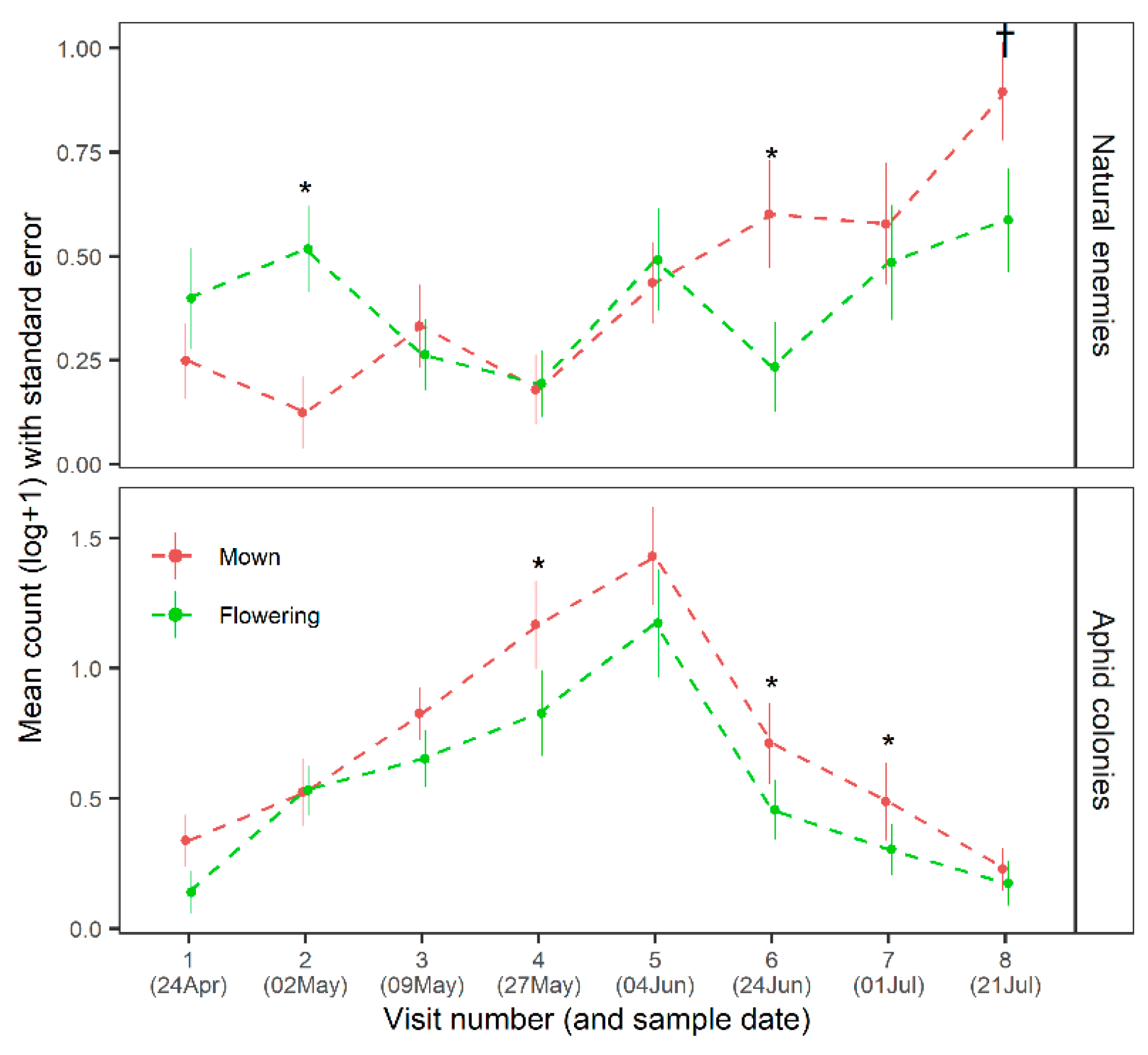
3.1. Effects on Functional Groups, Fruit Damage and Pollination in Apple Trees
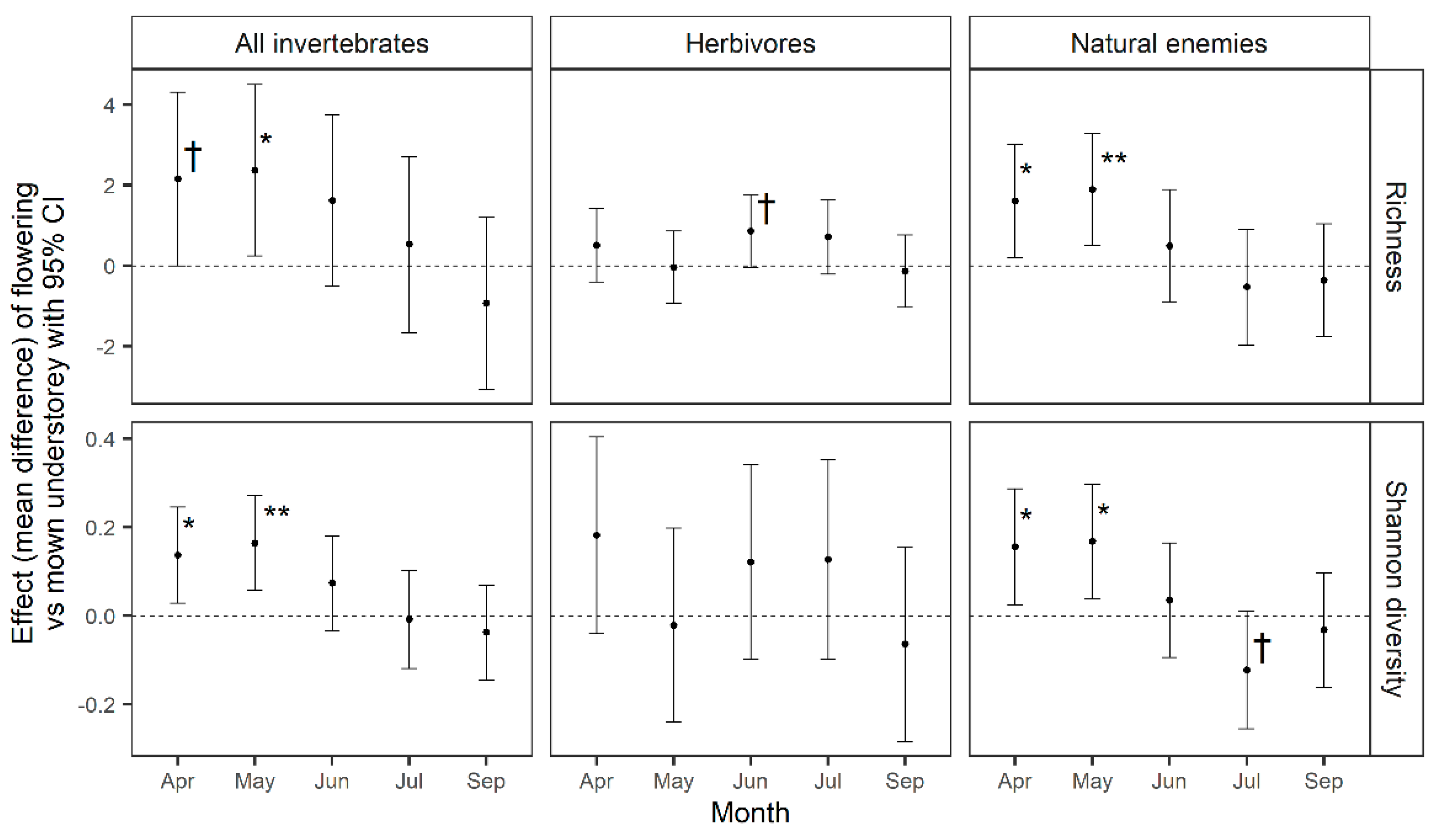
3.2. Effects on the Arable Community and Productivity
3.3. Financial Modelling
4. Discussion
4.1. Effects in the Apple Trees: Natural Pest Control and Pollination
4.2. Effects in the Arable Crop: Invertebrate Diversity and Natural Pest Control
4.3. Financial Implications
4.4. Constraints
4.5. Potential Disadvantages of Flowering Understoreys
4.6. Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C. Agricultural Landscape Simplification Reduces Natural Pest Control: A Quantitative Synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Deguines, N.; Jono, C.; Baude, M.; Henry, M.; Julliard, R.; Fontaine, C. Large-Scale Trade-off between Agricultural Intensification and Crop Pollination Services. Front. Ecol. Environ. 2014, 12, 212–217. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop Pollination from Native Bees at Risk from Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural Sustainability: Concepts, Principles and Evidence. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Pretty, J.; Benton, T.G.; Bharucha, Z.P.; Dicks, L.V.; Flora, C.B.; Godfray, H.C.J.; Goulson, D.; Hartley, S.; Lampkin, N.; Morris, C.; et al. Global Assessment of Agricultural System Redesign for Sustainable Intensification. Nat. Sustain. 2018, 1, 441–446. [Google Scholar] [CrossRef]
- Tittonell, P. Ecological Intensification of Agriculture-Sustainable by Nature. Curr. Opin. Environ. Sustain. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- Gordon, A.M.; Newman, S.M.; Coleman, B.R.W.; Thevathasan, N.V. Temperate agroforestry: An overview. In Temperate Agroforestry Systems; Gordon, A.M., Newman, S.M., Coleman, B.R.W., Eds.; CABI: Wallingford, UK, 2018; pp. 1–6. [Google Scholar]
- Torralba, M.; Fagerholm, N.; Burgess, P.J.; Moreno, G.; Plieninger, T. Do European Agroforestry Systems Enhance Biodiversity and Ecosystem Services? A Meta-Analysis. Agric. Ecosyst. Environ. 2016, 230, 150–161. [Google Scholar] [CrossRef]
- Tsonkova, P.; Böhm, C.; Quinkenstein, A.; Freese, D. Ecological Benefits Provided by Alley Cropping Systems for Production of Woody Biomass in the Temperate Region: A Review. Agrofor. Syst. 2012, 85, 133–152. [Google Scholar] [CrossRef]
- Smith, J.; Pearce, B.D.; Wolfe, M.S. Reconciling Productivity with Protection of the Environment: Is Temperate Agroforestry the Answer? Renew. Agric. Food Syst. 2013, 28, 80–92. [Google Scholar] [CrossRef]
- Staton, T.; Walters, R.J.; Smith, J.; Girling, R.D. Evaluating the Effects of Integrating Trees into Temperate Arable Systems on Pest Control and Pollination. Agric. Syst. 2019, 176, 102676. [Google Scholar] [CrossRef]
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of Agroforestry on Pest, Disease and Weed Control: A Meta-Analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Varah, A.; Jones, H.; Smith, J.; Potts, S.G. Temperate Agroforestry Systems Provide Greater Pollination Service than Monoculture. Agric. Ecosyst. Environ. 2020, 301, 107031. [Google Scholar] [CrossRef]
- Jose, S. Agroforestry for Conserving and Enhancing Biodiversity. Agrofor. Syst. 2012, 85, 1–8. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Kennedy, C.M.; Kremen, C.; Batáry, P.; Berendse, F.; Bommarco, R.; Bosque-Pérez, N.A.; Carvalheiro, L.G.; Snyder, W.E.; Williams, N.M.; et al. A Global Synthesis of the Effects of Diversified Farming Systems on Arthropod Diversity within Fields and across Agricultural Landscapes. Glob. Chang. Biol. 2017, 23, 4946–4957. [Google Scholar] [CrossRef]
- Boinot, S.; Poulmarc’h, J.; Mézière, D.; Lauri, P.É.; Sarthou, J.P. Distribution of Overwintering Invertebrates in Temperate Agroforestry Systems: Implications for Biodiversity Conservation and Biological Control of Crop Pests. Agric. Ecosyst. Environ. 2019, 285, 106630. [Google Scholar] [CrossRef]
- Hatt, S.; Francis, F.; Xu, Q.; Wang, S.; Osawa, N. Perennial Flowering Strips for Conservation Biological Control of Insect Pests: From Picking and Mixing Flowers to Tailored Functional Diversity. In Integrative Biological Control. Progress in Biological Control; Hokkanen, H., Gao, Y., Eds.; Springer: Cham, Switzerland, 2020; Volume 20, pp. 57–71. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Ganser, D.; Albrecht, M.; Knop, E. Wildflower Strips Enhance Wild Bee Reproductive Success. J. Appl. Ecol. 2020, 58, 486–495. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H.; et al. The Effectiveness of Flower Strips and Hedgerows on Pest Control, Pollination Services and Crop Yield: A Quantitative Synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Ganser, D.; Knop, E.; Albrecht, M. Sown Wildflower Strips as Overwintering Habitat for Arthropods: Effective Measure or Ecological Trap? Agric. Ecosyst. Environ. 2019, 275, 123–131. [Google Scholar] [CrossRef]
- Burgess, P.J.; Incoll, L.D.; Hart, B.J.; Beaton, A.; Piper, R.W.; Seymour, I.; Reynolds, F.H.; Wright, C.; Pilbeam, D.J.; Graves, A.R. The Impact of Silvoarable Agroforestry with Poplar on Farm Profitability and Biological Diversity. Final Report to DEFRA; Cranfield University: Bedfordshire, UK, 2003; pp. 1–63. [Google Scholar]
- Smits, N.; Dupraz, C.; Dufour, L. Unexpected Lack of Influence of Tree Rows on the Dynamics of Wheat Aphids and Their Natural Enemies in a Temperate Agroforestry System. Agrofor. Syst. 2012, 85, 153–164. [Google Scholar] [CrossRef]
- Kleijn, D.; Bommarco, R.; Fijen, T.P.M.; Garibaldi, L.A.; Potts, S.G.; van der Putten, W.H. Ecological Intensification: Bridging the Gap between Science and Practice. Trends Ecol. Evol. 2019, 34, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.M.; Pilbeam, D.J.; Briggs, S. Agroforestry in the UK. In Temperate Agroforestry Systems; Gordon, A.M., Newman, S.M., Coleman, B.R.W., Eds.; CABI: Wallingford, UK, 2018; pp. 72–97. [Google Scholar]
- Reubens, B. Managing the Tree Row Understorey in Agroforestry Systems; AFINET: Lugo, Spain, 2018; pp. 1–2. [Google Scholar]
- Gao, L.; Xu, H.; Bi, H.; Xi, W.; Bao, B.; Wang, X.; Bi, C.; Chang, Y. Intercropping Competition between Apple Trees and Crops in Agroforestry Systems on the Loess Plateau of China. PLoS ONE 2013, 8, e70739. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B. Apple Pollination: Demand Depends on Variety and Supply Depends on Pollinator Identity. PLoS ONE 2016, 11, e0153889. [Google Scholar] [CrossRef]
- Samnegård, U.; Alins, G.; Boreux, V.; Bosch, J.; García, D.; Happe, A.K.; Klein, A.M.; Miñarro, M.; Mody, K.; Porcel, M.; et al. Management Trade-Offs on Ecosystem Services in Apple Orchards across Europe: Direct and Indirect Effects of Organic Production. J. Appl. Ecol. 2019, 56, 802–811. [Google Scholar] [CrossRef]
- Graves, A.R.; Burgess, P.J.; Liagre, F.; Pisanelli, A.; Paris, P.; Moreno, G.; Bellido, M.; Mayus, M.; Postma, M.; Schindler, B.; et al. Farmer Perceptions of Silvoarable Systems in Seven European Countries. Adv. Agrofor. 2008, 6, 67–86. [Google Scholar] [CrossRef]
- García de Jalón, S.; Burgess, P.J.; Graves, A.; Moreno, G.; McAdam, J.; Pottier, E.; Novak, S.; Bondesan, V.; Mosquera-Losada, R.; Crous-Durán, J. How Is Agroforestry Perceived in Europe? An Assessment of Positive and Negative Aspects by Stakeholders. Agrofor. Syst. 2018, 92, 829–848. [Google Scholar] [CrossRef]
- Cranfield University the Soils Guide. Available online: https://www.landis.org.uk (accessed on 11 January 2021).
- Staton, T.; Walters, R.J.; Smith, J.; Breeze, T.D.; Girling, R.D. Evaluating a Trait-based Approach to Compare Natural Enemy and Pest Communities in Agroforestry vs. Arable Systems. Ecol. Appl. 2021, e2294. [Google Scholar] [CrossRef]
- Thomas, C.F.G.; Brown, N.J.; Kendall, D.A. Carabid Movement and Vegetation Density: Implications for Interpreting Pitfall Trap Data from Split-Field Trials. Agric. Ecosyst. Environ. 2006, 113, 51–61. [Google Scholar] [CrossRef]
- Webber, S.M.; Garratt, M.P.D.; Lukac, M.; Bailey, A.P.; Huxley, T.; Potts, S.G. Quantifying Crop Pollinator-Dependence and Pollination Deficits: The Effects of Experimental Scale on Yield and Quality Assessments. Agric. Ecosyst. Environ. 2020, 304, 1–8. [Google Scholar] [CrossRef]
- Lang, A. The Pitfalls of Pitfalls: A Comparison of Pitfall Trap Catches and Absolute Density Estimates of Epigeal Invertebrate Predators in Arable Land. J. Pest Sci. 2000, 73, 99–106. [Google Scholar] [CrossRef]
- Woodcock, B.A. Pitfall trapping in ecological studies. In Insect Sampling in Forest Ecosystems; Leather, S., Ed.; Blackwell: Oxford, UK, 2005; pp. 37–57. [Google Scholar]
- AHDB. Encyclopaedia of Pests and Natural Enemies in Field Crops; AHDB: Kenilworth, UK, 2015; pp. 1–200. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 1–495. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 27 March 2021).
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Greenop, A.; Woodcock, B.A.; Wilby, A.; Cook, S.M.; Pywell, R.F. Functional Diversity Positively Affects Prey Suppression by Invertebrate Predators: A Meta-Analysis. Ecology 2018, 99, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Lampkin, N.; Measures, M.; Padel, S. 2017 Organic Farm Management Handbook, 11th ed.; Organic Research Centre: Newbury, UK, 2017; pp. 1–256. [Google Scholar]
- Garratt, M.P.D.; Fountain, M.T.; McKerchar, M.; Webber, S.M. Valuing Insect Pollinators for UK Apple Production; University of Reading: Reading, UK, 2016. [Google Scholar] [CrossRef]
- Defra National Average Wholesale Prices of Home-Grown Horticultural Produce. Available online: https://www.gov.uk/government/statistical-data-sets/wholesale-fruit-and-vegetable-prices-weekly-average (accessed on 9 February 2021).
- Redman, G. John Nix Pocketbook for Farm Management 2018, 48th ed.; Agro Business Consultants: Melton Mowbray, UK, 2017. [Google Scholar]
- Cahenzli, F.; Sigsgaard, L.; Daniel, C.; Herz, A.; Jamar, L.; Kelderer, M.; Jacobsen, S.K.; Kruczyńska, D.; Matray, S.; Porcel, M.; et al. Perennial Flower Strips for Pest Control in Organic Apple Orchards—A Pan-European Study. Agric. Ecosyst. Environ. 2019, 278, 43–53. [Google Scholar] [CrossRef]
- Herz, A.; Cahenzli, F.; Penvern, S.; Pfiffner, L.; Tasin, M.; Sigsgaard, L. Managing Floral Resources in Apple Orchards for Pest Control: Ideas, Experiences and Future Directions. Insects 2019, 10, 247. [Google Scholar] [CrossRef]
- Rousselin, A.; Bevacqua, D.; Sauge, M.H.; Lescourret, F.; Mody, K.; Jordan, M.O. Harnessing the Aphid Life Cycle to Reduce Insecticide Reliance in Apple and Peach Orchards. A Review. Agron. Sustain. Dev. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Cahenzli, F.; Pfiffner, L.; Daniel, C. Reduced Crop Damage by Self-Regulation of Aphids in an Ecologically Enriched, Insecticide-Free Apple Orchard. Agron. Sustain. Dev. 2017, 37, 1–8. [Google Scholar] [CrossRef]
- Pfiffner, L.; Wyss, E. Use of sown wildflower strips to enhance natural enemies of agricultural pests. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; Gurr, G.M., Wratten, S.D., Altieri, M.A., Eds.; CABI: Wallingford, UK, 2004; pp. 165–186. [Google Scholar]
- Wyss, E. The Effects of Artificial Weed Strips on Diversity and Abundance of the Arthropod Fauna in a Swiss Experimental Apple Orchard. Agric. Ecosyst. Environ. 1996, 60, 47–59. [Google Scholar] [CrossRef]
- Markó, V.; Jenser, G.; Mihályi, K.; Hegyi, T.; Balázs, K. Flowers for Better Pest Control? Effects of Apple Orchard Groundcover Management on Mites (Acari), Leafminers (Lepidoptera, Scitellidae), and Fruit Pests. Biocontrol Sci. Technol. 2012, 22, 39–60. [Google Scholar] [CrossRef]
- Sigsgaard, L. Conservation Biological Control of Codling Moth, Cydia Pomonella. IOBC/WPRS Bull. 2014, 100, 123–126. [Google Scholar]
- Gardner, E.; Breeze, T.D.; Clough, Y.; Smith, H.G.; Baldock, K.C.R.; Campbell, A.; Garratt, M.P.D.; Gillespie, M.A.K.; Kunin, W.E.; McKerchar, M.; et al. Reliably Predicting Pollinator Abundance: Challenges of Calibrating Process-Based Ecological Models. Methods Ecol. Evol. 2020, 11, 1673–1689. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F.L. Do Sown Flower Strips Boost Wild Pollinator Abundance and Pollination Services in a Spring-Flowering Crop? A Case Study from UK Cider Apple Orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef]
- Sutter, L.; Albrecht, M.; Jeanneret, P. Landscape Greening and Local Creation of Wildflower Strips and Hedgerows Promote Multiple Ecosystem Services. J. Appl. Ecol. 2018, 55, 612–620. [Google Scholar] [CrossRef]
- Timberlake, T.P.; Vaughan, I.P.; Memmott, J. Phenology of Farmland Floral Resources Reveals Seasonal Gaps in Nectar Availability for Bumblebees. J. Appl. Ecol. 2019, 56, 1585–1596. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored Flower Strips Promote Natural Enemy Biodiversity and Pest Control in Potato Crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Ditner, N.; Balmer, O.; Beck, J.; Blick, T.; Nagel, P.; Luka, H. Effects of Experimentally Planting Non-Crop Flowers into Cabbage Fields on the Abundance and Diversity of Predators. Biodivers. Conserv. 2013, 22, 1049–1061. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Redhead, J.; Vanbergen, A.J.; Hulmes, L.; Hulmes, S.; Peyton, J.; Nowakowski, M.; Pywell, R.F.; Heard, M.S. Impact of Habitat Type and Landscape Structure on Biomass, Species Richness and Functional Diversity of Ground Beetles. Agric. Ecosyst. Environ. 2010, 139, 181–186. [Google Scholar] [CrossRef]
- O’Connor, R.S.; Kunin, W.E.; Garratt, M.P.D.; Potts, S.G.; Roy, H.E.; Andrews, C.; Jones, C.M.; Peyton, J.M.; Savage, J.; Harvey, M.C.; et al. Monitoring Insect Pollinators and Flower Visitation: The Effectiveness and Feasibility of Different Survey Methods. Methods Ecol. Evol. 2019, 10, 2129–2140. [Google Scholar] [CrossRef]
- Jonsson, M.; Kaartinen, R.; Straub, C.S. Relationships between Natural Enemy Diversity and Biological Control. Curr. Opin. Insect Sci. 2017, 20, 1–6. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A Global Synthesis Reveals Biodiversity-Mediated Benefits for Crop Production. Sci. Adv. 2019, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Briggs, S.; Knight, I. The economic case for agroforestry. In The Agroforestry Handbook; Raskin, B., Osborn, S., Eds.; Soil Association Limited: Bristol, UK, 2019; pp. 95–141. [Google Scholar]
- Prasifka, J.R.; Hellmich, R.L.; Dively, G.P.; Lewis, L.C. Assessing the Effects of Pest Management on Nontarget Arthropods: The Influence of Plot Size and Isolation. Environ. Entomol. 2005, 34, 1181–1192. [Google Scholar] [CrossRef]
- Merckx, T.; Feber, R.E.; Dulieu, R.L.; Townsend, M.C.; Parsons, M.S.; Bourn, N.A.D.; Riordan, P.; Macdonald, D.W. Effect of Field Margins on Moths Depends on Species Mobility: Field-Based Evidence for Landscape-Scale Conservation. Agric. Ecosyst. Environ. 2009, 129, 302–309. [Google Scholar] [CrossRef]
- Bennett, A.B.; Gratton, C. Measuring Natural Pest Suppression at Different Spatial Scales Affects the Importance of Local Variables. Environ. Entomol. 2012, 41, 1077–1085. [Google Scholar] [CrossRef]
- Pfiffner, L.; Jamar, L.; Cahenzli, F.; Korsgaard, M.; Swiergiel, W.; Sigsgaard, L. Perennial Flower Strips-a Tool for Improving Pest Control in Fruit Orchards; Research Institute of Organic Agriculture: Frick, Switzerland, 2018; pp. 1–16. [Google Scholar]

| Purpose | Sampling Technique | Temporal Replication |
|---|---|---|
| Invertebrate natural enemies and pests in the tree component | Visual searches of trees | Eight visits between May and July |
| Apple pest and disease damage | Visual pest and disease assessment | One visit (July for diseases and pests except aphids, September for aphid damage) |
| Apple pollinators | Flower visitation counts | Two complete visits plus one partial visit to a block still in flower (late April and early May) |
| Apple pollination | Apple seed counts [38] | One visit in September |
| Apple yield | Fruit count and width | One visit in September |
| Invertebrate diversity, natural enemies, and pests 1 in the arable component | Pitfall traps [39,40] | Five visits (April, May, June, July, September) |
| Sticky traps | Three visits (May, June, July) | |
| Arable yield | Grain samples | One visit in August |
| Response | Sampling Methods | Fixed Effects | Random Effects | |||
|---|---|---|---|---|---|---|
| Treatment | Treatment vs. Visit Interaction | Distance from Boundary | Block | Visit | ||
| Pooled aphid colonies in apple trees–seasonal effects | Visual searches | ● | ● | |||
| Pooled aphid colonies in apple trees–overall effects | Visual searches | ● | ● | ● | ||
| Pooled natural enemies in apple trees | Visual searches | ● | ● | |||
| Apple damage by aphids, other insects, and scab | Visual searches | ● | ● | |||
| Pollinator visitation to apple flowers | Flower visitation counts | ● | ● | ● | ||
| Apple seed count | Apple seed count | ● | ● (nested with sample tree) | |||
| Apple yield | Apple yield | ● | ● | |||
| Richness and Shannon diversity (separately for herbivores, natural enemies, pooled invertebrates) | Separately for pitfall and sticky traps | ● | (●) | ● | ||
| Abundances of six arable pest taxa | See Table S4 | ● | ● | ● | ||
| Pooled aerial insect captures | Sticky traps | ● | ● | ● | ||
| Arable yield | Grain samples | ● | ● | |||
| Sampling Method | Total Count/Number of Specimens | Taxonomic Groups |
|---|---|---|
| Apple pest and natural enemy visual counts | 969 1 | 27 |
| Pollinator counts | 184 | 5 |
| Pitfall traps | 15318 | 121 |
| Sticky traps | 11899 | 74 |
| Apple Damage | Estimate | Standard Error | Z Value | P-Value | R2 Marginal | R2 Conditional |
|---|---|---|---|---|---|---|
| Aphids | −0.846 | 0.127 | −6.678 | <0.001 | 0.047 | 0.155 |
| Other insect damage | 0.093 | 0.237 | 0.392 | 0.695 | <0.001 | <0.001 |
| Scab | 0.002 | 0.163 | 0.009 | 0.993 | <0.001 | 0.114 |
| Apple Variety | Predicted Increase in Income from Reduced Apple Yield Loss to Aphids | Income from Flower Mix Grant | Reduction in Mowing Costs | Total Predicted Increase in Income from Flowering Understoreys Relative to Mown Understoreys |
|---|---|---|---|---|
| Lord Derby | 580.22 | 53.90 | 13.50 | 647.62 |
| Spartan | −11.69 | 55.71 | ||
| King of the Pippins | −32.23 | 35.17 | ||
| Bramley’s Seedling | 173.72 | 241.12 | ||
| D’Arcy Spice | 108.07 | 175.47 | ||
| Mean | 163.62 | 53.90 | 13.50 | 231.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staton, T.; Walters, R.; Smith, J.; Breeze, T.; Girling, R. Management to Promote Flowering Understoreys Benefits Natural Enemy Diversity, Aphid Suppression and Income in an Agroforestry System. Agronomy 2021, 11, 651. https://doi.org/10.3390/agronomy11040651
Staton T, Walters R, Smith J, Breeze T, Girling R. Management to Promote Flowering Understoreys Benefits Natural Enemy Diversity, Aphid Suppression and Income in an Agroforestry System. Agronomy. 2021; 11(4):651. https://doi.org/10.3390/agronomy11040651
Chicago/Turabian StyleStaton, Tom, Richard Walters, Jo Smith, Tom Breeze, and Robbie Girling. 2021. "Management to Promote Flowering Understoreys Benefits Natural Enemy Diversity, Aphid Suppression and Income in an Agroforestry System" Agronomy 11, no. 4: 651. https://doi.org/10.3390/agronomy11040651
APA StyleStaton, T., Walters, R., Smith, J., Breeze, T., & Girling, R. (2021). Management to Promote Flowering Understoreys Benefits Natural Enemy Diversity, Aphid Suppression and Income in an Agroforestry System. Agronomy, 11(4), 651. https://doi.org/10.3390/agronomy11040651






