Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Phenotypic Analysis
2.3. Measurements of MDA, H2O2 and Proline, Soluble Sugar Content
2.4. Determination of GSH, NADP+ and NADPH Content
2.5. Determination of G6PDH Activity
2.6. Plasma Membrane (PM) NADPH Oxidase Activity Assay
2.7. Determination of NO3− Content and N Metabolism-Related Enzyme Activities
2.8. Total RNA Isolation and Quantitative RT-PCR
2.9. Statistical Analysis
3. Results
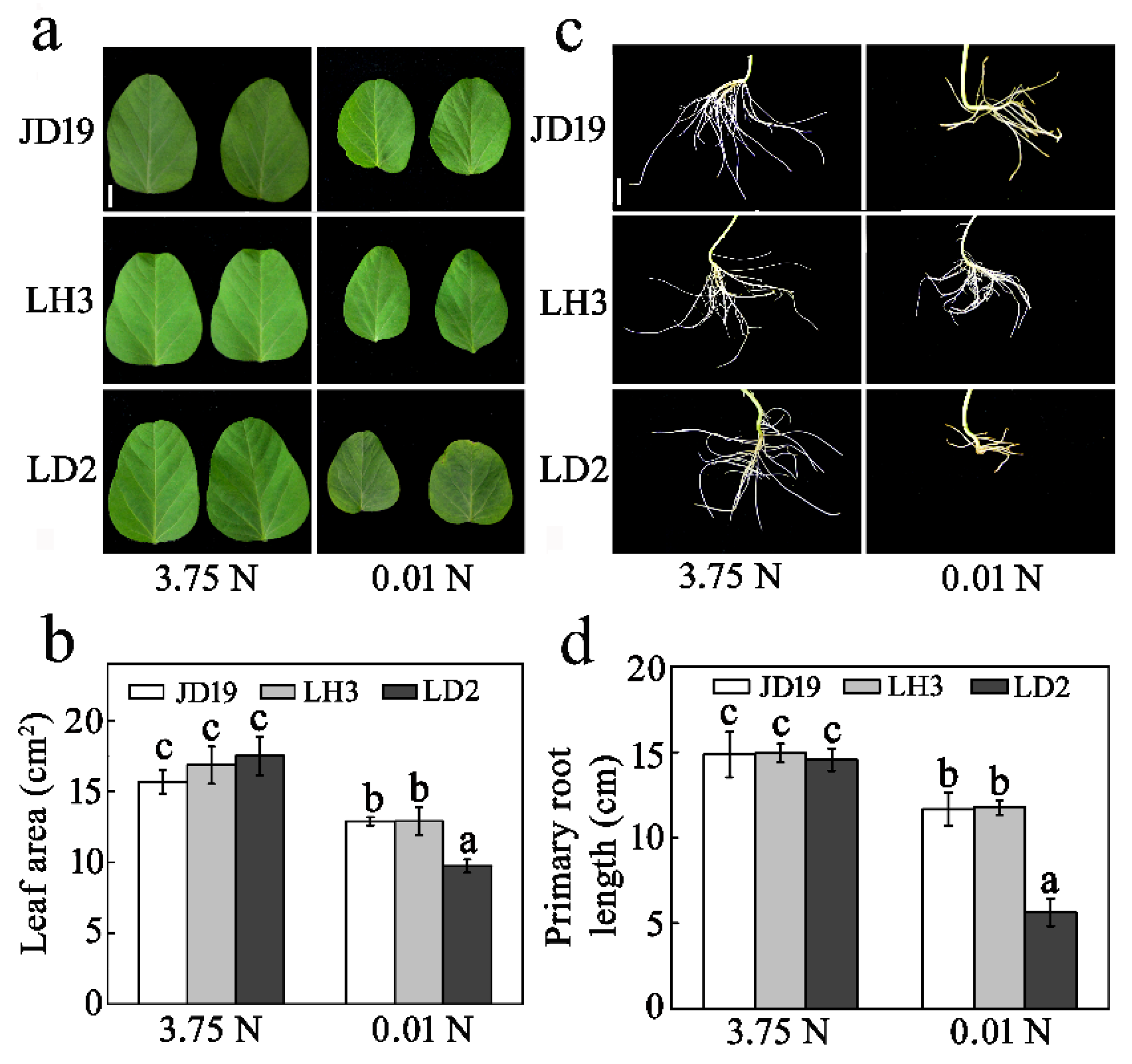
3.1. Low-N Stress Affects Soybean Growth
3.2. Low-N Stress Increases MDA and H2O2 Content but Decreases Proline and Soluble Sugar Content in Three Soybean Cultivars
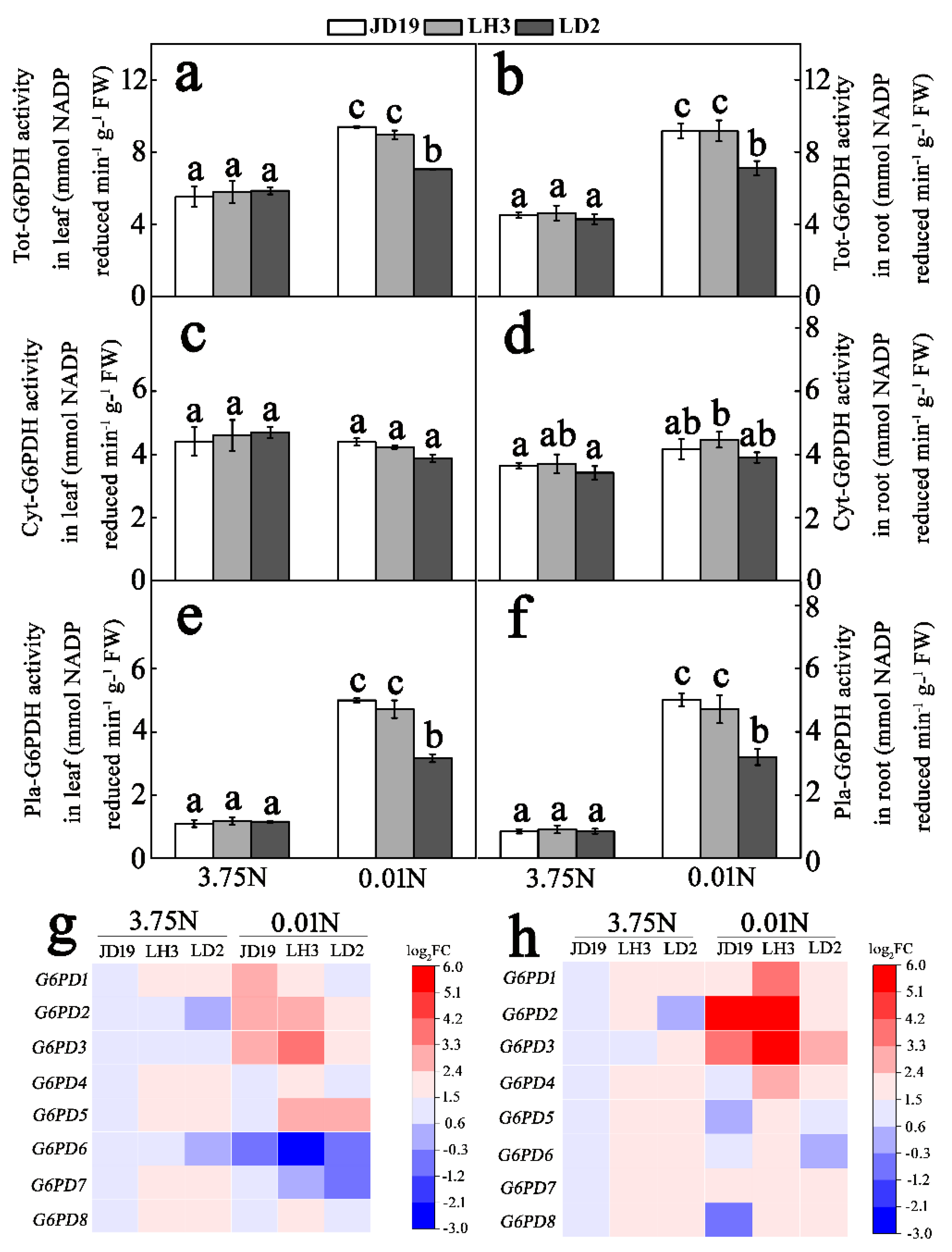
3.3. Low-N Stress Affects G6PDH Activity and G6PDH Transcript Levels in Three Soybean Cultivars
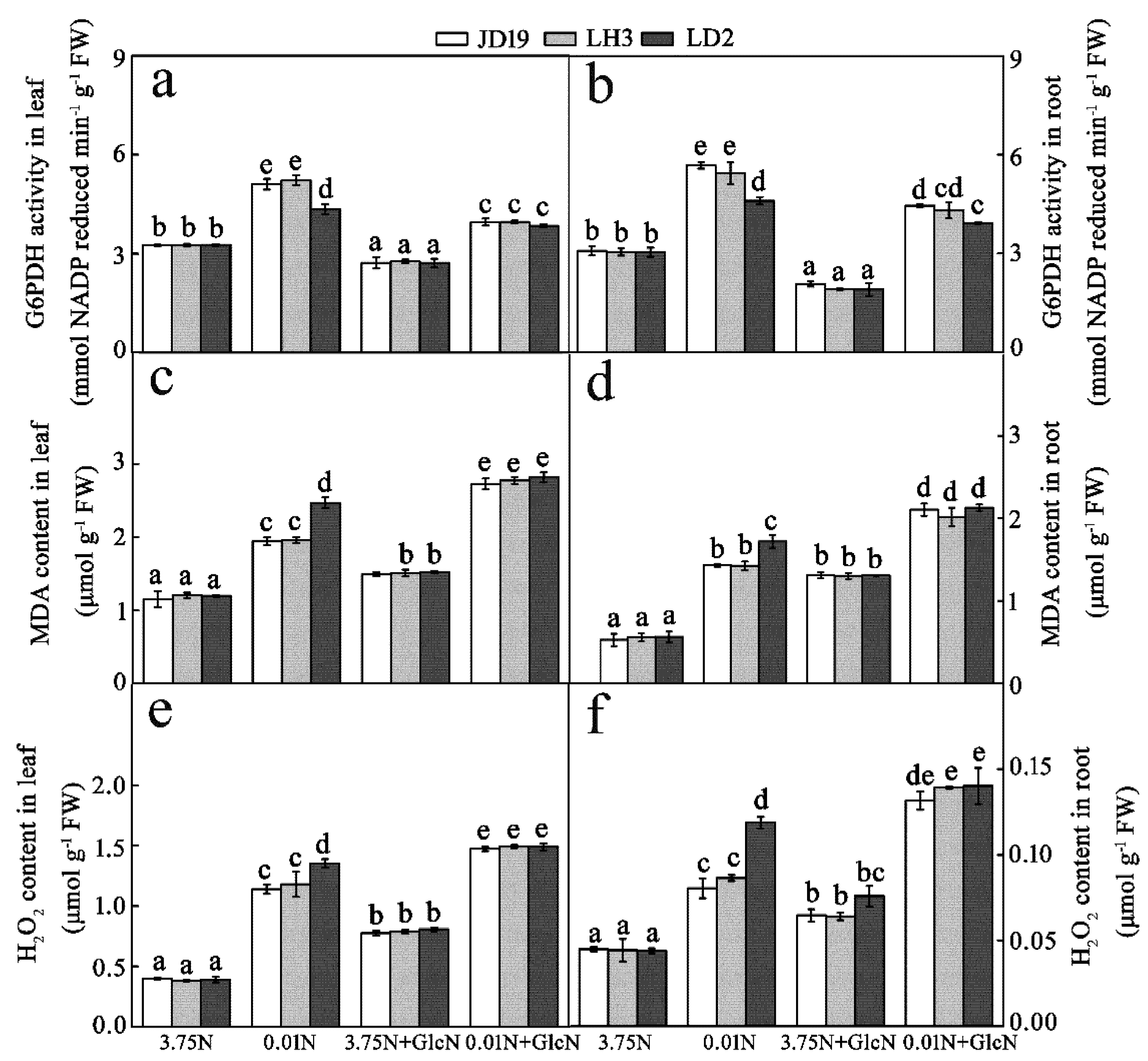
3.4. Low-N stress Increases ROS Levels after Dysfunction of G6PDH in Three Soybean Cultivars
3.5. Changes of the Redox Status under Low-N Stress
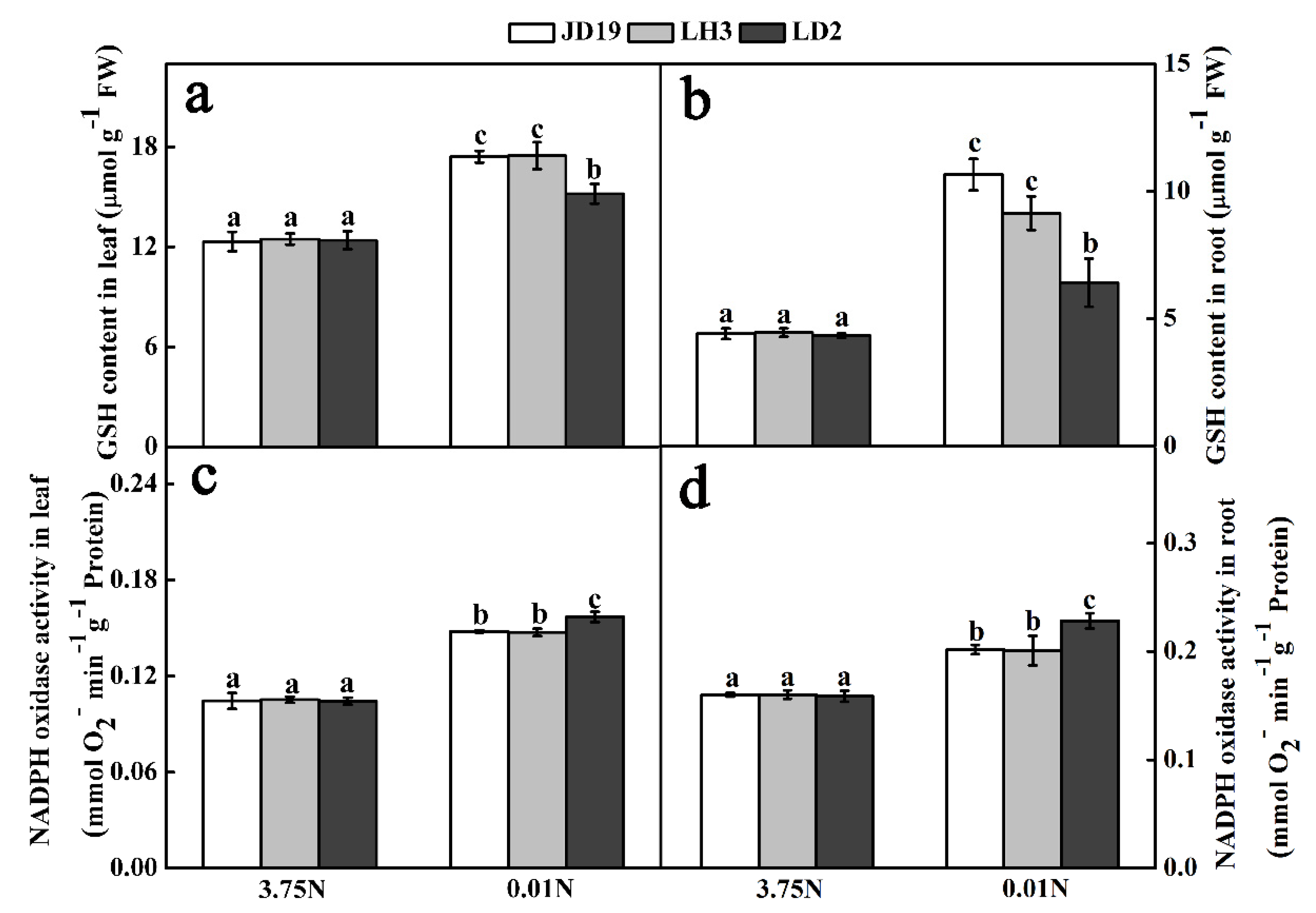
3.6. Effects of Low-N Stress on GSH Content and Plasma Membrane NADPH Oxidase Activity
3.7. Principal Component Analysis
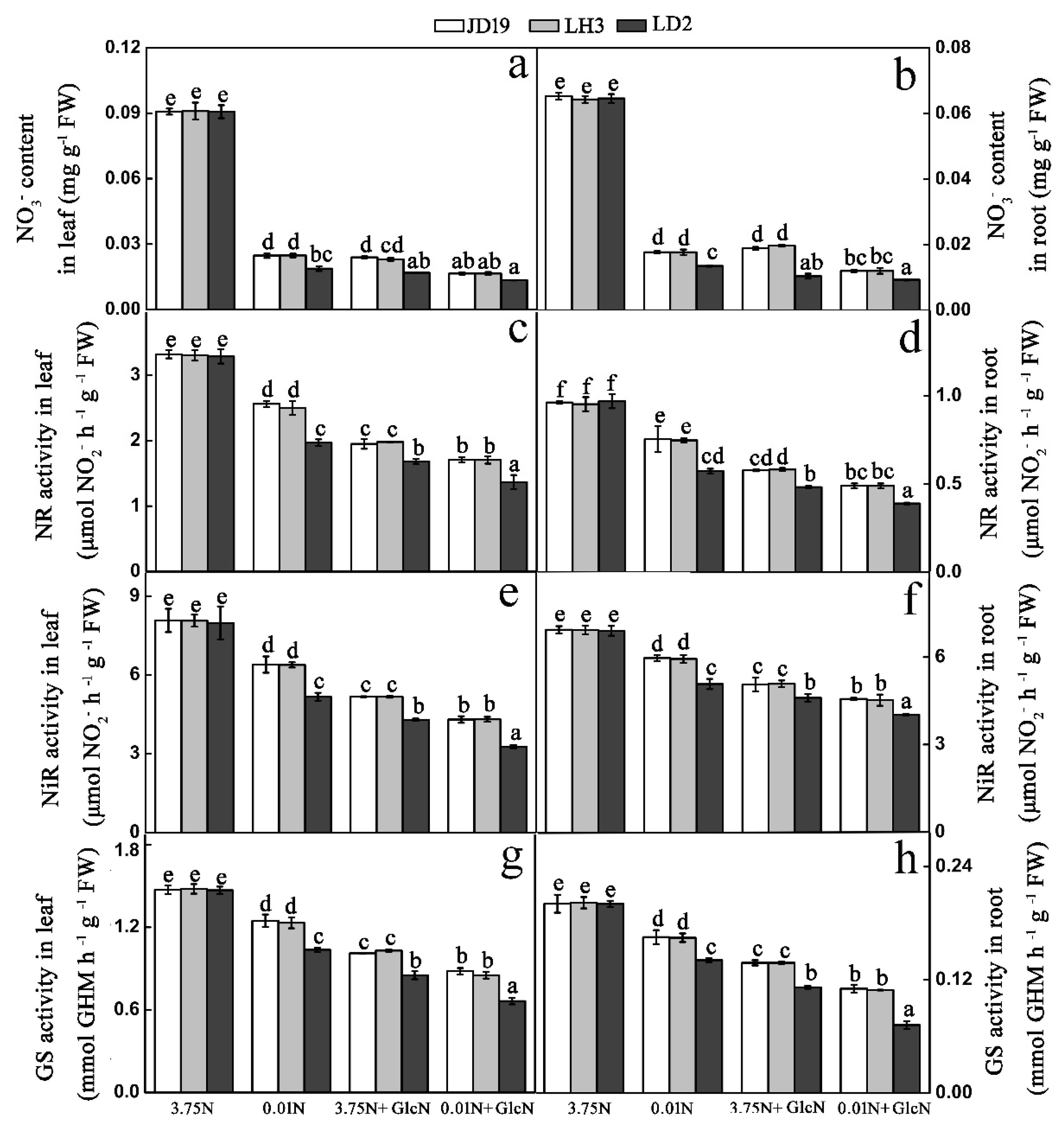
3.8. Low-N Stress Affects Nitrate Assimilation after Dysfunction of G6PDH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crawford, N.M.; Forde, B.G. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef]
- Li, H.; Hu, B.; Chu, C.C. Nitrogen use efficiency in crops, lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Crawford, N.M. Nitrate, nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Effects of row-type, row-spacing, seeding rate, soil-type, and cultivar differences on soybean seed nutrition under us Mississippi Delta conditions. PLoS ONE 2015, 10, e0129913. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans, a review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Miller, A.; Cramer, M. Root Nitrogen Acquisition and Assimilation. In Root Physiology: From Gene to Function; Springer: Dordrecht, The Netherlands, 2005; pp. 1–36. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Oxidative Stress and Antioxidant Responses in Young Leaves of Mulberry Plants Grown Under Nitrogen, Phosphorus or Potassium Deficiency. J. Int. Plant Biol. 2007, 49, 313–322. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione, Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Yang, L.D.; Yan, L.; Hou, J.J.; Huang, J.J.; Liang, W.H. Involvement of ABA-and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 2016, 107, 126. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Andre, C.; Benning, C. Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiol. 2008, 146, 277. [Google Scholar] [CrossRef]
- Wang, X.M.; Ma, Y.Y.; Huang, C.H.; Li, J.S.; Wan, Q.; Bi, Y.R. Involvement of glucose-6-phosphate dehydrogenase in reduced glutathione maintenance and hydrogen peroxide signal under salt stress. Plant Signal. Behav. 2008, 3, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Cairns, N.G.; Pasternak, M.; Wachter, A.; Cobbett, C.S.; Meyer, A.J. Maturation of Arabidopsis seeds is dependent on glutathione biosynthesis within the embryo. Plant Physiol. 2006, 141, 446–455. [Google Scholar] [CrossRef]
- Freeman, J.L.; Persans, M.W.; Nieman, K.; Albrecht, C.; Peer, W.; Pickering, I.J.; Salt, D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in thlaspi nickel hyperaccumulators. Plant Cell. 2004, 16, 2176–2191. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S. Heavy metals toxicity in plants, an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought, can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Chen, X.; Wang, Z.; Wang, S.M.; Wang, Y.P.; Zhu, Q.S.; Li, S.G.; Xiang, C.B. Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol. 2013, 162, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Saradhi, P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1991, 138, 554–558. [Google Scholar]
- Castiglia, D.; Cardi, M.; Landi, S.; Cafasso, D.; Esposito, S. Expression and characterization of a cytosolic glucose 6 phosphate dehydrogenase isoform from barley (Hordeum vulgare) roots. Protein Expr. Purif. 2015, 112, 8–14. [Google Scholar] [CrossRef]
- Corpas, J.F.; Barroso, B.J.; Sandalio, M.L.; Distefano, S.; Palma, M.J.; Lupiáñez, J.A.; Río, A.L.D. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem. J. 1998, 330, 777–784. [Google Scholar] [CrossRef]
- Esposito, S.; Carfagna, S.; Massaro, G.; Vona, V.; Rigano, V.D.M. Glucose-6-phosphate dehydrogenase in barley roots, kinetic properties and localisation of the isoforms. Planta 2001, 212, 627–634. [Google Scholar] [CrossRef]
- Knight, J.S.; Emes, M.J.; Debnam, P.M. Isolation and characterisation of a full-length genomic clone encoding a plastidic glucose 6-phosphate dehydrogenase from Nicotiana Tab. Planta 2001, 212, 499–507. [Google Scholar] [CrossRef]
- Wakao, S.; Benning, C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 2005, 41, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Chen, G.C.; Wang, X.M.; Zhang, Y.L.; Jia, H.L.; Bi, Y.R. Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol. Plant 2011, 141, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Wang, X.M.; Bi, Y.R. Glucose-6-phosphate dehydrogenase acts as a regulator of cell redox;balance in rice suspension cells under salt stress. Plant Growth Regul. 2013, 69, 139–148. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Wang, X.M.; Wang, X.Y.; Wu, K.L.; Li, P.; Chang, N.; Wang, J.F.; Wang, F.; Li, J.L.; Bi, Y.R. Glucose-6-phosphate dehydrogenase and alternative oxidase are involved in the cross tolerance of highland barley to salt stress and UV-B radiation. J. Plant Physiol. 2015, 181, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Hou, J.J.; Li, Y.; Zhang, Y.Y.; Huang, J.J.; Liang, W.H. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil. 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Wang, X.M.; Ruan, M.J.; Wan, Q.; He, W.L.; Yang, L.; Liu, X.Y.; He, L.; Yan, L.L.; Bi, Y.R. Nitric oxide and hydrogen peroxide increase glucose-6-phosphate dehydrogenase activities and expression upon drought stress in soybean roots. Plant Cell Rep. 2020, 39, 63–73. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.M.; Chang, N.; Nan, W.B.; Wang, S.W.; Ruan, M.J.; Sun, L.L.; Li, S.F.; Bi, Y.R. Cytosolic glucose-6-phosphate dehydrogenase is involved in seed germination and root growth under salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef]
- Salvemini, F.; Franzé, A.; Iervolino, A.; Filosa, S.; Salzano, S.; Ursini, M.V. Enhanced glutathione levels and oxidoresistance mediated by increased glucose-6-phosphate dehydrogenase expression. J. Biol. Chem. 1999, 274, 2750–2757. [Google Scholar] [CrossRef]
- Landi, S.; Nurcato, R.; De Lillo, A.; Lentini, M.; Grillo, S.; Esposito, S. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol. Biochem. 2016, 105, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Stampfl, H.; Fritz, M.; Dal Santo, S.; Jonak, C. The GSK3/Shaggy-like kinase ASKα contributes to pattern-triggered immunity. Plant Physiol. 2016, 171, 1366–1377. [Google Scholar] [PubMed]
- Chu, S.H.; Noh, H.N.; Kim, S.; Kim, K.H.; Hong, S.W.; Lee, H. Enhanced drought tolerance in Arabidopsis via genetic manipulation aimed at the reduction of glucosamine-induced ROS generation. Plant Mol. Biol. 2010, 74, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jin, Z.M.; Liu, Z.P.; Gong, W.J. Physiological and ecological characters studies on Aloe vera under soil salinity and seawater irrigation. Process Biochem. 2007, 42, 710–714. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.M.; Zhao, C.Z.; Wang, J.F.; Li, P.; Dou, Y.Q.; Bi, Y.R. Alternative pathway is involved in the tolerance of highland barley to the low-nitrogen stress by maintaining the cellular redox homeostasis. Plant Cell Rep. 2016, 35, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.S.; Su, X.F. The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Funct. Plant Biol. 1998, 25, 923–928. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Bai, L.; Zhao, Z.G.; Zhang, G.P.; Cheng, F.M.; Jiang, L.X.; Chen, K.M. Drought-stimulated activity of plasma membrane nicotinamide adenine dinucleotide phosphate oxidase and its catalytic properties in rice. J. Integr. Plant Biol. 2009, 51, 1104–1115. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, J.F.; Nan, Z.B.; Christensen, M.J.; Zhang, X.X.; Tian, P.; Zhang, Z.X.; Niu, X.L.; Gao, P.; Chen, T.; Ma, L.X. Effect of Epichloë gansuensis endophyte on the nitrogen metabolism, nitrogen use efficiency, and stoichiometry of Achnatherum inebrians under nitrogen limitation. J. Agric. Food Chem. 2018, 66, 4022–4031. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Y.; Lin, X.Y.; Wang, Y.; Tang, C.X. Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ. 2008, 31, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Zhang, Y.; Lu, J.A.; Shao, H.B. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling, a metabolic interface between stress perception and physiological responses. Plant Cell. 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci. 2008, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Chen, H.; Jie, L.; He, W.L.; Nan, W.B.; Gong, H.L.; Bi, Y.R. Hydrogen peroxide is involved in the regulation of rice (Oryza sativa L.) tolerance to salt stress. Acta Physiol. Plant. 2013, 35, 891–900. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism, biochemistry and physiology of the pentose phosphate pathway. Bio-REV 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Yoshioka, M.; Nomura, H.; Tone, C.; Nakajima, K.; Nakane, E.; Doke, N.; Yoshioka, H.A. plastidic glucose-6-phosphate dehydrogenase is responsible for hypersensitive response cell death and reactive oxygen species production. J. Gen. Plant Pathol. 2011, 77, 152–162. [Google Scholar] [CrossRef]
- Santo, S.D.; Stampfl, H.; Krasensky, J.; Kempa, S.; Gibon, Y.; Petutschnig, E.; Rozhon, W.; Heuck, A.; Clausen, T.; Jonak, C. Stress-Induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 2012, 24, 3380–3392. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.M.; Hu, Y.F.; Hu, W.; Bi, Y.R. Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 2013, 32, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Padmasree, K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003, 8, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Zhang, Z.S.; Gao, H.Y.; Meng, X.L.; Cheng, Y.; Liu, J.G.; Meng, Q.W. The mitochondrial alternative oxidase pathway protects the photosynthetic apparatus against photodamage in Rumex K-1 leaves. BMC Plant Biol. 2012, 12, 40. [Google Scholar] [CrossRef]
- Zhang, L.T.; Zhang, Z.S.; Gao, H.Y.; Xue, Z.C.; Yang, C.; Meng, X.L.; Meng, Q.W. Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiol. Plant. 2011, 143, 396–407. [Google Scholar] [CrossRef]
- Au, S.; Naylor, C.; Gover, S.; Vandeputte-Rutten, L.; Scopes, D.; Mason, P.; Luzzatto, L.; Lam, V.; Adams, M. Solution of the structure of tetrameric human glucose 6-phosphate dehydrogenase by molecular replacement. Acta Crystallogr. 1999, 55, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, K.; Qin, J.; Yan, L.; Wang, S.; Zhang, G.; Wang, X.; Bi, Y.R. The response mechanism to salt stress in Arabidopsis transgenic lines over-expressing of GmG6PD. Plant Physiol. Biochem. 2021, 162, 74–85. [Google Scholar] [CrossRef]
- Van, G.P.; Asard, H.; Caubergs, R.J. Solubilization and separation of a plant plasma membrane NADPH-O2-synthase from other NAD(P)H oxidoreductases. Plant Physiol. 1997, 115, 543–550. [Google Scholar]
- Esposito, S. Nitrogen assimilation, abiotic stress and glucose 6-phosphate dehydrogenase: The full circle of reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Wang, X.; Wang, J.; Li, K.; Wang, S.; Zhang, W.; Zhang, G.; Bi, Y. Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress. Agronomy 2021, 11, 637. https://doi.org/10.3390/agronomy11040637
Jin J, Wang X, Wang J, Li K, Wang S, Zhang W, Zhang G, Bi Y. Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress. Agronomy. 2021; 11(4):637. https://doi.org/10.3390/agronomy11040637
Chicago/Turabian StyleJin, Jie, Xiaomin Wang, Jianfeng Wang, Keke Li, Shengwang Wang, Wenya Zhang, Guohong Zhang, and Yurong Bi. 2021. "Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress" Agronomy 11, no. 4: 637. https://doi.org/10.3390/agronomy11040637
APA StyleJin, J., Wang, X., Wang, J., Li, K., Wang, S., Zhang, W., Zhang, G., & Bi, Y. (2021). Glucose-6-Phosphate Dehydrogenase Is Involved in the Tolerance of Soybean Seedlings to Low Nitrogen Stress. Agronomy, 11(4), 637. https://doi.org/10.3390/agronomy11040637





