Investigating the Efficacy of Selected Very-Long-Chain Fatty Acid-Inhibiting Herbicides on Iowa Waterhemp (Amaranthus tuberculatus) Populations with Evolved Multiple Herbicide Resistances
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination Chamber Dose–Response Assay
2.2. Field Dose–Response Assay
2.3. Statistical Analysis
3. Results and Discussion
3.1. Reponses of MHR Waterhemp Populations to VLCFA-Inhibiting Herbicides under Germination Chamber Conditions
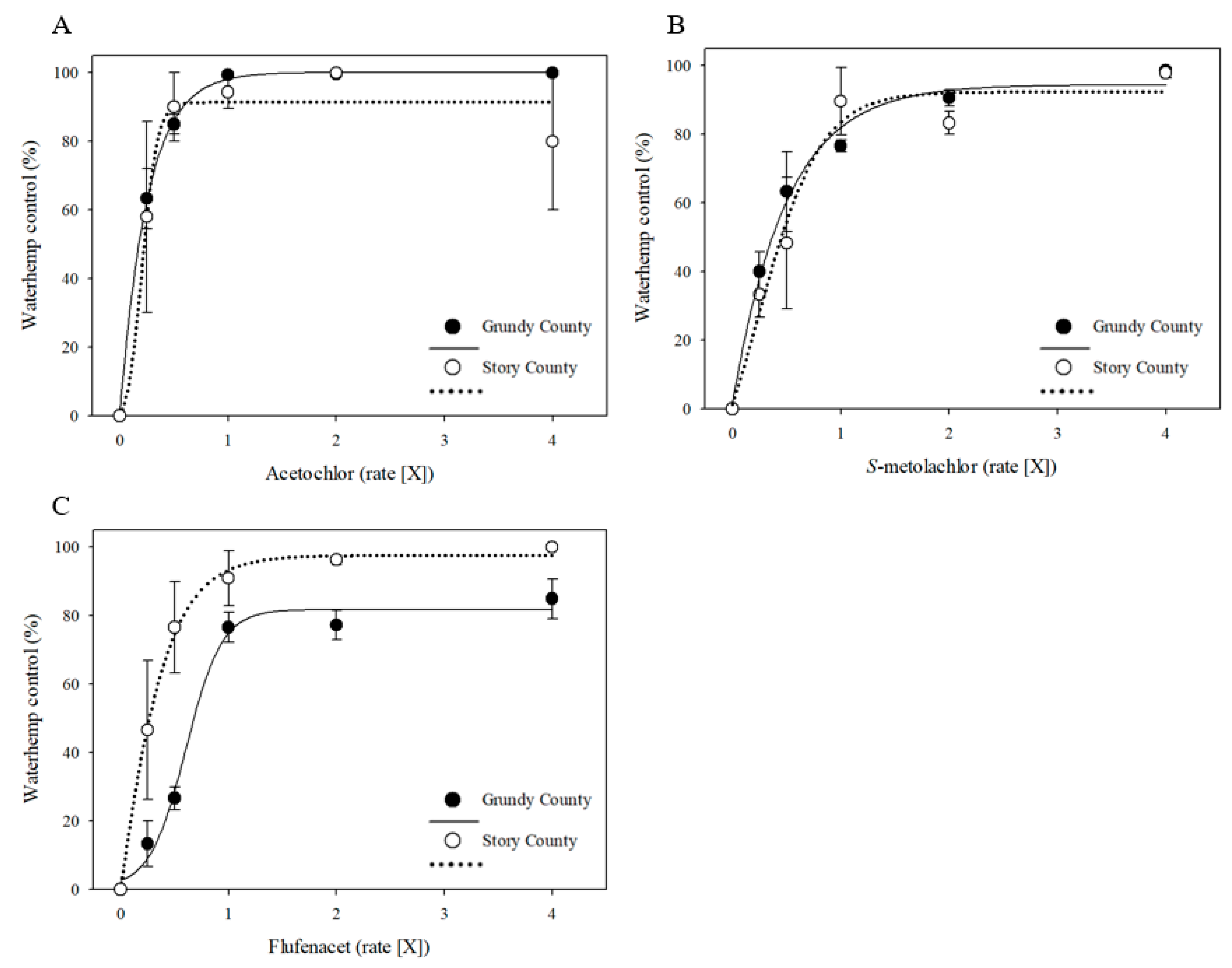
3.2. Responses of MHR Waterhemp Populations to VLCFA-Inhibiting Herbicides under Field Conditions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böger, P. Mode of action for chloroacetamides and functionally related compounds. J. Pestic. Sci. 2003, 28, 324–329. [Google Scholar] [CrossRef]
- Tanetani, Y.; Kaku, K.; Kawai, K.; Fujioka, T.; Shimizu, T. Action mechanism of a novel herbicide, pyroxasulfone. Pestic. Biochem. Physiol. 2009, 95, 47–55. [Google Scholar] [CrossRef]
- Hamm, P.C. Discovery, development, and current status of the chloroacetamide herbicides. Weed Sci. 1974, 22, 541–545. [Google Scholar] [CrossRef]
- Busi, R. Resistance to herbicides inhibiting the biosynthesis of very-long-chain fatty acids. Pest Manag. Sci. 2014, 70, 1378–1384. [Google Scholar] [CrossRef]
- Trenkamp, S.; Martin, W.; Tietjen, K. Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc. Natl. Acad. Sci. USA 2004, 101, 11903–11908. [Google Scholar] [CrossRef]
- Halsam, T.M.; Kunst, L. Extending the story of very-long-chain fatty acid elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: www.weedscience.org/in.asp (accessed on 28 January 2021).
- Brabham, C.; Norsworthy, J.K.; Houston, M.M.; Varanasi, V.K.; Barber, T. Confirmation of S-metolachlor resistance in Palmer amaranth (Amaranthus palmeri). Weed Technol. 2019, 33, 720–726. [Google Scholar] [CrossRef]
- Strom, S.A.; Gonzini, L.C.; Mitsdarfer, C.; Davis, A.S.; Riechers, D.E.; Hager, A.G. Characterization of multiple herbicide–resistant waterhemp (Amaranthus tuberculatus) populations from Illinois to VLCFA-inhibiting herbicides. Weed Sci. 2019, 67, 369–379. [Google Scholar] [CrossRef]
- Tranel, P.J.; Riggins, C.W.; Bell, M.S.; Hager, A.G. Herbicide resistances in Amaranthus tuberculatus: A call for new options. J. Agric. Food Chem. 2011, 59, 5808–5812. [Google Scholar] [CrossRef]
- Owen, M.D.K. Pest resistance: Overall principles and implications on evolved herbicide resistance in Iowa. In Proceedings of the Iowa Crop Management Conference, Ames, IA, USA, 4 December 2013; Volume 25, pp. 125–136. [Google Scholar]
- Shergill, L.S.; Barlow, B.R.; Bish, M.D.; Bradley, K.W. Investigations of a 2,4-D and mulitple herbicide resistance in a Missouri waterhemp (Amaranthus tuberculatus) population. Weed Sci. 2018, 66, 386–394. [Google Scholar] [CrossRef]
- Steckel, L.E.; Sprague, C.L.; Hager, A.G. Common waterhemp (Amaranthus rudis) control in corn (Zea mays) With single preemergence and sequential applications of residual herbicides1. Weed Technol. 2002, 16, 755–761. [Google Scholar] [CrossRef]
- Hausman, N.E.; Tranel, P.J.; Riechers, D.E.; Maxwell, D.J.; Gonzini, L.C.; Hager, A.G. Responses of an HPPD inhibitor-resistant waterhemp (Amaranthus tuberculatus) population to soil-residual herbicides. Weed Technol. 2013, 27, 704–711. [Google Scholar] [CrossRef]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2012, 69, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Letouzé, A.; Gasquz, J. Enhanced activity of several herbicide-degrading enzymes: A suggested mechanism responsible for multiple resistance in blackgrass (Alopecurus myosuriodes Huds.). Agronomie 2003, 23, 601–608. [Google Scholar] [CrossRef]
- Cummins, I.; Cole, D.J.; Edwards, R. A role for glutathione transfereases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 1999, 18, 285–292. [Google Scholar] [CrossRef]
- Yu, Q.; Abdallah, I.; Han, H.; Owen, M.; Powles, S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta 2009, 230, 713–723. [Google Scholar] [CrossRef]
- McMullan, P.M.; Green, J.M. Identification of a tall waterhemp (Amaranthus tuberculatus) biotype resistant to HPPD-inhibiting herbicides, atrazine, and thifensulfuron in Iowa. Weed Technol. 2011, 25, 514–518. [Google Scholar] [CrossRef]
- Kohlhase, D.R.; O’Rourke, J.A.; Owen, M.D.K.; Graham, M.A. Using RNA-seq to characterize responses to 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor herbicide resistance in waterhemp (Amaranthus tuberculatus). BMC Plant Biol. 2019, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.M.; Shoup, D.E.; Peterson, D.E. Palmer amaranth (Amaranthus palmeri) and common waterhemp (Amaranthus rudis) control with very-long-chain fatty acid inhibiting herbicides. Crop. Forage Turfgrass Manag. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Johnson, W.G.; Chahal, G.S.; Regehr, D.L. Efficacy of various corn herbicides applied preplant incorporated and preemergence. Weed Technol. 2012, 26, 220–229. [Google Scholar] [CrossRef]
- Burgos, N.R. Whole-plant and seed bioassays for resistance confirmation. Weed Sci. 2015, 63, 152–165. [Google Scholar] [CrossRef]
- Mahoney, D.J.; Jordan, D.L.; Burgos, N.R.; Jennings, K.M.; Leon, R.G.; Vann, M.C.; Everman, W.J.; Cahoon, C.W. Susceptibility of Palmer amaranth (Amaranthus palmeri) to herbicides in accessions collected from the North Carolina Coastal Plain. Weed Sci. 2020, 68, 582–593. [Google Scholar] [CrossRef]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 6th ed.; Penguin Books: London, UK, 1859. [Google Scholar]
- Pratt, D.B.; Clark, L.G. Amaranthus rudis and A. tuberculatus, One Species or Two? J. Torrey Bot. Soc. 2001, 128, 282. [Google Scholar] [CrossRef]
- Sauer, J.D. Revision of the dioecious amaranths. Madroño 1957, 13, 5–46. [Google Scholar]
- Meyer, C.J.; Norsworthy, J.K.; Young, B.G.; Steckel, L.E.; Bradley, K.W.; Johnson, W.G.; Loux, M.M.; Davis, V.M.; Kruger, G.R.; Bararpour, M.T.; et al. Herbicide program approaches for managing glyphosate-resistant Palmer amaranth (Amaranthus palmeri) and waterhemp (Amaranthus tuberculatus) in future soybean-trait technologies. Weed Technol. 2015, 29, 716–729. [Google Scholar] [CrossRef]
- Kohrt, J.R.; Sprague, C.L. Herbicide management strategies in field corn for a three-way herbicide-resistant palmer amaranth (Amaranthus palmeri) population. Weed Technol. 2017, 31, 364–372. [Google Scholar] [CrossRef]
- Jhala, A.J.; Malik, M.S.; Willis, J.B. Weed control and crop tolerance of micro-encapsulated acetochlor applied sequentially in glyphosate-resistant soybean. Can. J. Plant Sci. 2015, 95, 973–981. [Google Scholar] [CrossRef]
- Gressel, J. Evolving understanding of the evolution of herbicide resistance. Pest Manag. Sci. 2009, 65, 1164–1173. [Google Scholar] [CrossRef]
- Heap, I. Herbicide resistant weeds. In Integrated Pest Management Pesticide Problems; Springer: New York, NY, USA, 2014; Volume 3, pp. 281–301. [Google Scholar]
- Sandermann, H. Plant metabolism of xenobiotics. Trends Biochem. Sci. 1992, 17, 82–84. [Google Scholar] [CrossRef]
| Classification | Abbreviation | Herbicide Group [HG] Resistance Profile | Location |
|---|---|---|---|
| Herbicide-Susceptible | C | Susceptible | Story County, Iowa, USA |
| 3-Way Resistant | 3A | 2, 5, 27 | Henry County, Iowa, USA |
| 3-Way Resistant | 3B | 2, 5, 27 | Cherokee County, Iowa, USA |
| 4-Way Resistant | 4A | 2, 5, 9, 27 | Monona County, Iowa, USA |
| 4-Way Resistant | 4B | 2, 5, 9, 27 | Plymouth County, Iowa, USA |
| 5-Way Resistant | 5A | 2, 5, 9, 14, 27 | Not Recorded |
| 5-Way Resistant | 5B | 2, 5, 9, 14, 27 | Woodbury County, Iowa, USA |
| Population a | LD50 b | LD90 b | R/S c | Efficacy Curve |
|---|---|---|---|---|
| C | 1.2 | NA d | Y = 45.8 + 18.6 × ln(X), r2 = 0.81 | |
| 3A | 1.1 | 6.1 | 1.1 | Y = 49.6 + 22.4 × ln(X), r2 = 0.75 |
| 3B | 0.5 | 5.6 | 0.4 | Y = 62.3 + 16.2 × ln(X), r2 = 0.78 |
| 4A | 1.5 | NA | 1.3 | Y = 41.1 + 20.6 × ln(X), r2 = 0.86 |
| 4B | 0.9 | 6.2 | 0.8 | Y = 52.9 + 20.3 × ln(X), r2 = 0.94 |
| 5A | 0.8 | 6.3 | 0.7 | Y = 55.2 + 18.9 × ln(X), r2 = 0.77 |
| 5B | 1.4 | 10.0 | 1.2 | Y = 44.0 + 20.0 × ln(X), r2 = 0.77 |
| Population a | LD50 b | LD90 b | R/S c | Efficacy Curve |
|---|---|---|---|---|
| C | 1.2 | NA d | Y = 41.2 + 14.6 × ln(X), r2 = 0.84 | |
| 3A | 0.4 | 4.5 | 0.3 | Y = 66.3 + 15.7 × ln(X), r2 = 0.96 |
| 3B | 0.7 | 8.3 | 0.6 | Y = 55.5 + 16.2 × ln(X), r2 = 0.91 |
| 4A | 2.6 | NA | 2.2 | Y = 37.5 + 13.1 × ln(X), r2 = 0.83 |
| 4B | 3.2 | NA | 2.7 | Y = 37.5 + 13.1 × ln(X), r2 = 0.69 |
| 5A | 1.4 | NA | 1.2 | Y = 43.5 + 18.3 × ln(X), r2 = 0.72 |
| 5B | 2.1 | NA | 1.8 | Y = 39.7 + 14.2 × ln(X), r2 = 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, E.A.L.; Owen, M.D.K. Investigating the Efficacy of Selected Very-Long-Chain Fatty Acid-Inhibiting Herbicides on Iowa Waterhemp (Amaranthus tuberculatus) Populations with Evolved Multiple Herbicide Resistances. Agronomy 2021, 11, 595. https://doi.org/10.3390/agronomy11030595
Jones EAL, Owen MDK. Investigating the Efficacy of Selected Very-Long-Chain Fatty Acid-Inhibiting Herbicides on Iowa Waterhemp (Amaranthus tuberculatus) Populations with Evolved Multiple Herbicide Resistances. Agronomy. 2021; 11(3):595. https://doi.org/10.3390/agronomy11030595
Chicago/Turabian StyleJones, Eric A. L., and Micheal D. K. Owen. 2021. "Investigating the Efficacy of Selected Very-Long-Chain Fatty Acid-Inhibiting Herbicides on Iowa Waterhemp (Amaranthus tuberculatus) Populations with Evolved Multiple Herbicide Resistances" Agronomy 11, no. 3: 595. https://doi.org/10.3390/agronomy11030595
APA StyleJones, E. A. L., & Owen, M. D. K. (2021). Investigating the Efficacy of Selected Very-Long-Chain Fatty Acid-Inhibiting Herbicides on Iowa Waterhemp (Amaranthus tuberculatus) Populations with Evolved Multiple Herbicide Resistances. Agronomy, 11(3), 595. https://doi.org/10.3390/agronomy11030595





