Foliar Thidiazuron Promotes the Growth of Axillary Buds in Strawberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth
2.3. Chlorophyll
2.4. Soluble Sugar and Starch
2.5. RNA Preparation and Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
3.1. Growth
3.2. Chlorophyll
3.3. Soluble Sugar and Starch
3.4. The Expression Level of Genes in the Crown
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savini, G.; Neri, D.; Zucconi, F.; Sugiyama, N. Strawberry growth and flowering: An architectural model. Int. J. Fruit Sci. 2005, 5, 29–50. [Google Scholar] [CrossRef]
- Tenreira, T.; Lange, M.J.P.; Lange, T.; Bres, C.; Labadie, M.; Monfort, A.; Hernould, M.; Rothan, C.; Denoyes, B. A specific gibberellin 20-oxidase dictates the flowering-runnering decision in diploid strawberry. Plant Cell 2017, 29, 2168–2182. [Google Scholar] [CrossRef]
- Caruana, J.C.; Sittmann, J.W.; Wang, W.; Liu, Z. Suppressor of runnerless encodes a della protein that controls runner formation for asexual reproduction in strawberry. Mol. Plant 2018, 11, 230–233. [Google Scholar] [CrossRef]
- Hytönen, T.; Palonen, P.; Mouhu, K.; Junttila, O. Crown branching and cropping potential in strawberry (fragaria ananassa duch.) can be enhanced by daylength treatments. J. Hortic. Sci. Biotechnol. 2004, 79, 466–471. [Google Scholar] [CrossRef]
- Booker, J.; Chatfield, S.; Leyser, O. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 2003, 15, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Sato, S.; Mori, H. Control of outgrowth and dormancy in axillary buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li, Y.; Feng, J.; Cheng, L.; Dai, C.; Liu, Z. Gene expression profiling of the shoot meristematic tissues in woodland strawberry fragaria vesca. Front. Plant Sci. 2019, 10, 1624. [Google Scholar]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef]
- Bertheloot, J.; Barbier, F.; Boudon, F.; Perez-Garcia, M.D.; Péron, T.; Citerne, S.; Dun, E.; Beveridge, C.; Godin, C.; Sakr, S. Sugar availability suppresses the auxin-induced strigolactone pathway to promote bud outgrowth. New Phytol. 2020, 225, 866–879. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Wei, H.; Jeong, B.R. A long-day photoperiod and 6-benzyladenine promote runner formation through upregulation of soluble sugar content in strawberry. Int. J. Mol. Sci. 2020, 21, 4917. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Barciszewski, J.; Massino, F.; Clark, B.F. Kinetin—A multiactive molecule. Int. J. Biol. Macromol. 2007, 40, 182–192. [Google Scholar] [CrossRef]
- Ren, B.; Hu, J.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Spraying exogenous synthetic cytokinin 6-benzyladenine following the waterlogging improves grain growth of waterlogged maize in the field. J. Agron. Crop Sci. 2019, 205, 616–624. [Google Scholar] [CrossRef]
- Nisler, J.; Kopečný, D.; Končitíková, R.; Zatloukal, M.; Bazgier, V.; Berka, K.; Zalabák, D.; Briozzo, P.; Strnad, M.; Spíchal, L. Novel thidiazuron-derived inhibitors of cytokinin oxidase/dehydrogenase. Plant Mol. Biol. 2016, 92, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga chlorella vulgaris (trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, J.; Wei, H.; Jeong, B.R. Supplementary light source affects growth and development of carnation ‘dreambyul’cuttings. Agronomy 2020, 10, 1217. [Google Scholar] [CrossRef]
- Nisler, J. TDZ: Mode of action, use and potential in agriculture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–59. [Google Scholar]
- Murthy, B.; Murch, S.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. Vitr. Cell. Dev. Biol. Plant 1998, 34, 267. [Google Scholar] [CrossRef]
- Sankhla, N.; Mackay, W.; Davis, T. Reduction of flower abscission and leaf senescence in cut phlox inflorescences by thidiazuron. In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture, Toronto, ON, Canada, 11–17 August 2002; Volume 628, pp. 837–841. [Google Scholar]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in rauvolfia tetraphylla l. Using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Mroginski, E.; Rey, H.Y.; Gonzalez, A.M.; Mroginski, L.A. Thidiazuron promotes in vitro plant regeneration of arachis correntina (leguminosae) via organogenesis. J. Plant Growth Regul. 2004, 23, 129–134. [Google Scholar] [CrossRef]
- Verma, M.; Bansal, Y.K. Effect of a potent cytokinin thidiazuron (tdz) on in vitro regeneration of hedychium coronarium j. Koenig—A valuable medicinal plant. Int. J. Rec. Biotech. 2014, 2, 38–44. [Google Scholar]
- Chernyad’ev, I. The protective action of cytokinins on the photosynthetic machinery and productivity of plants under stress. Appl. Bioch. Microbiol. 2009, 45, 351–362. [Google Scholar] [CrossRef]
- Hauska, G.; Trebst, A.; Kötter, C.; Schulz, H. 1, 2, 3-thiadiazolyl-pheny 1-ureas, new inhibitors of photosynthetic and respiratory energy conservation. Z. Für Nat. C 1975, 30, 505–510. [Google Scholar] [CrossRef]
- Suttle, J.C. Involvement of ethylene in the action of the cotton defoliant thidiazuron. Plant Physiol. 1985, 78, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kiba, T.; Sakakibara, H. Metabolism and long-distance translocation of cytokinins. J. Integr. Plant Biol. 2010, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Sun, H.; Gu, P.; Liang, Z.; Chen, X.; Lou, J.; Xu, G.; Zhang, Y. A sensitive synthetic reporter for visualizing cytokinin signaling output in rice. Plant Methods 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Suzaki, T.; Ito, M.; Kawaguchi, M. Genetic basis of cytokinin and auxin functions during root nodule development. Front. Plant. Sci. 2013, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Markakis, M.N.; Boron, A.K.; Van Loock, B.; Saini, K.; Cirera, S.; Verbelen, J.-P.; Vissenberg, K. Characterization of a small auxin-up rna (saur)-like gene involved in arabidopsis thaliana development. PLoS ONE 2013, 8, e82596. [Google Scholar] [CrossRef]
- Bai, Q.; Hou, D.; Li, L.; Cheng, Z.; Ge, W.; Liu, J.; Li, X.; Mu, S.; Gao, J. Genome-wide analysis and expression characteristics of small auxin-up rna (saur) genes in moso bamboo (phyllostachys edulis). Genome 2017, 60, 325–336. [Google Scholar] [CrossRef]
- Ziliotto, F.; Corso, M.; Rizzini, F.M.; Rasori, A.; Botton, A.; Bonghi, C. Grape berry ripening delay induced by a pre-véraison naa treatment is paralleled by a shift in the expression pattern of auxin-and ethylene-related genes. BMC Plant. Biol. 2012, 12, 1–15. [Google Scholar] [CrossRef]
- Li, S.-B.; Xie, Z.-Z.; Hu, C.-G.; Zhang, J.-Z. A review of auxin response factors (arfs) in plants. Front. Plant Sci. 2016, 7, 47. [Google Scholar] [CrossRef]
- Raman, S.; Greb, T.; Peaucelle, A.; Blein, T.; Theres, K. Interplay of mir164, cup-shaped cotyledon genes and lateral suppressor controls axillary meristem formation in Arabidopsis thaliana. Plant. J. 2008, 55, 65–76. [Google Scholar] [CrossRef]
- Chahtane, H.; Vachon, G.; Le Masson, M.; Thévenon, E.; Périgon, S.; Mihajlovic, N.; Kalinina, A.; Michard, R.; Moyroud, E.; Monniaux, M. A variant of leafy reveals its capacity to stimulate meristem development by inducing rax1. Plant. J. 2013, 74, 678–689. [Google Scholar] [CrossRef]
- Cao, X.; Jiao, Y. Control of cell fate during axillary meristem initiation. Cell. Mol. Life Sci. 2020, 77, 2343–2354. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, Y. Regulation of axillary meristem initiation by transcription factors and plant hormones. Front. Plant. Sci. 2016, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced wus expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant. J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, E.; Laux, T.; Rensing, S.A. The wus homeobox-containing (wox) protein family. Genome Biol. 2009, 10, 248. [Google Scholar] [CrossRef]
- Alyssa, D.; Jung, H.S.; Sun, T.P. The DELLA motif is essential for gibberellin-induced degradation of RGA. Proc. Natl. Acad. Sci. USA 2001, 98, 14162–14167. [Google Scholar]
- Li, W.; Zhang, J.; Sun, H.; Wang, S.; Chen, K.; Liu, Y.; Li, H.; Ma, Y.; Zhang, Z. Fverga1, encoding a della protein, negatively regulates runner production in fragaria vesca. Planta 2018, 247, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mouhu, K.; Kurokura, T.; Koskela, E.A.; Albert, V.A.; Elomaa, P.; Hytönen, T. The fragaria vesca homolog of suppressor of overexpression of constans1 represses flowering and promotes vegetative growth. Plant. Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Hu, J.; Jeong, B.R. Method of silicon application affects quality of strawberry daughter plants during cutting propagation in hydroponic substrate system. Agronomy 2020, 10, 1753. [Google Scholar] [CrossRef]
- Gaston, A.; Perrotte, J.; Lerceteau-Köhler, E.; Rousseau-Gueutin, M.; Petit, A.; Hernould, M.; Rothan, C.; Denoyes, B. PFRU, a single dominant locus regulates the balance between sexual and asexual plant reproduction in cultivated strawberry. J. Exp. Bot. 2013, 64, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
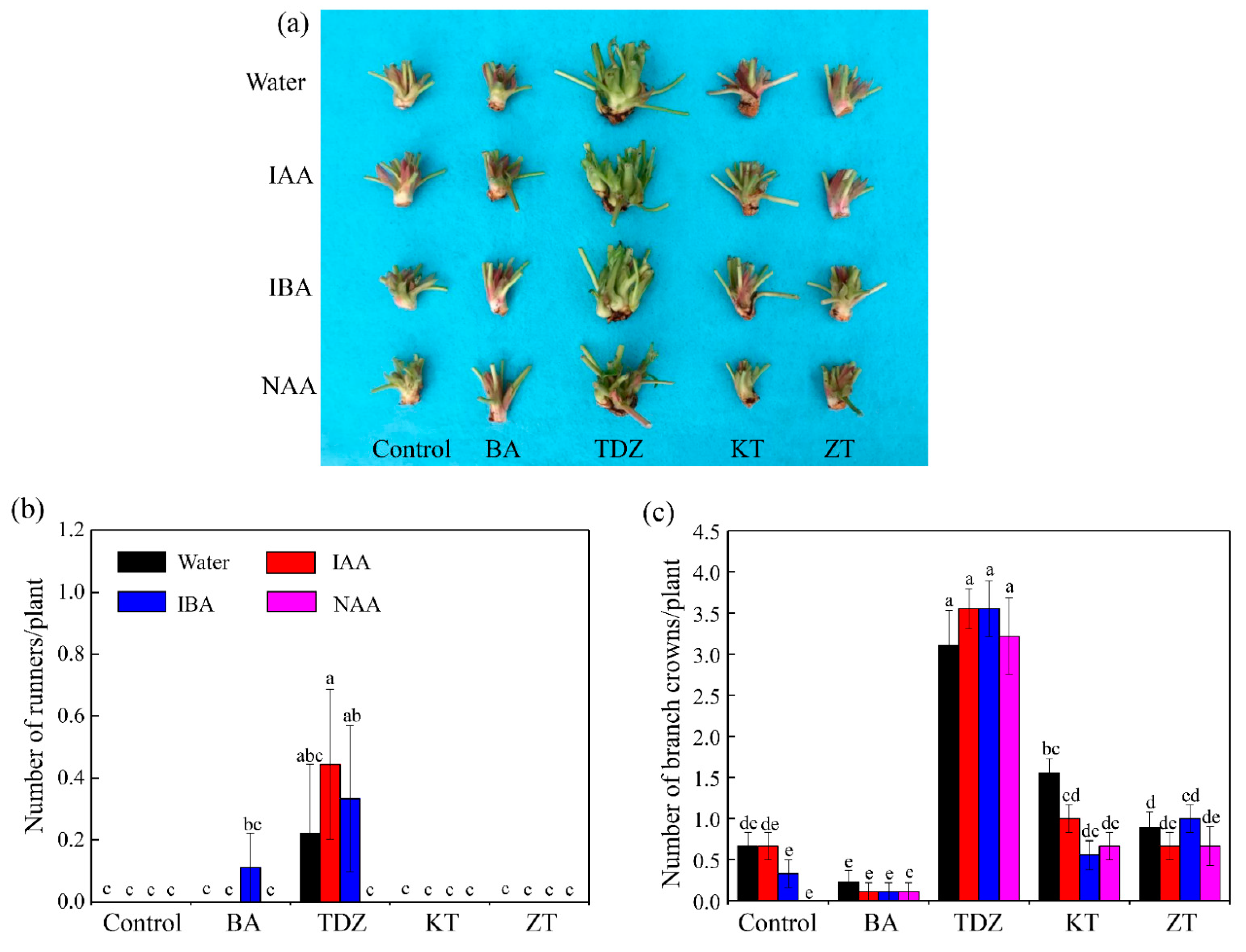


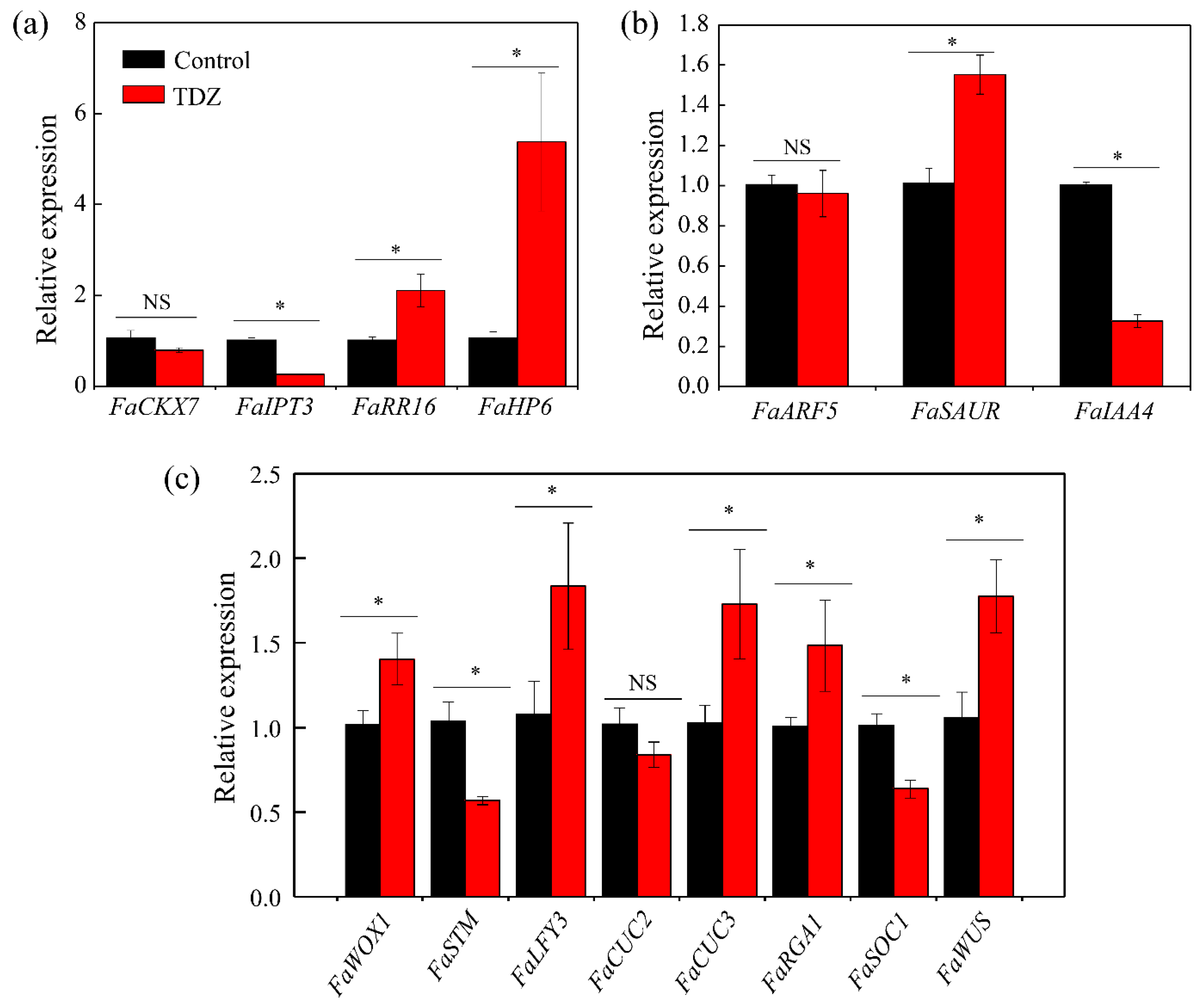
| Gene Name | Forward Primers (5′ to 3′) | Reverse Primers (5′ to 3′) |
|---|---|---|
| FaCKX7 | ATTATGGGAAGACGTGCTGAAA | TAACGACTTCAAGCTCCGTTAC |
| FaIPT3 | GTGAAAGACAATACGTGCAAGT | ACTTCGGCCTTGTTATTGTAGA |
| FaRR16 | GCAAGAGCATTGGAGTTTCTAG | TATCCTGTCATTCCTGGCATAC |
| FaHP6 | GGGGATCCATTTGAATCAGTTG | GATCTGCTGTATTGGGTATCGA |
| FaARF5 | TGGTTGGCATAAGGAGAGCA | GGCATGCCCTTGGATTGTAG |
| FaSAUR | CTTTCAGCCAGATTAGGAGCTA | CAAACCTCTTCTTCTTGCCTTC |
| FaIAA4 | CCAAGCCTCCTCCTTCCAAA | GAACATCCCACCGCTTTCTC |
| FaWOX1 | GATGAAGATCAGACTCAAACGC | GATTCAGTGACGATTTCTCAGC |
| FaSTM | TGGCTAGGTTAGAGGATGCG | GCTCTGGGTCTGGTTCTGAT |
| FaLFY3 | CCACCAAGGTCACAAACCAG | GCGTAGCAGTGAACGTAGTG |
| FaCUC2 | CCCAGGAAGAGTGGGTCATT | GAGACCGAGGAGGAAGAAGG |
| FaCUC3 | TCTGCTGCTACTGCTTCTGT | GCAGCTGGGAGTTTGATGAG |
| FaRGA1 | AAGCCGTCCAGCAGAACAA | GGTAAGGGCAGGTCTCGTAG |
| FaSOC1 | GCAACTAGCATGATGAAGCAGATA | TGTTCACACTCCTCTCCAACTG |
| FaWUS | CACCAATGGAGCCACAAC | TCCCTCGATCTTCCCGTA |
| Cytokinin | Auxin | Number of Flowers | Plant Height (cm) | Leaf Length (cm) | Leaf Width (cm) |
|---|---|---|---|---|---|
| Control | Water | 3.4 c–e | 15.6 f–h | 6.30 c,d | 5.18 a–e |
| IAA | 3.7 c–e | 14.1 h,i | 5.71 d–g | 4.68 c–g | |
| IBA | 1.8 e | 13.7 h,i | 5.43 e–h | 4.34 f–h | |
| NAA | 4.3 c–e | 12.8 i,j | 3.89 i | 3.12 i | |
| BA | Water | 4.0 c–e | 12.9 i,j | 5.37 f–h | 4.36 f–h |
| IAA | 3.9 c–e | 11.6 j | 4.81 h | 4.30 f–h | |
| IBA | 3.0 d,e | 19.2 c,d | 6.49 b,c | 4.66 c–h | |
| NAA | 3.4 c–e | 17.9 d,e | 5.10 g,h | 4.73 b–f | |
| TDZ | Water | 5.0 b–d | 24.0 a | 8.03 a | 5.74 a |
| IAA | 5.0 b–d | 23.7 a | 7.79 a | 5.38 a,b | |
| IBA | 4.8 b–d | 22.2 a,b | 8.20 a | 5.83 a | |
| NAA | 5.4 a–d | 20.7 b,c | 7.08 b | 5.33 a–c | |
| KT | Water | 5.9 a–d | 14.9 g,h | 5.73 d–g | 4.61 d–h |
| IAA | 5.1 a–d | 16.7 e–g | 6.18 c,d | 4.78 b–f | |
| IBA | 6.2 a–c | 18.4 d,e | 6.47 b,c | 4.98 b–f | |
| NAA | 8.0 a | 18.3 d,e | 5.34 f–h | 4.04 g,h | |
| ZT | Water | 7.3 a,b | 17.4 d–f | 6.04 c–f | 4.88 b–f |
| IAA | 7.6 a,b | 17.8 d,e | 6.10 c–e | 5.23 a–d | |
| IBA | 6.2 a–c | 15.2 g,h | 5.62 d–g | 4.52 e–h | |
| NAA | 4.9 b–d | 15.2 g,h | 4.84 h | 3.98 h | |
| F-test | Cytokinin | *** | *** | *** | *** |
| Auxin | NS | * | *** | *** | |
| Cytokinin x Auxin | NS | *** | * | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hu, J.; Xiao, J.; Guo, G.; Jeong, B.R. Foliar Thidiazuron Promotes the Growth of Axillary Buds in Strawberry. Agronomy 2021, 11, 594. https://doi.org/10.3390/agronomy11030594
Li Y, Hu J, Xiao J, Guo G, Jeong BR. Foliar Thidiazuron Promotes the Growth of Axillary Buds in Strawberry. Agronomy. 2021; 11(3):594. https://doi.org/10.3390/agronomy11030594
Chicago/Turabian StyleLi, Yali, Jiangtao Hu, Jie Xiao, Ge Guo, and Byoung Ryong Jeong. 2021. "Foliar Thidiazuron Promotes the Growth of Axillary Buds in Strawberry" Agronomy 11, no. 3: 594. https://doi.org/10.3390/agronomy11030594
APA StyleLi, Y., Hu, J., Xiao, J., Guo, G., & Jeong, B. R. (2021). Foliar Thidiazuron Promotes the Growth of Axillary Buds in Strawberry. Agronomy, 11(3), 594. https://doi.org/10.3390/agronomy11030594








