L-3,4-dihydroxyphenylalanine Accumulation in Faba Bean (Vicia faba L.) Tissues during Different Growth Stages
Abstract
1. Introduction
2. Materials and Methods
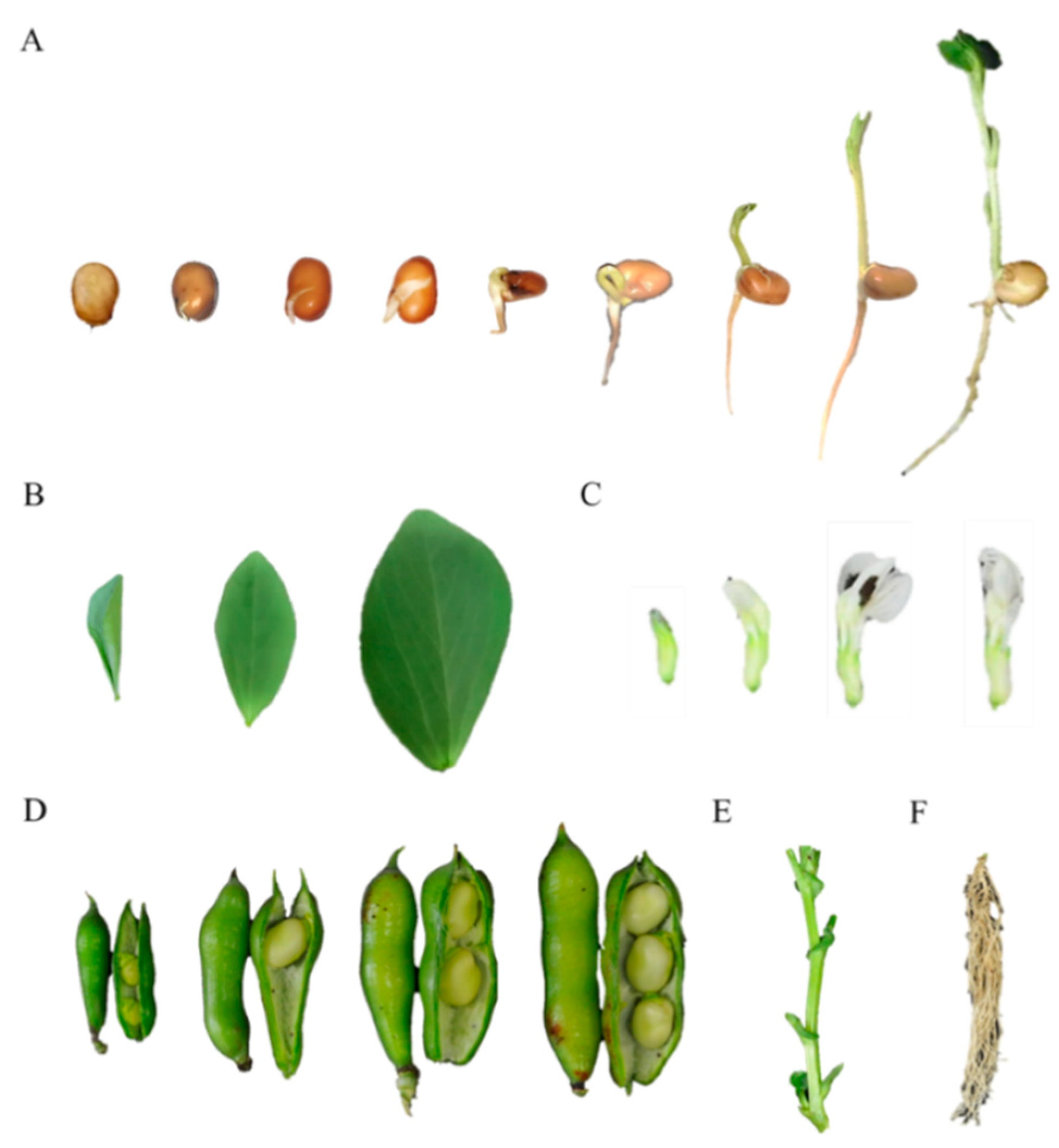
2.1. Samples
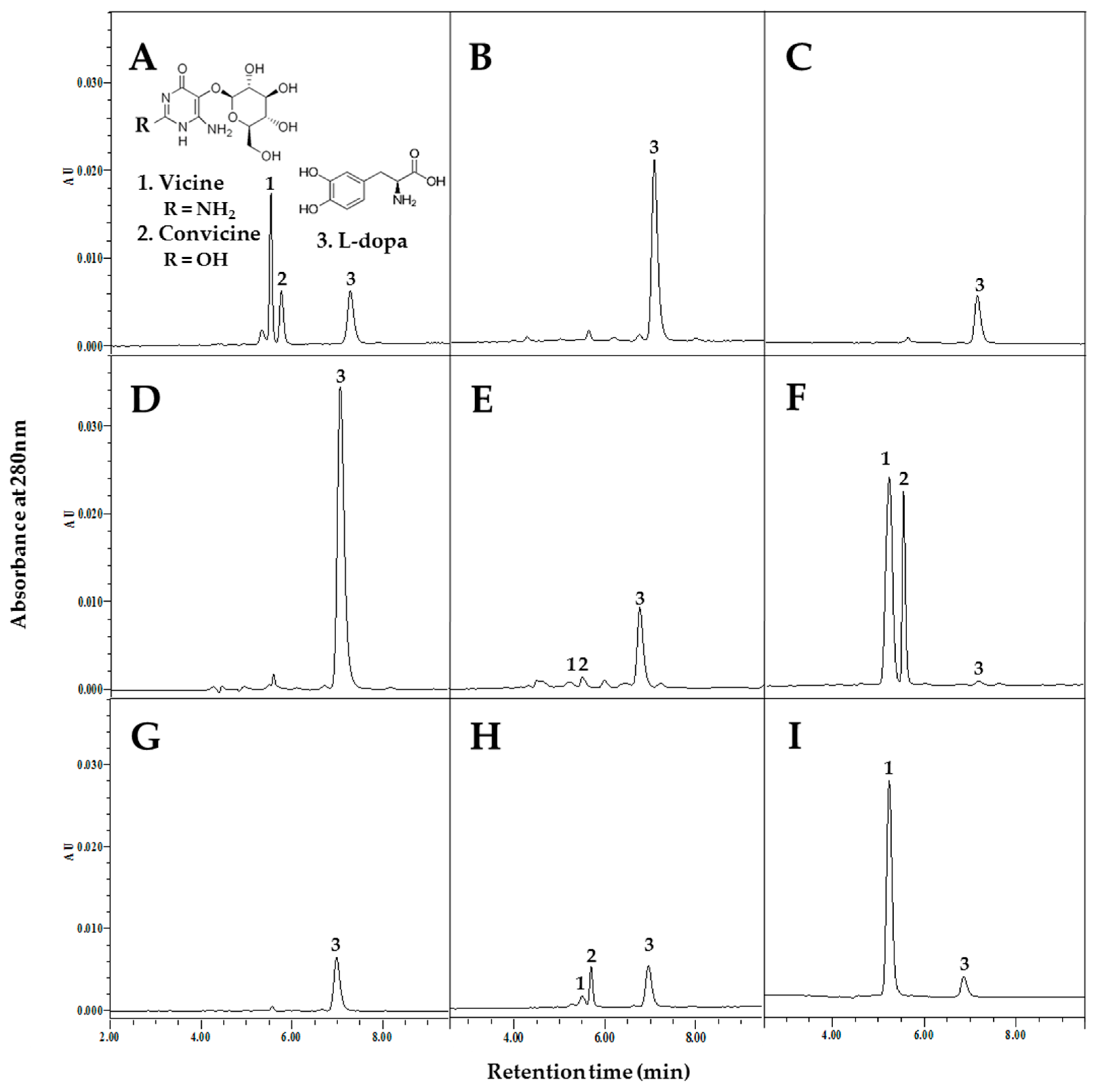
2.2. Analysis of L-dopa, Vicine, and Convicine Contents in Plant Tissues
2.3. Statistical Analysis
3. Results and Discussion
3.1. Accumulation of L-dopa, Vicine, and Convicine during Seed Germination
3.2. Accumulation of L-dopa, Vicine, and Convicine in Leaves
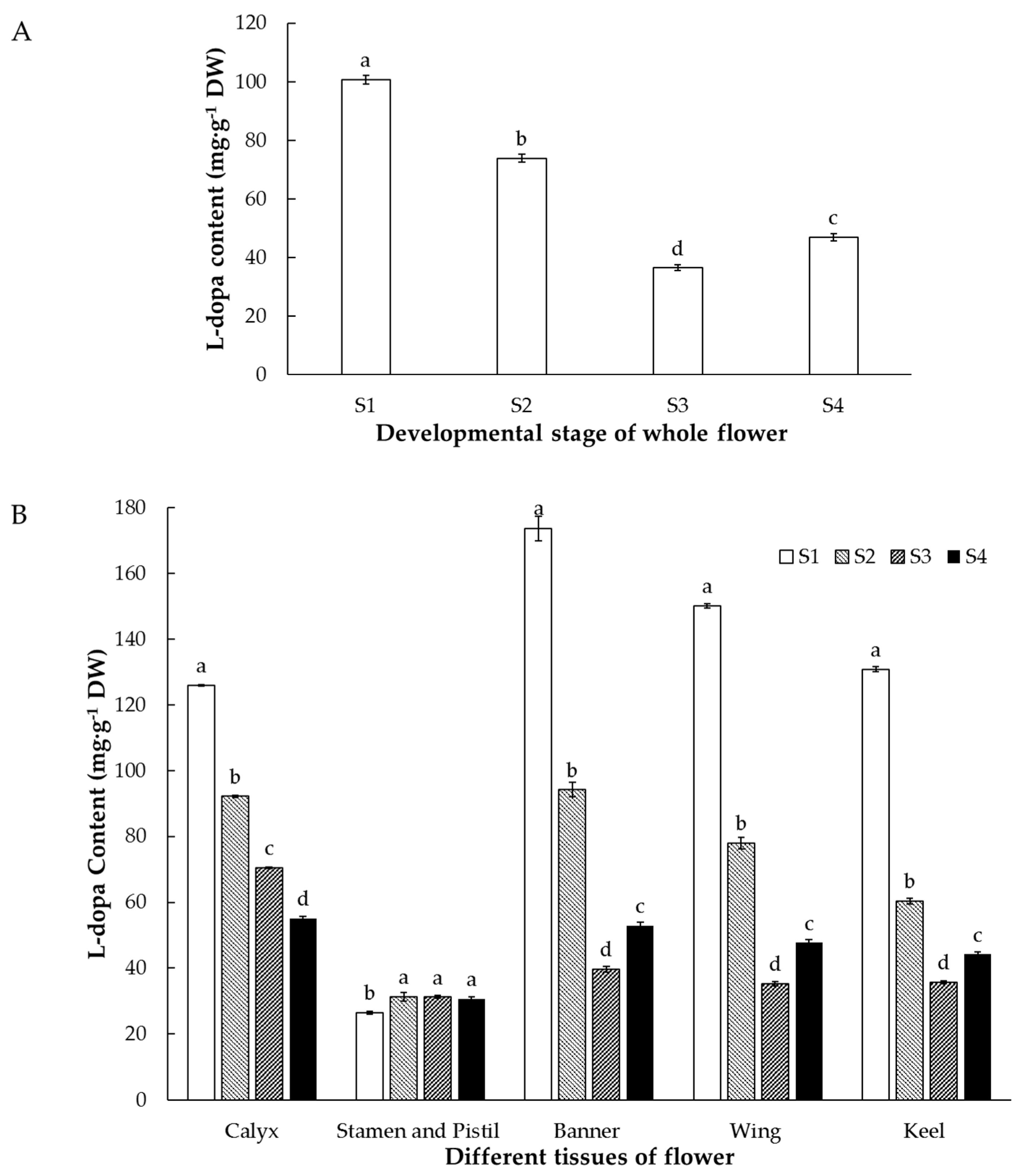
3.3. Accumulation of L-dopa, Vicine, and Convicine in Flowers
3.4. Accumulation of L-dopa, Vicine, and Convicine in Faba Bean Pods
3.5. Accumulation of L-dopa, Vicine, and Convicine in Other Faba Organs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: http://faostat.fao.org (accessed on 2 March 2020).
- Kumar, A.; Nidhi; Prasad, N.; Sinha, S.K. Nutritional and antinutritional attributes of faba bean (Vicia faba L.) germplasms growing in Bihar, India. Physiol. Mol. Biol. Plants 2015, 21, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Pulkkinen, M.; Wang, Y.J.; Lampi, A.M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT-Food Sci. Technol. 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Kim, D.-G.; Kim, J.M.; Kang, K.-Y.; Lee, M.-K.; Hong, M.J.; Kim, J.-B.; Eom, S.H.; Kang, S.-Y.; Ha, B.-K.; et al. Phytochemical compounds and antioxidant activity in the grain of selected faba bean (Vicia faba) genotypes. Plant Breed. Biotech. 2018, 6, 65–73. [Google Scholar] [CrossRef]
- Okumura, K.; Hosoya, T.; Kawarazaki, K.; Izawa, N.; Kumazawa, S. Antioxidant activity of phenolic compounds from Fava bean sprouts. J. Food Sci. 2016, 81, 1394–1398. [Google Scholar] [CrossRef]
- Renna, M.; Signore, A.; Paradiso, V.M.; Santamaria, P. Faba greens, globe artichoke’s offshoots, crenate broomrape and summer squash greens: Unconventional vegetables of Puglia (southern Italy) with good quality traits. Front. Plant Sci. 2018, 9, 378. [Google Scholar] [CrossRef]
- Allam, A.; Nafady, A.; Khedr, A.I.M.; Nakagawa, T.; Shimizu, K. Potential activities for constituents from Vicia faba. Trends Phytochem. Res. 2018, 2, 21–26. [Google Scholar]
- Renna, M.; De Cillis, F.; Leoni, B.; Acciardi, E.; Santamaria, P. From by-product to unconventional vegetable: Preliminary evaluation of fresh fava hulls highlights richness in L-dopa and low content of anti-nutritional factor. Foods 2020, 9, 159. [Google Scholar] [CrossRef]
- Neugart, S.; Rohn, S.; Schreiner, M. Identification of complex, naturally occurring flavonoid glycosides in Vicia faba and Pisum Sativum leaves by HPLC-DAD-ESI-MSn and the genotypic effect on their flavonoid profile. Food Res. Int. 2015, 76, 114–121. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Autio, W.R.; Mangan, F.X.; Zandvakili, O. Yield and accumulation trend of biomass and L-DOPA in different parts of eight faba bean cultivars. Crop Sci. 2018, 58, 2020–2028. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Randhir, R.; ZandVakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Poewe, W.; Antonini, A.; Zijlmans, J.C.; Burkhard, P.R.; Vingerhoets, F. Levodopa in the treatment of Parkinson’s disease: An old drug still going strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Park, K.; Park, D.-H.; Yoo, Y.J. Overview on the biotechnological production of L-DOPA. Appl. Microbiol. Biotechnol. 2015, 99, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.; Karouta, C.; Morgan, I.; Ashby, R. Effectiveness and safety of topical levodopa in a chick model of myopia. Sci. Rep. 2019, 9, 18345. [Google Scholar] [CrossRef]
- Saito, I.; Kawabe, H.; Hasegawa, C.; Iwaida, Y.; Yamakawa, H.; Saruta, T.; Takeshita, E.; Nagano, S.; Sekihara, T. Effect of L-dopa in young patients with hypertension. Angiology 1991, 42, 691–695. [Google Scholar] [CrossRef]
- Zech, P.; Collard, M.; Guey, A.; Plantier, J.; Beruard, M.; Berthoux, F.; Pinet, A.; Traeger, J. Renal hemodynamic response to L-dopa during acute renal failure in Man. Biomedicine 1975, 23, 456–460. [Google Scholar] [PubMed]
- Rabey, J.M.; Vered, Y.; Shabtai, H.; Graff, E.; Korczyn, A.D. Improvement of parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption. J. Neurol. Neurosurg. Psychiatry 1992, 55, 725–727. [Google Scholar] [CrossRef]
- Hu, J.; Kwon, S.-J.; Park, J.-J.; Landry, E.; Mattinson, D.S.; Gang, D.R. LC-MS determination of L-DOPA concentration in the leaf and flower tissues of six faba bean (Vicia faba L.) lines with common and rare flower colors. Funct. Foods Health Dis. 2015, 5, 243–250. [Google Scholar] [CrossRef]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Varela, A.; Guillamón, E.; Pedrosa, M.M.; Muzquiz, M. Content and distribution of vicine, convicine and L-DOPA during germination and seedling growth of two Vicia faba L. varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
- Geng, G. Analysis on content of L-DOPA in various tissues of horsebean in field production. North. Hortic. 2012, 2, 26–27. [Google Scholar]
- Hussein, M.A. Anti-inflammatory effect of natural heterocycle glucoside vicine obtained from Vicia faba L. its aglucone (divicine) their effect on some oxidative stress biomarkers in Albino rats. Free Radic. Antioxid. 2012, 2, 44–54. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Maya-Ocaña, K.; Ortiz-Moreno, A.; Herrera-Cabrera, B.E.; Dávila-Ortiz, G.; Múzquiz, M.; Martín-Pedrosa, M.; Burbano, C.; Cuadrado, C.; Jiménez-Martínez, C. Effect of roasting and boiling on the content of vicine, convicine and L-3,4-Dihydroxyphenylalanine in Vicia faba L. J. Food Qual. 2012, 35, 419–428. [Google Scholar] [CrossRef]
- Purves, R.W.; Zhang, H.; Khazaei, H.; Vandenberg, A. Rapid analysis of medically relevant compounds in faba bean seeds using FAIMS and mass spectrometry. Int. J. Ion Mobil. Spec. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- Purves, R.W.; Khazaei, H.; Vandenberg, A. Quantification of vicine and convicine in faba bean seeds using hydrophilic interaction liquid chromatography. Food Chem. 2018, 240, 1137–1145. [Google Scholar] [CrossRef]
- Burbano, C.; Cuadrado, C.; Muzquiz, M.; Cubero, J.I. Variation of favism-inducing factors (vicine, convicine and L-DOPA) during pod development in Vicia faba L. Plant Foods Hum. Nutr. 1995, 47, 265–275. [Google Scholar] [CrossRef]
- Ramsay, G.; Griffiths, D.W. Accumulation of vicine and convicine in vicia faba and V. narbonensis. Phytochemistry 1996, 42, 63–67. [Google Scholar] [CrossRef]
- Lee, M.-K.; Kim, D.-G.; Kim, J.M.; Ryu, J.; Eom, S.H.; Hong, M.J.; Hong, M.J.; Jang, Y.E.; Ha, B.-K.; Kwon, S.-J. Selection of the elite lines with high adaptability to autumn sowing of South Korea in faba bean. Plant Breed. Biotech. 2018, 6, 57–64. [Google Scholar] [CrossRef][Green Version]
- Vora, R.; Joshi, A.N.; Joshi, N.C. Comparison of extraction efficiency of various methods to extract L-DOPA from Mucuna pruriens (L.) DC. Int. J. Bioassays 2017, 6, 5343. [Google Scholar] [CrossRef][Green Version]
- Polanowska, K.; Łukasik, R.; Kuligowski, M.; Nowak, J. Development of a sustainable, simple, and robust method for efficient l-DOPA extraction. Molecules 2019, 24, 2325. [Google Scholar] [CrossRef]
- Labaneiah, M.E.O.; Luh, B.S. Changes of starch, crude fiber, and oligosaccharides in germinating dry beans. Cereal Chem. 1981, 58, 135–138. [Google Scholar]
- Oviedo-Silva, C.; Elso-Freudenberg, M.; Aranda-Bustos, M. L-DOPA trends in different tissues at early stages of Vicia faba growth: Effect of tyrosine treatment. Appl. Sci. 2018, 8, 2431. [Google Scholar] [CrossRef]
- Björnsdotter, E.; Nadzieja, M.; Chang, W.; Escobar-Herrera, L.; Mancinotti, D.; Angra, D.; Khazaei, H.; Crocoll, C.; Vandenberg, A.; Stoddard, F.L.; et al. VC1 catalyzes a key step in the biosynthesis of vicine from GTP in faba bean. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, D.-G.; Lee, M.-K.; Kim, J.M.; Hong, M.J.; Kang, K.-Y.; Eom, S.H.; Kang, S.-Y.; Kim, J.-B.; Kwon, S.-J. Fatty acid composition, isoflavone and L-3,4-dihydroxyphenylalanine (L-dopa) contents in different parts of faba bean (Vicia faba) genotypes. Plant Breed. Biotech. 2017, 5, 314–324. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, C.; Chen, K.; Li, X. Flavonoids, phenolics, and antioxidant capacity in the flower of Eriobotrya japonica Lindl. Int. J. Mol. Sci. 2011, 12, 2935–2945. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.-B.; Xu, X.-Y.; Qiu, S.-L.; Li, A.-P. Study on L-dopa content in faba bean flowers. Legume Res. 2016, 39, 931–934. [Google Scholar]
- Topal, N.; Bulduk, I.; Mut, Z.; Bozodlu, H.; Tosun, Y.K. Flowers, pollen and honey for use in the treatment of Parkinson’s disease. Rev. Chim. 2020, 71, 308–319. [Google Scholar] [CrossRef]
- Belsey, M.A. The epidemiology of favism. Bull. World Health Organ. 1973, 48, 1–13. [Google Scholar]
- Topal, N.; Bozoğlu, H. Determination of L-dopa (L-3, 4-dihydroxyphenylalanine) content of some faba bean (Vicia faba L.) genotypes. J. Agric. Sci. 2016, 22, 145–151. [Google Scholar] [CrossRef]
- De Cillis, F.; Leoni, B.; Massaro, M.; Renna, M.; Santamaria, P. Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between Italian landraces and commercial varieties. Agriculture 2019, 9, 253. [Google Scholar] [CrossRef]


| Germination Time (Days) | L-dopa (mg∙g−1 DW) | Vicine (mg∙g−1 DW) | Convicine (mg∙g−1 DW) |
|---|---|---|---|
| 1 | 0.43 ± 0.00 d | 10.88 ± 0.08 abc | 4.43 ± 0.08 abc |
| 2 | 0.47 ± 0.01 d | 9.75 ± 0.17 c | 4.19 ± 0.08 bc |
| 3 | 0.51 ± 0.03 d | 10.26 ± 0.35 abc | 4.01 ± 0.16 c |
| 4 | 0.70 ± 0.01 d | 11.11 ± 0.19 ab | 4.19 ± 0.11 bc |
| 5 | 1.03 ± 0.03 d | 10.85 ± 0.05 abc | 4.67 ± 0.04 ab |
| 6 | 2.63 ± 0.09 cd | 10.88 ± 0.18 abc | 4.72 ± 0.07 ab |
| 7 | 4.50 ± 0.07 c | 9.74 ± 0.22 c | 4.18 ± 0.98 bc |
| 8 | 11.11 ± 0.32 b | 11.38 ± 0.36 a | 5.00 ± 0.24 a |
| 12 | 16.87 ± 1.58 a | 10.07 ± 0.44 bc | 4.85 ± 0.13 a |
| Plant Part | Stage of Plant | L-dopa (mg∙g−1 DW) | Vicine (mg∙g−1 DW) | Convicine (mg∙g−1 DW) |
|---|---|---|---|---|
| Stem | Seedling | 71.48 ± 1.04 a | 9.59 ± 0.28 a | 7.92 ± 0.22 a |
| Vegetative | 17.67 ± 0.06 b | 1.41 ± 0.08 b | 0.75 ± 0.05 b | |
| Flowering | 6.81 ± 0.24 d | N.D. | N.D. | |
| Ripening | 12.92 ± 0.23 c | N.D. | N.D. | |
| Root | Seedling | 55.15 ± 0.66 a | 3.81 ± 0.03 a | 8.90 ± 0.17 a |
| Vegetative | 28.82 ± 0.24 b | 2.67 ± 0.01 b | 5.81 ± 0.15 b | |
| Flowering | 9.61 ± 0.42 c | 0.67 ± 0.02 d | 2.01 ± 0.02 d | |
| Ripening | 8.73 ± 0.69 c | 1.37 ± 0.06 c | 3.18 ± 0.69 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Kwon, S.J.; Lim, Y.J.; Gil, C.S.; Jin, C.; Eom, S.H. L-3,4-dihydroxyphenylalanine Accumulation in Faba Bean (Vicia faba L.) Tissues during Different Growth Stages. Agronomy 2021, 11, 502. https://doi.org/10.3390/agronomy11030502
Duan S, Kwon SJ, Lim YJ, Gil CS, Jin C, Eom SH. L-3,4-dihydroxyphenylalanine Accumulation in Faba Bean (Vicia faba L.) Tissues during Different Growth Stages. Agronomy. 2021; 11(3):502. https://doi.org/10.3390/agronomy11030502
Chicago/Turabian StyleDuan, Shucheng, Soon Jae Kwon, You Jin Lim, Chan Saem Gil, Chengwu Jin, and Seok Hyun Eom. 2021. "L-3,4-dihydroxyphenylalanine Accumulation in Faba Bean (Vicia faba L.) Tissues during Different Growth Stages" Agronomy 11, no. 3: 502. https://doi.org/10.3390/agronomy11030502
APA StyleDuan, S., Kwon, S. J., Lim, Y. J., Gil, C. S., Jin, C., & Eom, S. H. (2021). L-3,4-dihydroxyphenylalanine Accumulation in Faba Bean (Vicia faba L.) Tissues during Different Growth Stages. Agronomy, 11(3), 502. https://doi.org/10.3390/agronomy11030502






