Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design: Experimental Site Description, Plant Material, and Maintenance
2.2. Eno-Carpological Description
2.3. Wine Production and Characterization
2.4. Statistic Data Processing
3. Results
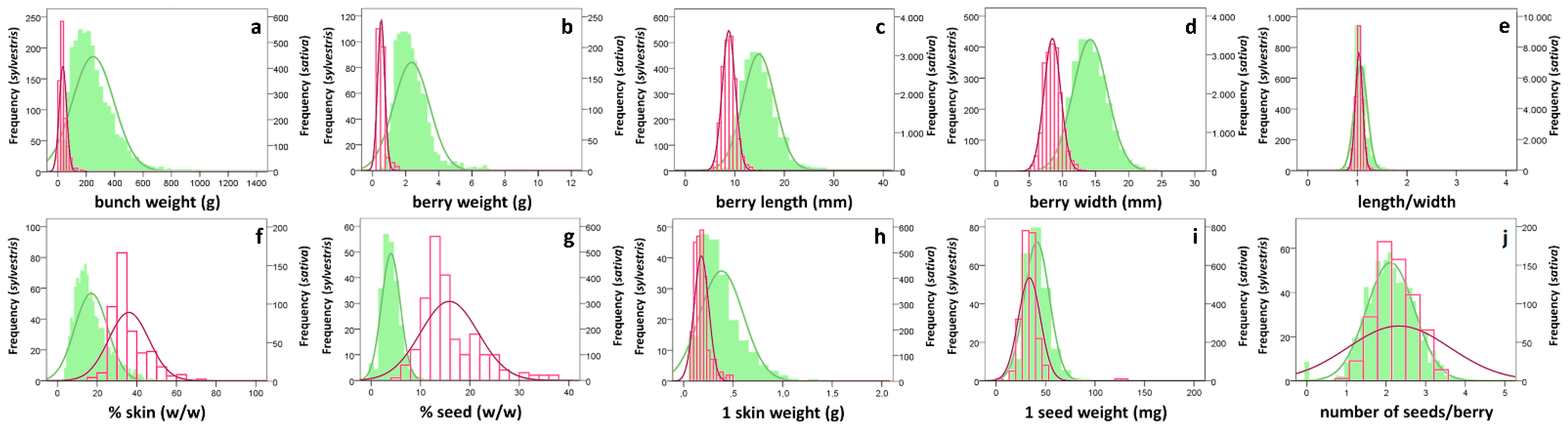
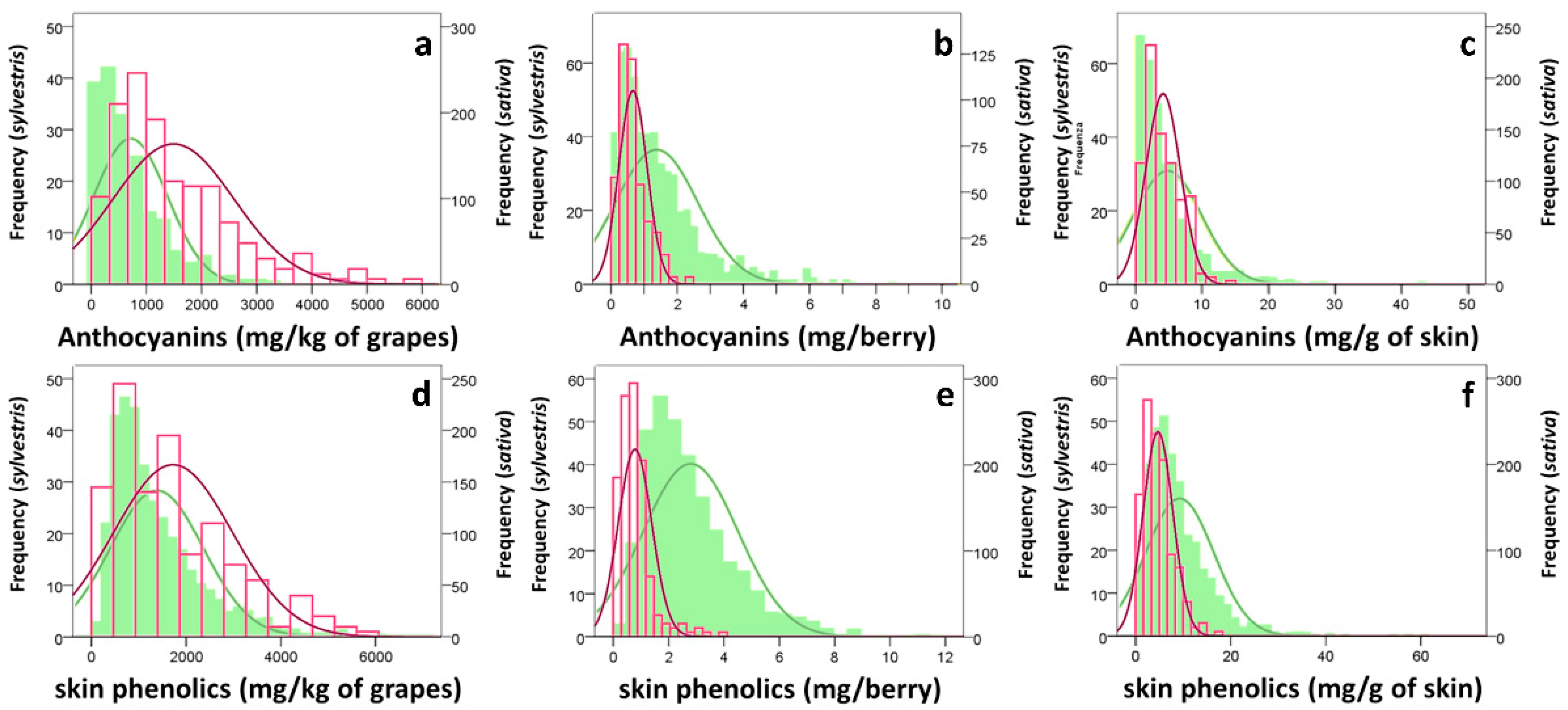
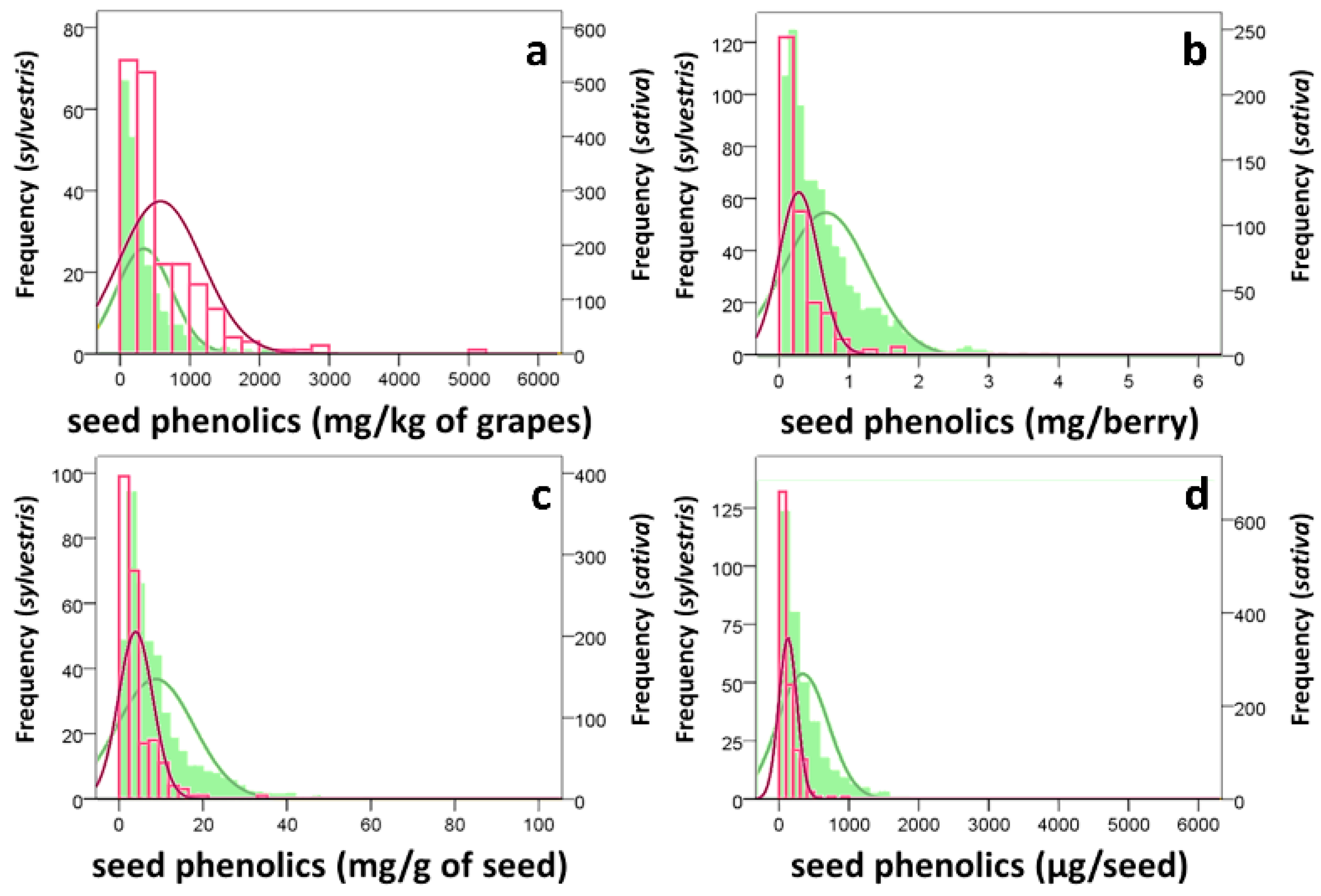
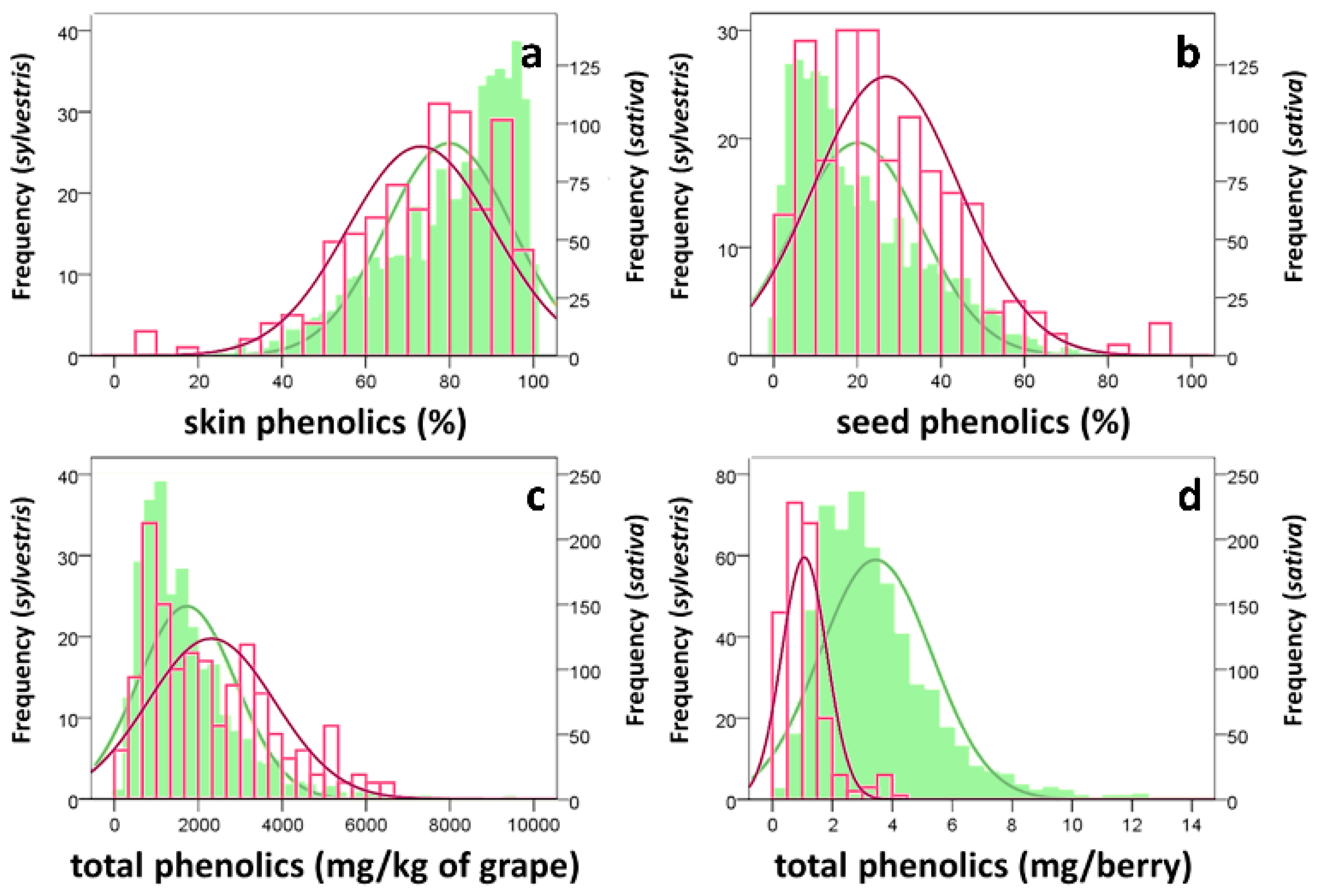
3.1. Grape Characterization
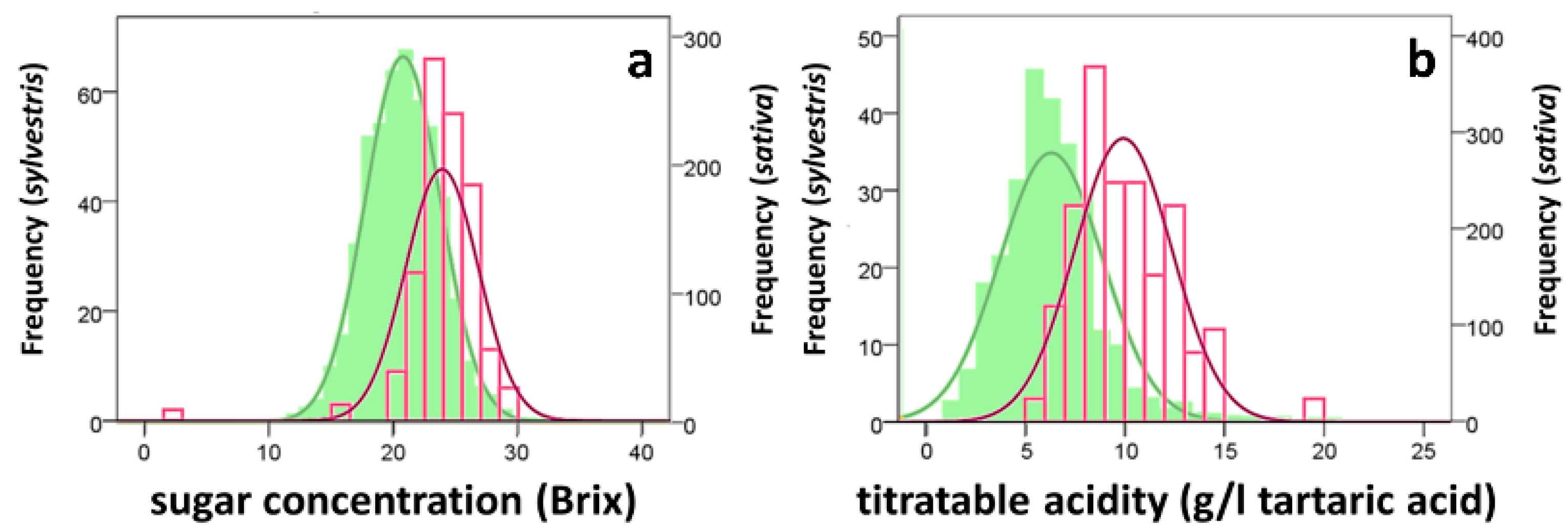
3.2. Composition of Musts and Wines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early neolithic wine of georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef]
- Maghradze, D.; Aslanishvili, A.; Mdinaradze, I.; Tkemaladze, D.; Mekhuzla, L.; Lordkipanidze, D.; Jalabadze, M.; Kvavadze, E.; Rusishvili, N.; McGovern, P.; et al. Progress for research of grape and wine culture in Georgia; the South Caucasus. BIO Web Conf. 2019, 12, 03003. [Google Scholar] [CrossRef]
- Forni, G.P. The Origin of ‘Old World’ Viticulture. In Caucasus and Northern Black Sea Region Ampelography; Maghradze, D., Rustioni, L., Turok, J., Scienza, A., Failla, O., Eds.; Vitis: Quedlinburg, Germany, 2012; pp. 27–38. [Google Scholar]
- Vavilov, N. Centry proiskhozhdenia kulturnikh rastenii (The centers of origin for cultivated plants). Tr. Po Prikl. Bot. Genet. I Sel. Proc. Appl. Bot. Genet. Breed. 1926, 16, 133–137. [Google Scholar]
- Stummer, A. Zur Urgeschichte der Rebe und des Weinbaues. Mitt. Der Anthropol. Ges. Wien 1911, 41, 238–296. [Google Scholar]
- Bouby, L.; Wales, N.; Jalabadze, M.; Rusishvili, N.; Bonhomme, V.; Ramos-Madrigal, J.; Evin, A.; Ivorra, S.; Lacombe, T.; Pagnoux, C.; et al. Tracking the history of grapevine cultivation in Georgia by combining geometric morphometric and ancient DNA. Veget. Hist. Archaeobot. 2020. [Google Scholar] [CrossRef]
- Ucchesu, M.; Orrù, M.; Grillo, O.; Venora, G.; Paglietti, G.; Ardu, A.; Bacchetta, G. Predictive method for correct identification of archaeological charred grape seeds: Support for advances in knowledge of grape domestication process. PLoS ONE 2016, 11, e0149814. [Google Scholar] [CrossRef] [PubMed]
- Valamoti, S.M.; Mangafa, M.; Koukouli-Chrysanthaki, C.; Malamidou, D. Grape-pressing from northern Greece: The earliest wine in the Aegean? Antiquity 2007, 81, 54–61. [Google Scholar] [CrossRef]
- Pagnoux, C.; Bouby, L.; Valamoti, S.M.; Bonhomme, V.; Ivora, S.; Gkatzogia, E.; Karathanou, A.; Kotsachristou, D.; Kroll, H.; Terral, J.F. Local domestication of diffusion? Insights into viticulture in Greece from Neolithic to Archaic times; using geometric morphometric analyses of archaeological grape seeds. J. Archaeol. Sci. 2021, 125, 105263. [Google Scholar] [CrossRef]
- Arroyo-García, R.; Cantos, M.; Lara, M.; López, M.A.; Gallardo, A.; Ocete, C.A.; Pérez, A.; Bánáti, H.; García, J.L.; Ocete, R. Characterization of the largest relic Eurasian wild grapevine reservoir in Southern Iberian Peninsula. Span. J. Agric. Res. 2016, 14, e0708. [Google Scholar] [CrossRef]
- Gamba, J.F. Voyage Dans La Russie Méridionale Et Particulièrement Dans Les Provinces Situées Au-Delà du CAUCASE; Fait Depius 1820 Jusqu’en 1824. C.J.; Trouvé: Paris, France, 1826; Volume 3. [Google Scholar]
- Lovicu, G.; Farci, M.; Bacchetta, G.; Orrú, M.; Pérez, M.A.; Gómez, J.; Ocete, R. Hábitats; estado sanitario y caracterización enológica de la vid silvestre [Vitis vinifera L. subespecie sylvestris (Gmelin) Hegi] en Cerdeña (Insula vini). Enólogos 2009, 62, 30–35. [Google Scholar]
- Guramishvili, D. Davitiani (Book of David), 1st ed.; Khelovneba: Tbilisi, GA, USA; Mistectvo: Kiev, Ukraine, 1980; 85p. [Google Scholar]
- Lara, M.; Iriarte-Chiapusso, M.J.; Cantos, M.; García Jiménez, J.L.; Morales, R.; Ocete, C.A.; López, M.A.; Salinas, J.A.; Rubio, I.; Hidalgo, J.; et al. La vid silvestre. Un importante recurso fitogenético sin protección legal en España. Rev. Iberoam. Vitic. Agroind. Y Rural. 2017, 4, 46–68. [Google Scholar]
- Schumann, F. Berichte über die Verwendung der Wildrebe Vitis vinifera L.var. silvestris Gmelin. Die Weinwissenschaft 1971, 26, 212–218. [Google Scholar]
- Anzani, R.; Failla, O.; Scienza, A.; De Micheli, L. Individuazione e conservazione del germoplasma di vite selvatica (Vitis vinifera sylvestris) in Italia. Vignevini 1993, 6, 51–61. [Google Scholar]
- Ocete, C.A.; Ocete, R.F.; Ocete, R.; Lara, M.; Renobales, G.; Valle, J.M.; Rodríguez-Miranda, A.; Morales, R. Traditional medicinal uses of the Eurasian wild grapevine in the Iberian Peninsula. An. Jard. Bot. Madr. 2020, 77, e102. [Google Scholar] [CrossRef]
- Zdunić, G.; Maul, E.; Dias, J.E.; Organero, G.M.; Carka, F.; Maletić, E.; Savvide, S.; Jahnke, G.G.; Nagy, Z.A.; Nikolić, D.; et al. Guiding principles for identification, evaluation and conservation of Vitis vinifera L. subsp. Sylvestris. Vitis 2017, 56, 127–131. [Google Scholar]
- Meléndez, E.; Puras, P.; Garcí, J.L.; Cantos, M.; Gómez-Rodrígues, J.A.; Íňiguez, M.; Rodrígues, A.; Valle, J.M.; Arnold, C.; Ocete, C.A.; et al. Evolution of wild and feral vines from the Ega River gallery forest (Basque Country and Navarra; Spain) from 1995 to 2015. J. Int. Sci. Vigne Vin 2016, 50, 65–75. [Google Scholar] [CrossRef]
- Ocete, C.A.; Zapater, J.M.; Ocete, R.; Lara, M.; Cantos, M.; Arroyo, R.; Morales, M.; Iriarte-Chiapusso, J.; Hidalgo, J.; Valle, J.M.; et al. La vid silvestre euroasiática; un recurso fitogenético amenazado ligado a la historia de la humanidad. Enoviticultura 2018, 50, 1–16. [Google Scholar]
- Maghradze, D.; Malyan, G.; Salimov, V.; Chipashvili, R.; Íñiguez, M.; Puras, P.; Melendez, E.; Vaca, R.; Ocete, C.A.; Rivera, D.; et al. Wild grapevine (Vitis sylvestris C.C.Gmel.) wines from the Southern Caucasus region. OENO ONE 2020, 54, 849–862. [Google Scholar] [CrossRef]
- Ocete, C.A.; Ocete, R.; Ayala, M.C.; del Rio, J.M.; Lara, M.; Hidalgo, J.; Valle, J.M.; Rordígues-Miranda, Á. Microvinification in wild grapevine relict populations of Spain and France. Munibe Cienc. Nat. 2020, 68, 59–75. [Google Scholar] [CrossRef]
- Derosas, P.; Graviano, O.; Farci, M.; Delpiano, D.; Piras, F.; Damasco, G.; Lovicu, G. Risultati preliminari sulla vinificazione di alcune accessioni di uva selvatica (Vitis vinifera L. ssp. sylvestris) in Sardegna. In Proceedings of the CONAVI, San Michele all’Adige, Italy, 5–9 July 2010. [Google Scholar]
- De Lorenzis, G.; Chipashvili, R.; Failla, O.; Maghradze, D. Study of genetic variability in Vitis vinifera germplasm by high-throughput Vitis18kSNP array: The case of Georgian genetic resources. BMC Plant Biol. 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Cola, G.; Failla, O.; Maghradze, D.; Megrelidze, L.; Mariani, L. Grapevine phenology and climate change in Georgia. Int. J. Biometeorol. 2017, 61, 761–773. [Google Scholar] [CrossRef]
- Rustioni, L.; Cola, G.; Maghradze, D.; Abashidze, E.; Argiriou, A.; Aroutiounian, R.; Brazão, J.; Chipashvili, R.; Cocco, M.; Cornea, V.; et al. Description of the Vitis vinifera L. phenotypic variability in eno-carpological traits by a Euro-Asiatic collaborative network among ampelographic collections. Vitis 2019, 58, 37–46. [Google Scholar] [CrossRef]
- Rustioni, L.; Maghradze, D.; Popescu, C.F.; Cola, G.; Abashidze, E.; Aroutiounian, R.; Brazão, J.; Coletti, S.; Cornea, V.; Dejeu, L.; et al. First results of the European grapevine collections’ collaborative network: Validation of a standard eno-carpological phenotyping method. Vitis 2014, 53, 219–226. [Google Scholar]
- Abashidze, E.; Mdinaradze, I.; Chipashvili, R.; Vashakidze, L.; Maghradze, D.; Rustioni, L.; Failla, O. Evaluation of eno-carpological traits in Georgian grapevine varieties from Skra germplasm repository. Vitis 2015, 54, 151–154. [Google Scholar]
- Kikilashvili, S. Georgian Viticulture and Wine Making. Study of Genotypes of Wild Grapevines Vitis vinifera ssp. sylvestris Gmel. The Jighaura Experimental Station. Master’s Thesis, The Caucasus International University, Tbilisi, GA, USA, 22 July 2018. [Google Scholar]
- Fracassetti, D.; Gabrielli, M.; Tirelli, A. Characterisation of Vernaccia Nera (Vitis vinifera L.) grapes and wine. S. Afr. J. Enol. Vitic. 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Rustioni, L.; Fracassetti, D.; Prinsi, B.; Geuna, F.; Ancelotti, A.; Fauda, V.; Tirelli, A.; Espen, L.; Failla, O. Oxidations in white grape (Vitis vinifera L.) skins: Comparison between ripening process and photooxidative sunburn symptoms. Plant Physiol. Biochem. 2020, 150, 270–278. [Google Scholar] [CrossRef]
- Navarre, C.; Langlade, F. L’Oenologie Translated from French into Georgian by Samanishvili G.; Lavoisier: Paris, France, 2004; pp. 149–160. [Google Scholar]
- Sargolzaei, M.; Rustioni, L.; Cola, G.; Ricciardi, V.; Bianco, P.A.; Maghradze, D.; Failla, O.; Quaglino, F.; Toffolatti, S.L.; De Lorenzis, G. Georgian grapevine cultivars: An ancient source of biodiversity for the future viticulture. Front. Plant Sci. 2021, 12, 630122. [Google Scholar] [CrossRef]
- Ocete, R.; Muñoz, G.; Lopez, M.A.; Pérez, M.A.; Benito, A.; Cabello, F.; Valle, J.M. Environmental, sanitary and ampelographic characterization of wild grapevine in Western Pyrenées (Spain, France). J. Int. Sci. Vigne Vin 2011, 45, 1–12. [Google Scholar] [CrossRef]
- Gepts, P. Crop Domestication as a Long-Term Selection Experiment. In Plant Breeding Reviews: Part 2: Long-Term Selection: Crops, Animals, and Bacteria; Janick, J., Ed.; John Wiley & Sons; Inc.: Hoboken, NJ, USA, 2004; Volume 24, Chapter 1. [Google Scholar] [CrossRef]
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242. [Google Scholar] [CrossRef]
- Pratt, C. Reproductive anatomy in cultivated grapes—A review. Am. J. Enol. Vitic. 1971, 22, 92–109. [Google Scholar]
- Fuller, D.Q. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann Bot. 2007, 100, 903–924. [Google Scholar] [CrossRef]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Bokhochadze, A. Viticulture and Wine Making in Old Georgia Based on Archaeological Materials; Publication of the Academy of the Sciences of Georgia: Tbilisi, Georgia, 1963; Volume 207. [Google Scholar]
- Chilashvili, L. The Vine, Wine and the Georgians; Petite: Tbilisi, GA, USA, 2004. [Google Scholar]
- Batiuk, S.D. The fruits of migration: Understanding the ‘longue durée’ and the socio-economic relations of the early Transcaucasian culture. J. Anthropol. Archaeol. 2013, 32, 449–477. [Google Scholar] [CrossRef]
- Suklje, K.; Antalick, G.; Meeks, C.; Blackman, J.W.; Deloire, A.; Schmidtke, L.M. Grapes to wine: The nexus between berry ripening, composition and wine style. Int. Soc. Hortic. Sci. 2017, 43–50. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Antalick, G.; Šuklje, K.; Blackman, J.W.; Boccard, J.; Deloire, A. Cultivar, site or harvest date: The gordian knot of wine terroir. Metabolomics 2020, 16–52. [Google Scholar] [CrossRef]
- Ageorges, A.; Fernandez, L.; Vialet, S.; Merdinoglu, D.; Terrier, N.; Romieu, C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006, 170, 372–383. [Google Scholar] [CrossRef]
- Walker, A.R.; Lee, E.; Bogs, J.; McDavid, D.A.J.; Thomas, M.K.; Robinson, S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007, 49, 772e785. [Google Scholar] [CrossRef] [PubMed]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzis, G.; Rustioni, L.; Pozzi, C.; Failla, O. Disfunctions in the anthocyanin accumulation of Vitis vinifera L. varieties studied by a targeted resequencing approach. J. Berry Res. 2020, 10, 345–363. [Google Scholar] [CrossRef]
- Rustioni, L.; De Lorenzis, G.; Hârţa, M.; Failla, O. Pink berry grape (Vitis vinifera L.) characterization: Reflectance spectroscopy; HPLC. and molecular markers. Plant Physiol. Biochem. 2016, 98, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Rustioni, L.; Rossoni, M.; Failla, O.; Scienza, A. Anthocyanin esterification in Sangiovese grapes. Ital. J. Food Sci. 2013, 25, 133–141. [Google Scholar]
- Rustioni, L.; Rossoni, M.; Calatroni, M.; Failla, O. Influence of bunch exposure on anthocyanins extractability from grapes skins (Vitis vinifera L.). Vitis 2011, 50, 137–143. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Rustioni, L.; Parisi, S.G.; Zoli, F.; Brancadoro, L. Anthocyanin biosynthesis during berry development in corvina grape. Sci. Hortic. 2016, 212, 74–80. [Google Scholar] [CrossRef]
- Rustioni, L.; Rossoni, M.; Cola, G.; Mariani, L.; Failla, O. Bunch exposure to direct solar radiation increases ortho-diphenol anthocyanins in northern Italy climatic condition. J. Int. Sci. Vigne Vin. 2011, 45, 85–99. [Google Scholar] [CrossRef]
- Grassi, F.; Arroyo-Garcia, R. Editorial: Origins and domestication of the grape. Front. Plant Sci. 2020, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
| Must | Wild Grape | Cabernet Sauvignon | Saperavi | LS # |
|---|---|---|---|---|
| Sugar concentration (°Brix) | 25.1 ± 0.9 a | 24.1 ± 1.4 a | 22.1 ± 1.2 a | ns |
| Total acidity (g/L of tartaric acid) | 7.8 ± 0.8 a | 6.7 ± 0.7 a | 9.2 ± 1.3 a | ns |
| pH | 3.4 ± 0.2 a | 3.4 ± 0.2 a | 3.2 ± 0.2 a | ns |
| Wine | Wild Grape | Cabernet Sauvignon | Saperavi | LS # |
|---|---|---|---|---|
| Residual sugars (g/L) | 3.0 ± 0.9 a | 5.0 ± 4.5 a | 1.9 ± 0.2 a | ns |
| Total acidity (g/L of tartaric acid) | 7.1 ± 0.5 a | 6.2 ± 0.2 b | 7.2 ± 0.5 a | ** |
| Volatile acidity (g/L of acetic acid) | 0.5 ± 0.2 a | 0.6 ± 0.1 a | 0.6 ± 0.0 a | ns |
| pH | 3.6 ± 0.0 a | 3.3 ± 0.4 a | 3.3 ± 0.2 a | ns |
| Ethanol (%, v/v) | 14.2 ± 0.8 a | 13.8 ± 1.0 a | 13.7 ± 0.9 a | ns |
| Total phenol content (g/L of catechin) | 3.1 ± 1.1 a | 1.7 ± 0.4 b | 1.7 ± 0.4 b | * |
| Total dry extract (g/L) | 33.6 ± 3.7 a | 31.4 ± 5.3 a | 25.2 ± 1.9 b | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maghradze, D.; Kikilashvili, S.; Gotsiridze, O.; Maghradze, T.; Fracassetti, D.; Failla, O.; Rustioni, L. Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa. Agronomy 2021, 11, 472. https://doi.org/10.3390/agronomy11030472
Maghradze D, Kikilashvili S, Gotsiridze O, Maghradze T, Fracassetti D, Failla O, Rustioni L. Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa. Agronomy. 2021; 11(3):472. https://doi.org/10.3390/agronomy11030472
Chicago/Turabian StyleMaghradze, David, Shengeli Kikilashvili, Olan Gotsiridze, Tamar Maghradze, Daniela Fracassetti, Osvaldo Failla, and Laura Rustioni. 2021. "Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa" Agronomy 11, no. 3: 472. https://doi.org/10.3390/agronomy11030472
APA StyleMaghradze, D., Kikilashvili, S., Gotsiridze, O., Maghradze, T., Fracassetti, D., Failla, O., & Rustioni, L. (2021). Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa. Agronomy, 11(3), 472. https://doi.org/10.3390/agronomy11030472







