(Z)-3-Hexenyl Butyrate Induces Stomata Closure and Ripening in Vitis vinifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description, Plant Material and Maintenance
2.2. Experimental Design and Treatment Application
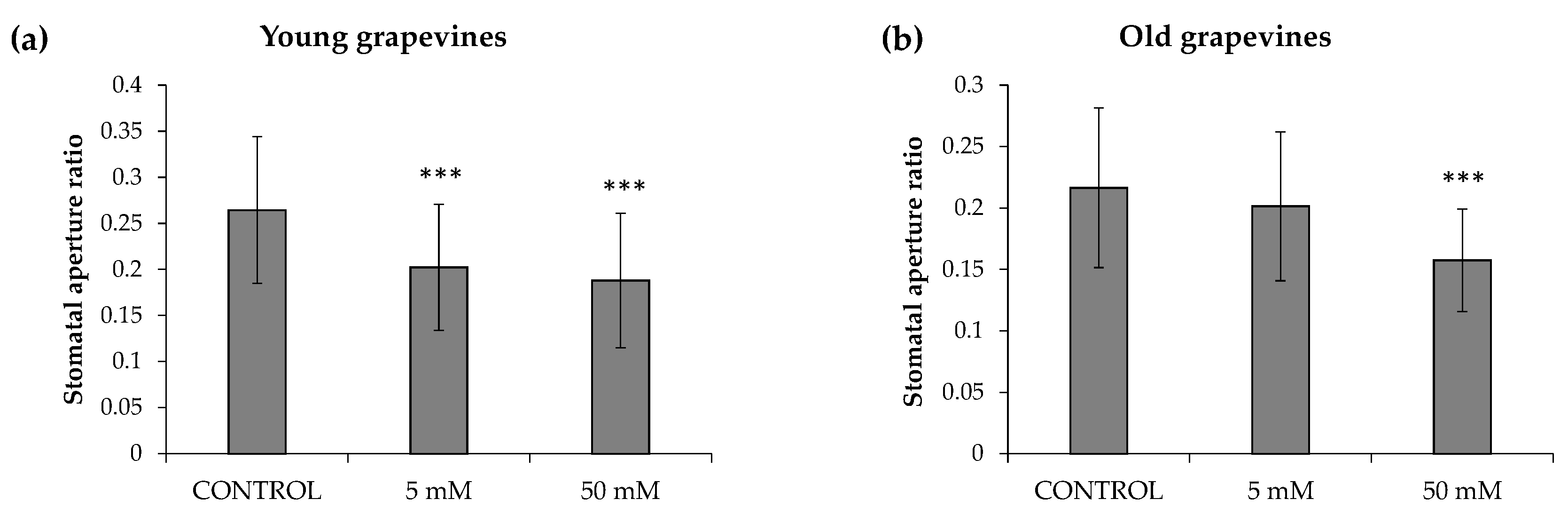
2.3. Stomatal Aperture Measurement
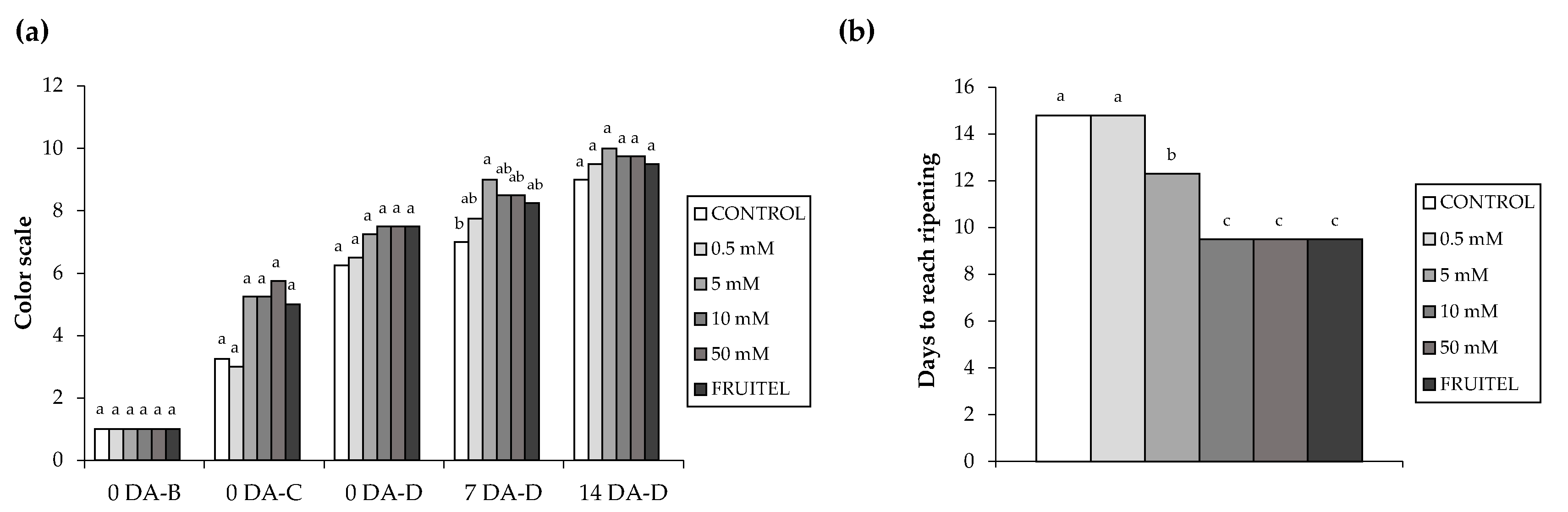
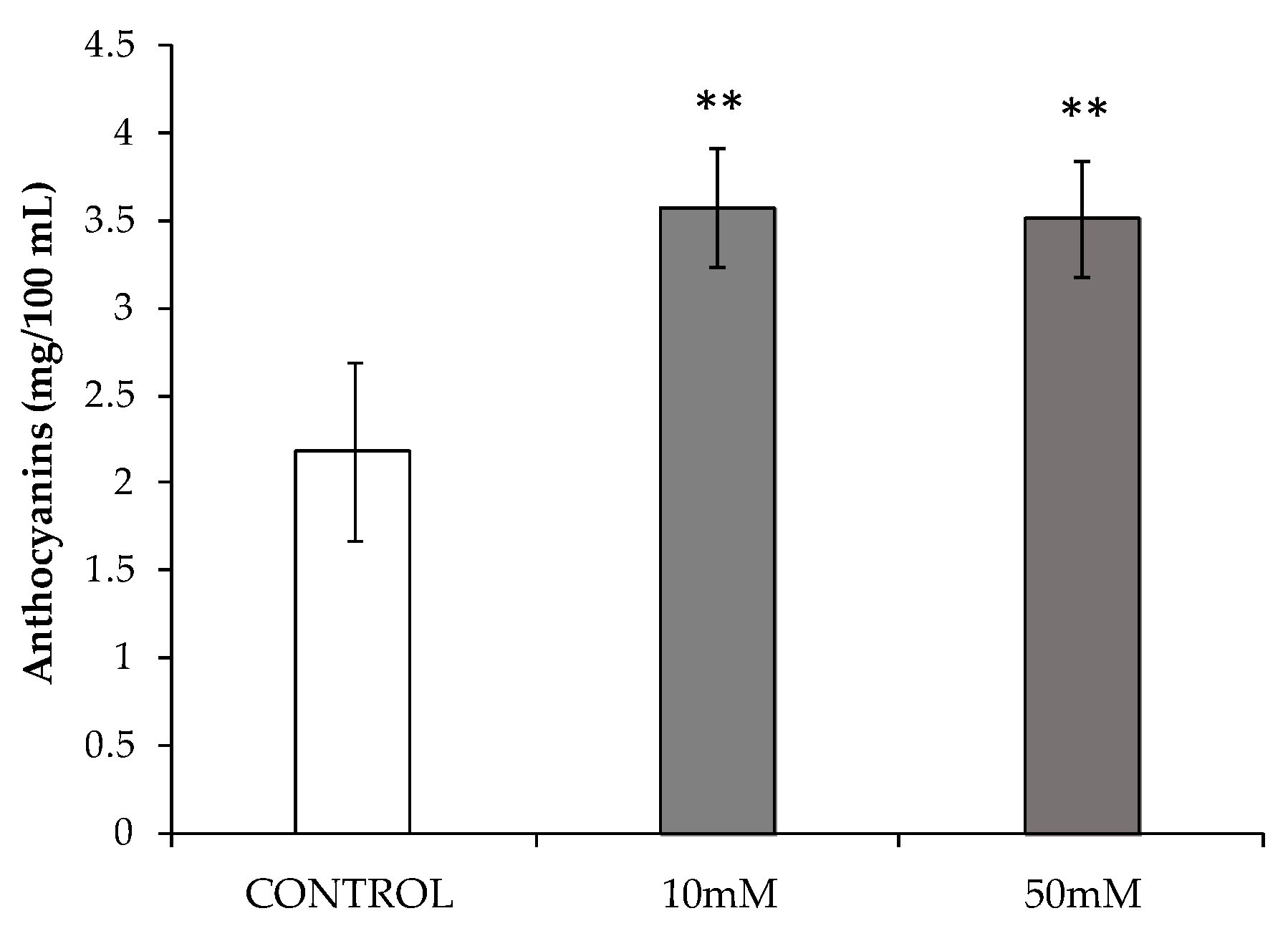
2.4. Color and Total Anthocyanin Content Assessment
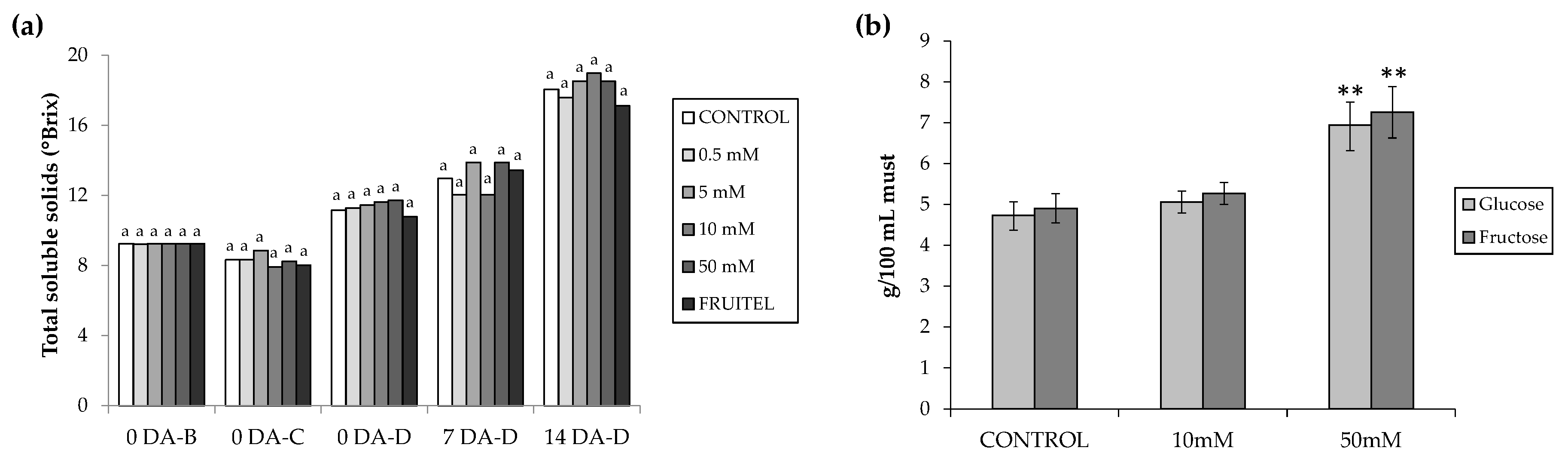
2.5. Sugar Content Determination
2.6. Grape Juice Acidity Analysis
2.7. (+)-Catechin and (−)-Epicatechin Analysis
2.8. Statistical Analysis
3. Results
3.1. Evalutation of HB Efficacy in Grapevine Plants under Open-Field Conditions
3.2. Study of the Effect of HB in Ripening and Color Assessment
3.3. Study of Phenolic Compounds Accumulation Induced by HB Treatments
3.4. Sugar Accumulation Determination
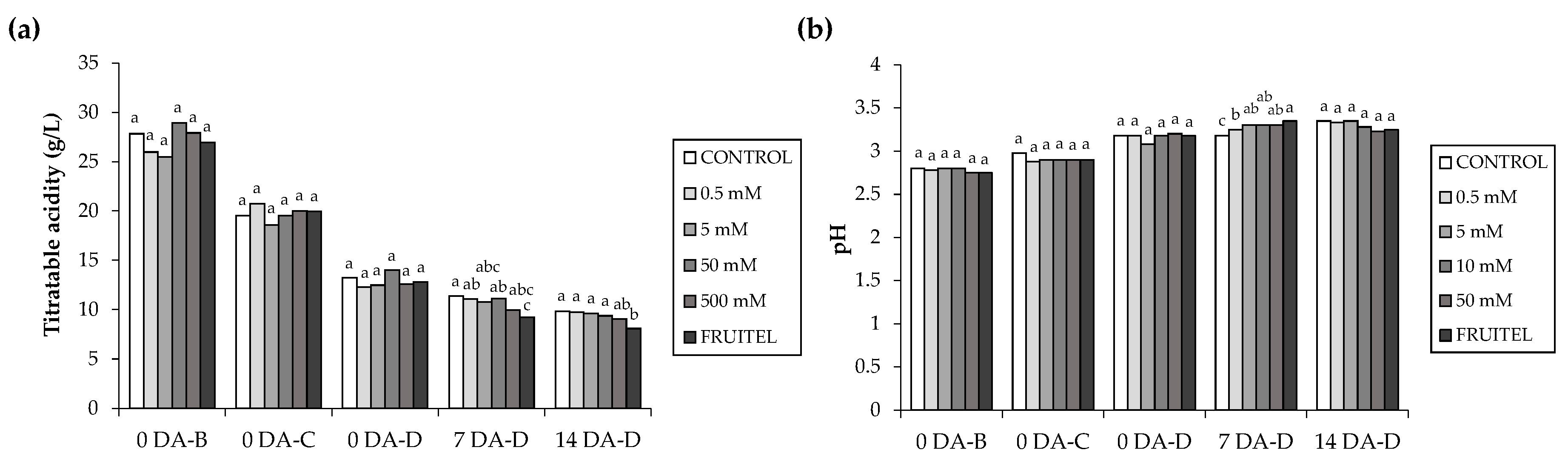
3.5. Acidity Evaluation
3.6. Effect of HB Treatments in Crop Level and Yield
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry development and fruit and wine quality. Food 2007, 1, 1–22. Available online: http://hdl.handle.net/1822/6820 (accessed on 14 March 2020).
- Zoccatelli, G.; Zenoni, S.; Savoi, S.; Dal Santo, S.; Tononi, P.; Zandonà, V.; Dal Cin, A.; Guantieri, V.; Pezzotti, M.; Tornielli, G. Skin pectin metabolism during the postharvest dehydration of berries from three distinct grapevine cultivars. Aust. J. Grape Wine Res. 2013, 19, 171–179. [Google Scholar] [CrossRef]
- Lund, S.T.; Bohlmann, J. The Molecular Basis for Wine Grape Quality-A Volatile Subject. Science 2006, 311, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; de lrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- Gerós, H.; Chaves, M.M.; Medrano, H.; Delrot, S. Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; John Wiley & Sons, Ltd.: West Sussex, UK, 2016. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Schultz, H.R. Climate Change and Viticulture: Research Needs for Facing the Future. J. Wine Res. 2010, 21, 113–116. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Mechlin, T.; Dami, I. Foliar Application of Abscisic Acid Induces Dormancy Responses in Greenhouse-grown Grapevines. HortSci. Horts 2011, 46, 1271–1277. [Google Scholar] [CrossRef]
- Jung, C.J.; Hur, Y.Y.; Yu, H.-J.; Noh, J.-H.; Park, K.-S.; Lee, H.J. Gibberellin application at pre-bloom in grapevines down-regulates the expressions of VvIAA9 and VvARF7, negative regulators of fruit set initiation, during parthenocarpic fruit development. PLoS ONE 2014, 9, e95634. [Google Scholar] [CrossRef] [PubMed]
- Deytieux-Belleau, C.; Gagné, S.; L’Hyvernay, A.; Donèche, B.; Geny, L. Possible roles of both abscisic acid and indol-acetic acid in controlling grape berry ripening process. OENO One 2007, 41, 141–148. [Google Scholar] [CrossRef]
- El-kenawy, M. Effect of chitosan, salicylic acid and fulvic acid on vegetative growth, yield and fruit quality of Thompson seedless grapevines. Egypt. J. Hortic. 2017, 44, 45–59. [Google Scholar] [CrossRef]
- Becatti, E.; Genova, G.; Ranieri, A.; Tonutti, P. Postharvest treatments with ethylene on Vitis vinifera (cv Sangiovese) grapes affect berry metabolism and wine composition. Food Chem. 2014, 159, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Yu, Y.; Shi, T.C.; Fu, Y.S.; Zhao, T.; Zhang, Z.W. Melatonin treatment of pre-veraison grape berries modifies phenolic components and antioxidant activity of grapes and wine. Food Sci. Technol. 2019, 39, 35–42. [Google Scholar] [CrossRef]
- Abdel-Salam, M.M. Effect of foliar application with humic acid and two antioxidants on ruby seedless grapevine. Middle East J. Agric. Res. 2016, 5, 123–131. [Google Scholar]
- Mirdehghan, S.H.; Rahimi, S. Pre-harvest application of polyamines enhances antioxidants and table grape (Vitis vinifera L.) quality during postharvest period. Food Chem. 2016, 196, 1040–1047. [Google Scholar] [CrossRef]
- Mencarelli, F.; Bellincontro, A. Recent advances in postharvest technology of the wine grape to improve the wine aroma. J. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Bellincontro, A.; Fardelli, A.; De Santis, D.; Botondi, R.; Mencarelli, F. Postharvest ethylene and 1-MCP treatments both affect phenols, anthocyanins, and aromatic quality of Aleatico grapes and wine. Aust. J. Grape Wine Res. 2006, 12, 41–149. [Google Scholar] [CrossRef]
- Botondi, R.; Lodola, L.; Mencarelli, F. Postharvest ethylene treatment affects berry dehydration, polyphenol and anthocyanin content by increasing the activity of cell wall enzymes in Aleatico wine grape. Eur. Food Res. Technol. 2011, 232, 679–685. [Google Scholar] [CrossRef]
- Williams, L.E.; Matthews, M.A. Grapevine. In Irrigation of Agricultural Crops. Agronomy Monograph No. 30; Stewart, B.A., Nielsen, D.R., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 1990; pp. 1019–1055. [Google Scholar]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.J.; Kim, S.J.; Lee, H.J. Stomatal and non-stomatal limitations to photosynthesis in field-grown grapevine cultivars. Biol. Plant. 2009, 53, 133–137. [Google Scholar] [CrossRef]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Lisón, P.; Campos, L.; Rodrigo, I.; Rambla, J.L.; Granell, A.; Conejero, V.; Bellés, J.M. A non-targeted metabolomics approach unravels the VOCs associated with the tomato immune response against Pseudomonas syringae. Front. Plant Sci. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Payá, C.; Ozáez, M.; Rodrigo, I.; Conejero, V.; Klee, H.; Bellés, J.M.; Lisón, P. A new role for green leaf volatile esters in tomato stomatal defense against pseudomonas syringe pv. Tomato. Front. Plant Sci. 2018, 871, 1–12. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- García-Hurtado, N.; Carrera, E.; Ruiz-Rivero, O.; López-Gresa, M.P.; Hedden, P.; Gong, F.; García-Martínez, J.L. The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J. Exp. Bot. 2012, 63, 5803–5813. [Google Scholar] [CrossRef]
- Coombe, B.G. Research on development and ripening of the grape berry. Am. J. Enol. Vitic. 1992, 43, 101–110. [Google Scholar]
- Fernández-López, J.A.; Almela, L.; Muñoz, J.A.; Hidalgo, V.; Carreño, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res. Int. 1998, 31, 667–672. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Troup, G.J.; Pilbrow, J.R.; Hutton, D.R.; Hewitt, D.; Hunter, C.R.; Ristic, R.; Iland, P.G.; Jones, G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000, 6, 244–254. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Jordan, W.R.; Brown, K.W.; Thomas, J.C. Leaf Age as a Determinant in Stomatal Control of Water Loss from Cotton during Water Stress. Plant Physiol. 1975, 56, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, D.; Barbour, M.M.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T. Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiol. 2011, 31, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Gamm, M.; Héloir, M.C.; Adrian, M. Trehalose and trehalose-6-phosphate induce stomatal movements and interfere with aba-induced stomatal closure in grapevine. J. Int. Sci. Vigne Vin 2015, 49, 165–171. [Google Scholar] [CrossRef]
- Di Vaio, C.; Marallo, N.; di Lorenzo, R.; Pisciotta, A. Anti-Transpirant effects on vine physiology, berry and wine composition of cv. Aglianico (Vitis vinifera L.) grown in South Italy. Agronomy 2019, 9, 244. [Google Scholar] [CrossRef]
- Di Vaio, C.; Villano, C.; Lisanti, M.T.; Marallo, N.; Cirillo, A.; Di Lorenzo, R.; Pisciotta, A. Application of Anti-Transpirant to Control Sugar Accumulation in Grape Berries and Alcohol Degree in Wines Obtained from Thinned and Unthinned Vines of cv. Falanghina (Vitis vinifera L.). Agronomy 2020, 10, 345. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.-M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evaluation of bitterness and astringency of (+)-catechin and (-)-epicatechin in red wine and in model solution. J. Sens. Stud. 1997, 12, 25–37. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing Kaolin and Pinolene to Improve Sustainable Grapevine Production during Drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Palliotti, A.; Panara, F.; Famiani, F.; Sabbatini, P.; Howell, G.S.; Silvestroni, O.; Poni, S. Postveraison Application of Antitranspirant Di-1-p-Menthene to Control Sugar Accumulation in Sangiovese Grapevines. Am. J. Enol. Vitic. 2013, 64, 378–385. [Google Scholar] [CrossRef]
- Fahey, D.J.; Rogiers, S.Y. Di-1-p-menthene reduces grape leaf and bunch transpiration. Aust. J. Grape Wine Res. 2019, 25, 134–141. [Google Scholar] [CrossRef]
- Martín, P.; Delgado, R.; González, M.R.; Gallegos, J.I. Colour of “Tempranillo” grapes affected by different nitrogen and potassium fertilization rates. Acta Hortic. 2004, 652, 153–160. [Google Scholar] [CrossRef]
- Fortes, A.M.; Teixeira, R.T.; Agudelo-Romero, P. Complex interplay of hormonal signals during grape berry ripening. Molecules 2015, 20, 9326–9343. [Google Scholar] [CrossRef]
- Griesser, M.; Savoi, S.; Supapvanich, S.; Dobrev, P.; Vankova, R.; Forneck, A. Phytohormone profiles are strongly altered during induction and symptom development of the physiological ripening disorder berry shrivel in grapevine. Plant Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Gomez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during the ripening period. J. Sci. Food Agric. 2017, 97, 977–983. [Google Scholar] [CrossRef]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative Insight upon Chitosan Solution and Chitosan Nanoparticles Application on the Phenolic Content, Antioxidant and Antimicrobial Activities of Individual Grape Components of Sousão Variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef]
- El-Kereamy, A.; Chervin, C.; Roustan, J.-P.; Cheynier, V.; Souquet, J.-M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J.-C.; et al. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiol. Plant. 2003, 119, 175–182. [Google Scholar] [CrossRef]
- Allègre, M.; Héloir, M.C.; Trouvelot, S.; Daire, X.; Pugin, A.; Wendehenne, D.; Adrian, M. Are grapevine stomata involved in the elicitor-induced protection against downy mildew? Mol. Plant-Microbe Interact. 2009, 22, 977–986. [Google Scholar] [CrossRef]
| Treatment | Yield (t/ha) |
|---|---|
| CONTROL | 19.72 a |
| 0.5 mM | 19.41 a |
| 5 mM | 20.53 a |
| 10 mM | 19.90 a |
| 50 mM | 19.91 a |
| FRUITEL | 19.80 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payá, C.; López-Gresa, M.P.; Intrigliolo, D.S.; Rodrigo, I.; Bellés, J.M.; Lisón, P. (Z)-3-Hexenyl Butyrate Induces Stomata Closure and Ripening in Vitis vinifera. Agronomy 2020, 10, 1122. https://doi.org/10.3390/agronomy10081122
Payá C, López-Gresa MP, Intrigliolo DS, Rodrigo I, Bellés JM, Lisón P. (Z)-3-Hexenyl Butyrate Induces Stomata Closure and Ripening in Vitis vinifera. Agronomy. 2020; 10(8):1122. https://doi.org/10.3390/agronomy10081122
Chicago/Turabian StylePayá, Celia, M. Pilar López-Gresa, Diego S. Intrigliolo, Ismael Rodrigo, José María Bellés, and Purificación Lisón. 2020. "(Z)-3-Hexenyl Butyrate Induces Stomata Closure and Ripening in Vitis vinifera" Agronomy 10, no. 8: 1122. https://doi.org/10.3390/agronomy10081122
APA StylePayá, C., López-Gresa, M. P., Intrigliolo, D. S., Rodrigo, I., Bellés, J. M., & Lisón, P. (2020). (Z)-3-Hexenyl Butyrate Induces Stomata Closure and Ripening in Vitis vinifera. Agronomy, 10(8), 1122. https://doi.org/10.3390/agronomy10081122







