Absorption and Elimination of the Allelochemical MBOA by Weeds during Seedling Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Allelochemicals
2.2. Seeds
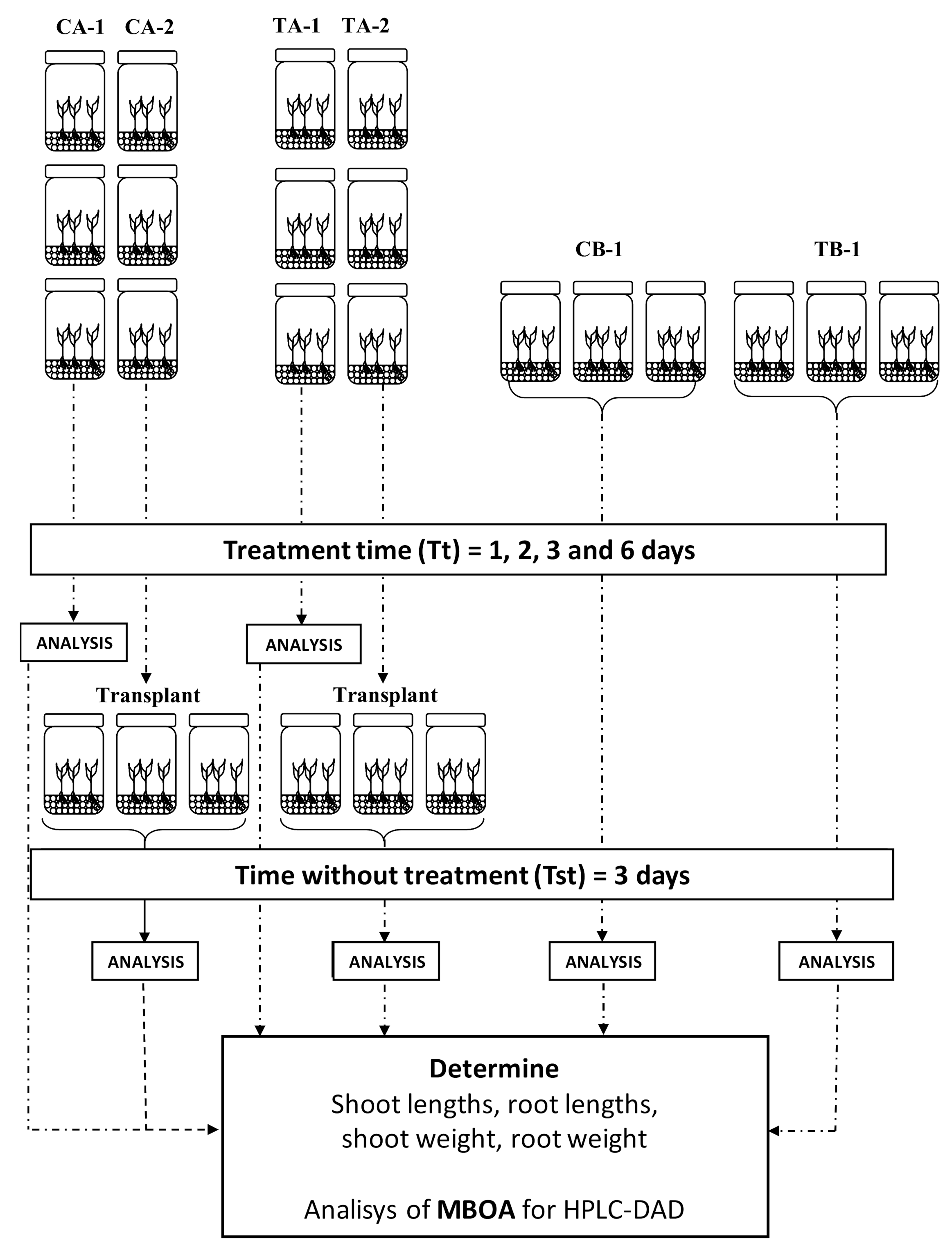
2.3. Bioassay Design
2.3.1. Absorption Studies for Seedlings
2.3.2. Absorption and Elimination of MBOA by Weed Seedlings
2.4. Quantitation by HPLC Analysis
2.5. MBOA in Solutions
2.6. Statistical Analysis
3. Results
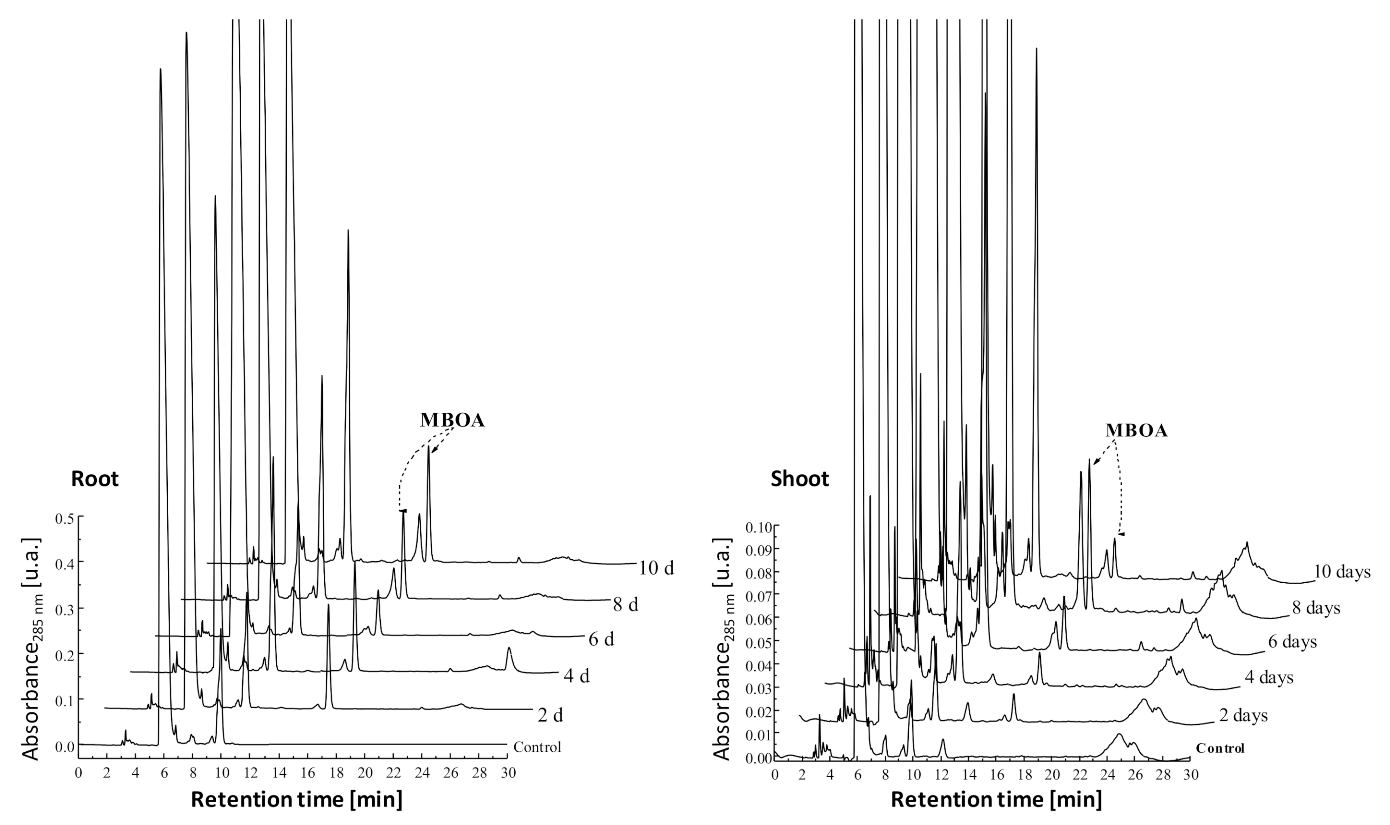
3.1. Occurrence of MBOA in the Control Treatments
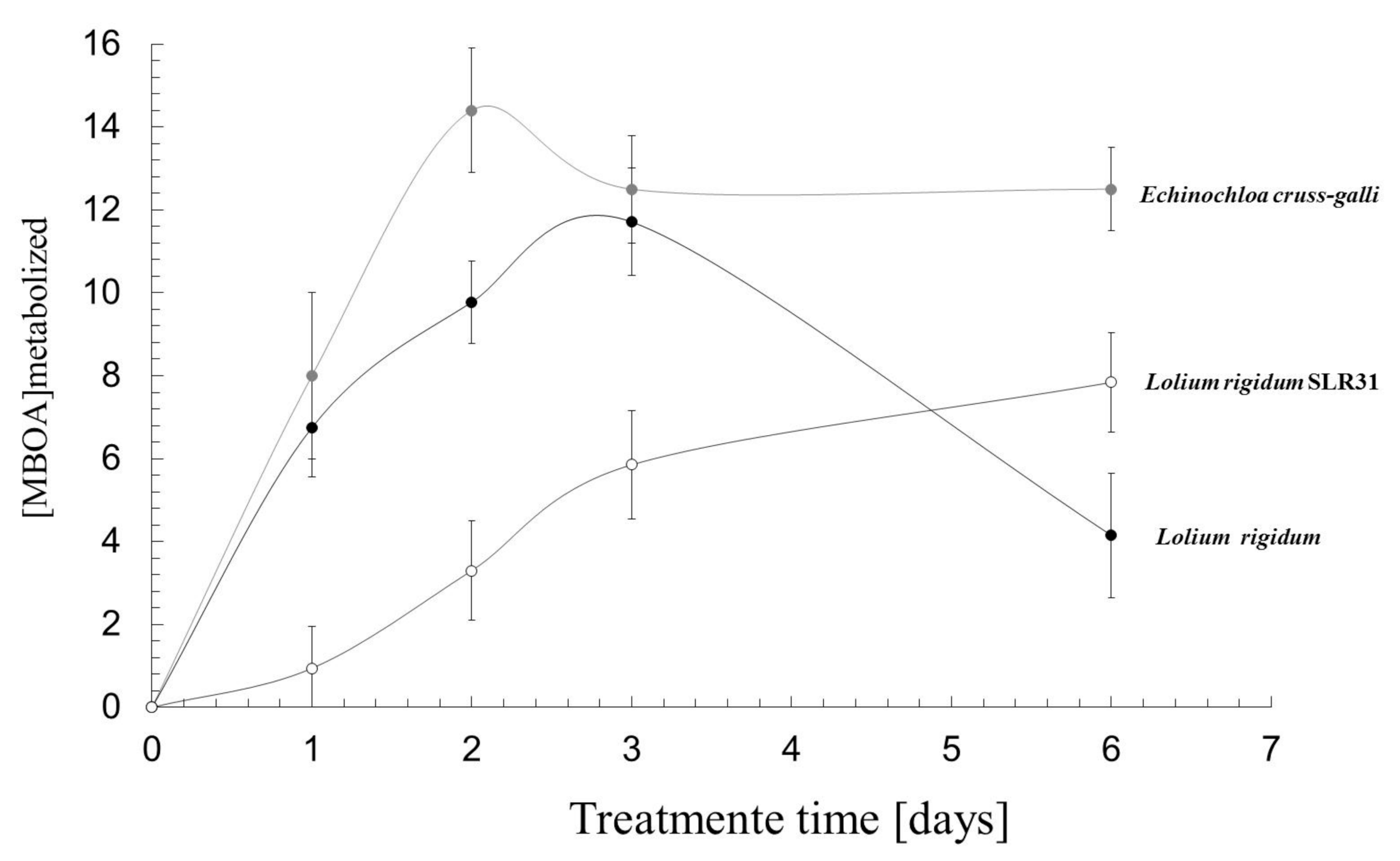
3.2. Absorption of MBOA by Weeds
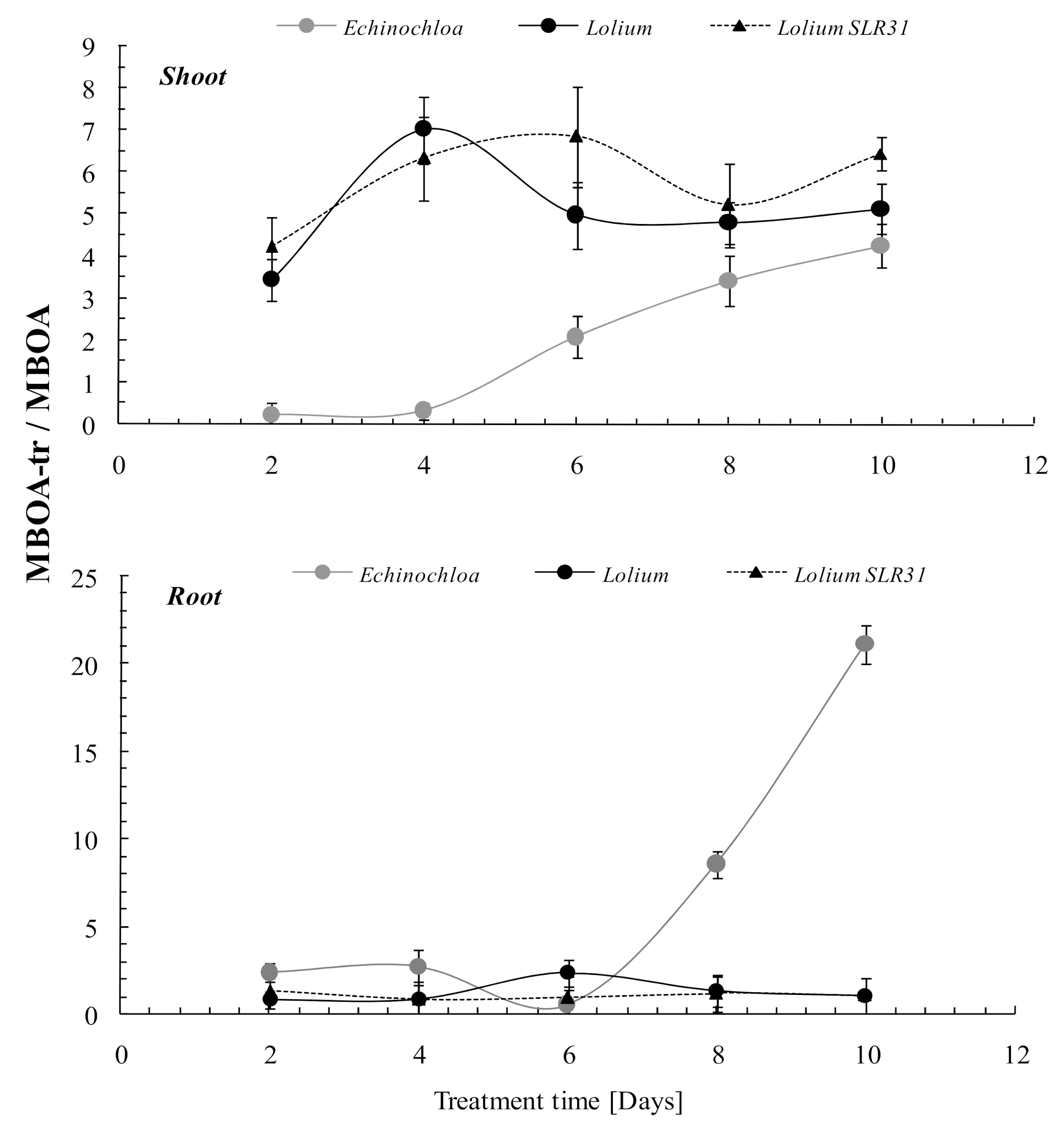
3.3. MBOA Detoxification
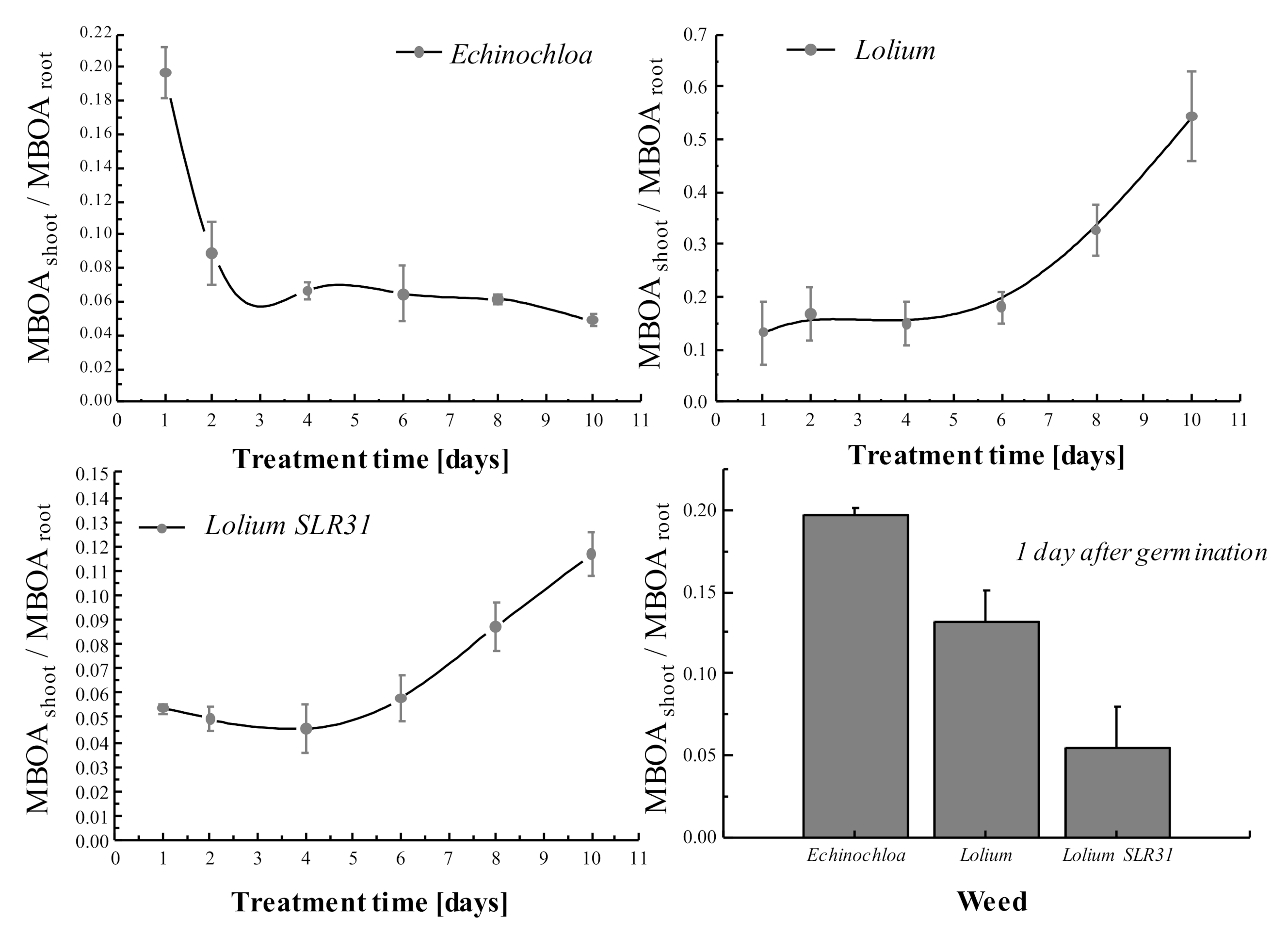
3.4. MBOA Translocation
3.5. Uptake and Elimination of MBOA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bakera, B.; Święcicka, M.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature. Agronomy 2020, 10, 1260. [Google Scholar] [CrossRef]
- Zhou, S.; Richter, A.; Jander, G. Beyond Defense: Multiple Functions of Benzoxazinoids in Maize Metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Pratt, K.; Kumar, P.; Chilton, W.S. Cyclic hydroxamic acids in dicotyledonous plants. Biochem. Syst. Ecol. 1995, 23, 781–785. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic Acids Derived from 2-Hydroxy-2H-1,4-Benzoxazin-3(4H)-one: Key Defense Chemicals of Cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G. Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Nat. Prod. Rep. 2009, 26, 478–489. [Google Scholar] [CrossRef]
- Macias, F.A.; Oliveros-Bastidas, A.; Marin, D.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Degradation studies on benzoxazinoids. Soil degradation dynamics of 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and its degradation products, phytotoxic allelochemicals from Gramineae. J. Agric. Food Chem. 2004, 52, 6402–6413. [Google Scholar] [CrossRef] [PubMed]
- Fomsgaard, I.S.; Mortensen, A.G.; Carlsen, S.C.K. Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals––A review. Chemosphere 2004, 54, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Zikmundova, M.; Drandarov, K.; Bigler, L.; Hesse, M.; Werner, C. Biotransformation of 2-benzoxazolinone and 2-hydroxy-1,4-benzoxazin-3-one by endophytic fungi isolated from Aphelandra tetragona. Appl. Environ. Microbiol. 2002, 68, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Marocco, A.; Tabaglio, V.; Macias, F.A.; Molinillo, J.M.G. Benzoxazinoids in Rye Allelopathy-From Discovery to Application in Sustainable Weed Control and Organic Farming. J. Chem. Ecol. 2013, 39, 154–174. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Macias, F.A.; Molinillo, J.M.G. Structure-activity relationship of benzoxazinones and related compounds with respect to the growth inhibition and α-amylase activity in cress seedlings. J. Plant Physiol. 2010, 167, 1221–1225. [Google Scholar] [CrossRef]
- Reberg-Horton, S.C.; Burton, J.D.; Danehower, D.A.; Ma, G.Y.; Monks, D.W.; Murphy, J.P.; Ranells, N.N.; Williamson, J.D.; Creamer, N.G. Changes over time in the allelochemical content of ten cultivars of rye (Secale cereale L.). J. Chem. Ecol. 2005, 31, 179–193. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Macias, F.A. Effects of 6-methoxy-2-benzoxazolinone on the germination and α-amylase activity in lettuce seeds. J. Plant Physiol. 2005, 162, 1304–1307. [Google Scholar] [CrossRef]
- Makowska, B.; Bakera, B.; Rakoczy-Trojanowska, M. The genetic background of benzoxazinoid biosynthesis in cereals. Acta Physiol. Plant. 2015, 37, 176. [Google Scholar] [CrossRef]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L.; Glauser, G.; Erb, M.; Flors, V.; Frey, M.; et al. Benzoxazinoid Metabolites Regulate Innate Immunity against Aphids and Fungi in Maize. Plant Physiol. 2011, 157, 317. [Google Scholar] [CrossRef] [PubMed]
- Tanwir, F.; Dionisio, G.; Adhikari, K.B.; Fomsgaard, I.S.; Gregersen, P.L. Biosynthesis and chemical transformation of benzoxazinoids in rye during seed germination and the identification of a rye Bx6-like gene. Phytochemistry 2017, 140, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Chinchilla, N.; Varela, R.M.; Oliveros-Bastidas, A.; Marin, D.; Molinillo, J.M.G. Structure-activity relationship studies of benzoxazinones and related compounds. Phytotoxicity on Echinochloa crus-galli (L.) P. Beauv. J. Agric. Food Chem. 2005, 53, 4373–4380. [Google Scholar] [CrossRef]
- Wu, H.W.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Distribution and exudation of allelochemicals in wheat Triticum aestivum. J. Chem. Ecol. 2000, 26, 2141–2154. [Google Scholar] [CrossRef]
- Wu, H.W.; Haig, T.; Pratley, J.; Lemerle, D.; An, M. Allelochemicals in wheat (Triticum aestivum L.): Production and exudation of 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one. J. Chem. Ecol. 2001, 27, 1691–1700. [Google Scholar] [CrossRef]
- Sicker, D.; Frey, M.; Schulz, M.; Gierl, A. Role of natural benzoxazinones in the survival strategy of plants. Int. Rev. Cytol. A Surv. Cell Biol. 2000, 198, 319–346. [Google Scholar]
- Schulz, M.; Marocco, A.; Tabaglio, V. BOA Detoxification of Four Summer Weeds during Germination and Seedling Growth. J. Chem. Ecol. 2012, 38, 933–946. [Google Scholar] [CrossRef]
- Macias, F.A.; Oliveros-Bastidas, A.; Marin, D.; Chinchilla, N.; Castellano, D.; Molinillo, J.M.G. Evidence for an Allelopathic Interaction Between Rye and Wild Oats. J. Agric. Food Chem. 2014, 62, 9450–9457. [Google Scholar] [CrossRef]
- Gents, M.B.; Nielsen, S.T.; Mortensen, A.G.; Christophersen, C.; Fomsgaard, I.S. Transformation products of 2-benzoxazolinone (BOA) in soil. Chemosphere 2005, 61, 74–84. [Google Scholar] [CrossRef]
- Christopher, J.T.; Preston, C.; Powles, S.B. Malathion Antagonizes Metabolism-Based Chlorsulfuron Resistance in Lolium rigidum. Pestic. Biochem. Physiol. 1994, 49, 172–182. [Google Scholar] [CrossRef]
- Eljarrat, E.; Guillamon, M.; Seuma, J.; Mogensen, B.B.; Fomsgaard, I.S.; Olivero-Bastidas, A.; Macias, F.A.; Stochmal, A.; Oleszek, W.; Shakaliene, O.; et al. First European interlaboratory study of the analysis of benzoxazinone derivatives in plants by liquid chromatography. J. Chromatogr. A 2004, 1047, 69–76. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Resistance cost of a cytochrome P450 herbicide metabolism mechanism but not an ACCase target site mutation in a multiple resistant Lolium rigidum population. New Phytol. 2005, 167, 787–796. [Google Scholar] [CrossRef]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Castellano, D.; Simonet, A.M.; Molinillo, J.M.G. Structure-activity relationship (SAR) studies of benzoxazinones, their degradation products, and analogues. Phytotoxicity on problematic weeds Avena fatua L. and Lolium rigidum Gaud. J. Agric. Food Chem. 2006, 54, 1040–1048. [Google Scholar] [CrossRef]
- Żebrowski, W.; Buszewski, B.; Lankmayr, E. Modeling of Uptake of Xenobiotics in Plants. Crit. Rev. Anal. Chem. 2004, 34, 147–164. [Google Scholar] [CrossRef]
- Hofmann, D.; Knop, M.; Hao, H.; Hennig, L.; Sicker, D.; Schulz, M. Glucosides from MBOA and BOA detoxification by Zea mays and Portulaca oleracea. J. Nat. Prod. 2006, 69, 34–37. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveros-Bastidas, A.; Molinillo, J.M.G.; Macias, F.A.; Chinchilla, N. Absorption and Elimination of the Allelochemical MBOA by Weeds during Seedling Growth. Agronomy 2021, 11, 471. https://doi.org/10.3390/agronomy11030471
Oliveros-Bastidas A, Molinillo JMG, Macias FA, Chinchilla N. Absorption and Elimination of the Allelochemical MBOA by Weeds during Seedling Growth. Agronomy. 2021; 11(3):471. https://doi.org/10.3390/agronomy11030471
Chicago/Turabian StyleOliveros-Bastidas, Alberto, José M. G. Molinillo, Francisco A. Macias, and Nuria Chinchilla. 2021. "Absorption and Elimination of the Allelochemical MBOA by Weeds during Seedling Growth" Agronomy 11, no. 3: 471. https://doi.org/10.3390/agronomy11030471
APA StyleOliveros-Bastidas, A., Molinillo, J. M. G., Macias, F. A., & Chinchilla, N. (2021). Absorption and Elimination of the Allelochemical MBOA by Weeds during Seedling Growth. Agronomy, 11(3), 471. https://doi.org/10.3390/agronomy11030471






