Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches
Abstract
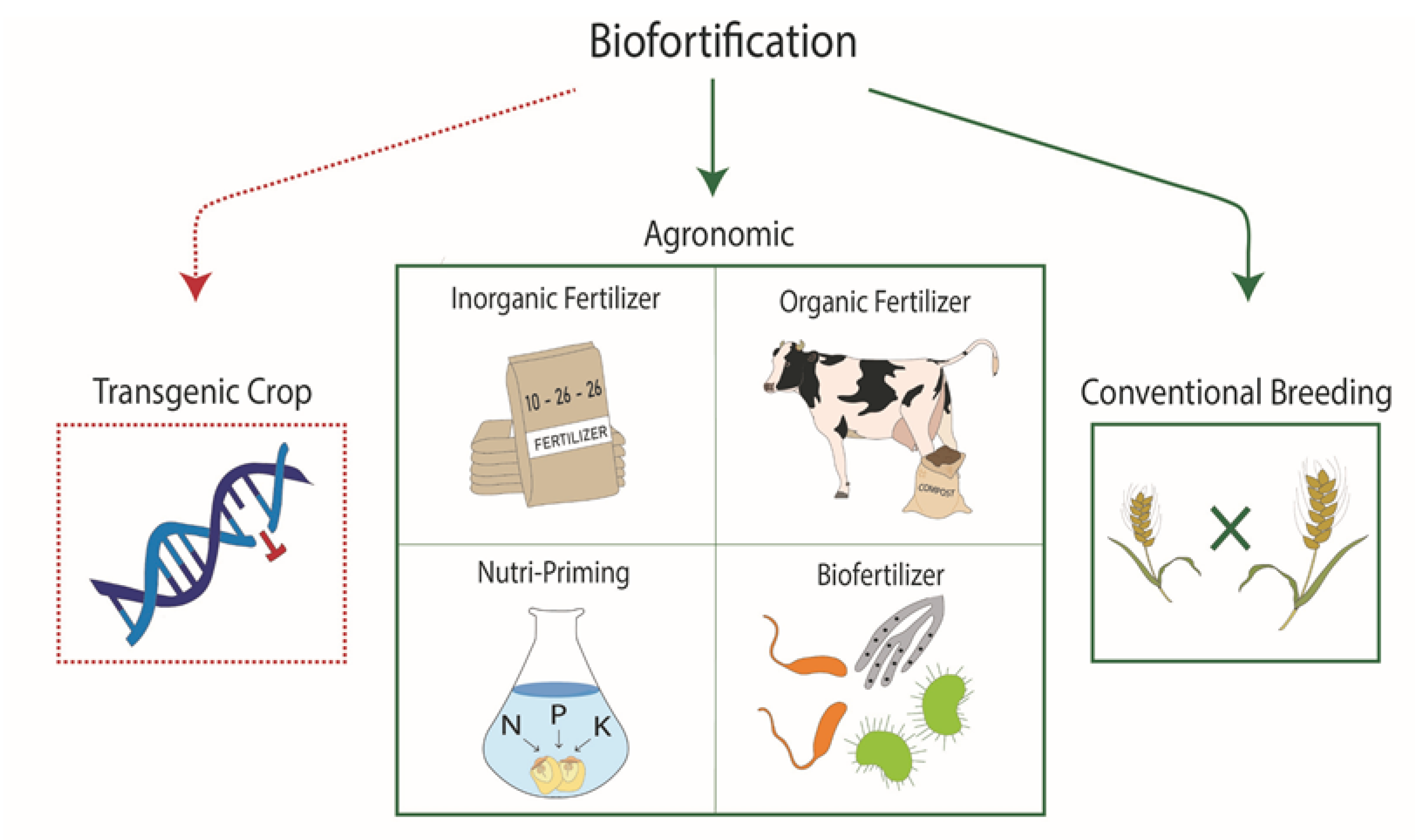
1. Introduction
2. Conventional Breeding
2.1. Benefits of Conventional Breeding
2.2. Limitations of Conventional Breeding
3. Agronomic Biofortification Approaches
3.1. Limitations of Inorganic Fertilizers to Biofortify Crops
3.2. Benefits and Limitations of Other Agronomic Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. The Future of Food and Agriculture, Trends and Challenges; Food and Agriculture Organization: Rome, Italy, 2017; Volume 4, ISBN 9789251095515. [Google Scholar]
- Food and Agriculture Organization (FAO). The State of Food and Agriculture—Executive Summary; FAO: Rome, Italy, 2013. [Google Scholar]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding crops for better nutrition. Crop. Sci. 2007, 47, S-88. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Wiesman, D.; Prasai, N.; Yohannes, Y.; Menon, P.; Thompson, J. 2014 Global Hunger Index: The Challenge of Hidden Hunger; Welthungerhilfe International Food Policy Research Institute (IFPRI) Concern Worldwide: Bonn, Germany; Washington, DC, USA; Dublin, Ireland, 2014. [Google Scholar]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop. Sci. 2010, 50, S-20–S-32. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef]
- Szakály, Z.; Szente, V.; Kövér, G.; Polereczki, Z.; Szigeti, O. The influence of lifestyle on health behavior and preference for functional foods. Appetite 2012, 58, 406–413. [Google Scholar] [CrossRef]
- Szakály, Z.; Kovács, S.; Pető, K.; Huszka, P.; Kiss, M. A modified model of the willingness to pay for functional foods. Appetite 2019, 138, 94–101. [Google Scholar] [CrossRef]
- Pappalardo, G.; Lusk, J.L. The role of beliefs in purchasing process of functional foods. Food Qual. Prefer. 2016, 53, 151–158. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional Foods: Trends and Development. Ital. J. Food Sci. 2016, 28, 338–352. [Google Scholar]
- Morris, D. Description and composition of flax. In Flax—A Health and Nutrition Primer; Flax Council of Canada: Winnipeg, MB, Canada, 2007; pp. 9–21. [Google Scholar]
- Flax Council of Canada. Chapter 11: Varieties Varietal Development in Canada Three. In Growing Flax Production, Management and Diagnostic Guide; Flax Council of Canada: Winnipeg, MB, Canada, 2015; pp. 49–53. [Google Scholar]
- Precedence Research. Functional Food Market Size, Share, Growth, Trends, Consumption, Regional Insights and Forecast 2020 to 2027; Precedence Research: Ottawa, ON, Canada, 2020. [Google Scholar]
- Scott, S.E.; Inbar, Y.; Rozin, P. Evidence for Absolute Moral Opposition to Genetically Modified Food in the United States. Perspect. Psychol. Sci. 2016, 11, 315–324. [Google Scholar] [CrossRef]
- Frewer, L.J.; van der Lans, I.A.; Fischer, A.R.H.; Reinders, M.J.; Menozzi, D.; Zhang, X.; van den Berg, I.; Zimmermann, K.L. Public perceptions of agri-food applications of genetic modification—A systematic review and meta-analysis. Trends Food Sci. Technol. 2013, 30, 142–152. [Google Scholar] [CrossRef]
- Shew, A.M.; Nalley, L.L.; Snell, H.A.; Nayga, R.M.; Dixon, B.L. CRISPR versus GMOs: Public acceptance and valuation. Glob. Food Secur. 2018, 19, 71–80. [Google Scholar] [CrossRef]
- Lucht, J.M. Public acceptance of plant biotechnology and GM crops. Viruses 2015, 7, 4254–4281. [Google Scholar] [CrossRef] [PubMed]
- Funk, B.Y.C.; Kennedy, B. The New Food Fights: US Public Divides over Food Science; Pew Research Center: Washington, DC, USA, 2016. [Google Scholar]
- Mabaya, E.; Fulton, J.; Simiyu-Wafukho, S.; Nang’ayo, F. Factors influencing adoption of genetically modified crops in Africa. Dev. South. Afr. 2015, 32, 577–591. [Google Scholar] [CrossRef]
- Acquaah, G. Conventional plant breeding principles and techniques. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2015; Volume 1, pp. 115–158. ISBN 9783319225210. [Google Scholar]
- Jiang, G.-L. Molecular Markers and Marker-Assisted Breeding in Plants in Plant Breeding from Laboratories to Fields. In Intech; Intech Open: London, UK, 2013. [Google Scholar]
- Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef]
- Mahender, A.; Anandan, A.; Pradhan, S.K.; Pandit, E. Rice grain nutritional traits and their enhancement using relevant genes and QTLs through advanced approaches. Springerplus 2016, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.P.M.; Rahman, M.A.; Inabangan-Asilo, M.A.; Amparado, A.; Manito, C.; Chadha-Mohanty, P.; Reinke, R.; Slamet-Loedin, I.H. Advances in breeding for high grain Zinc in Rice. Rice 2016, 9, 1–16. [Google Scholar] [CrossRef]
- International Potato Center. Sweetpotato Agri-Food Systems Program; International Potato Center: Lima, Peru, 2019. [Google Scholar]
- Low, J.W.; Mwanga, R.O.M.; Andrade, M.; Carey, E.; Ball, A.M. Tackling vitamin A deficiency with biofortified sweetpotato in sub-Saharan Africa. Glob. Food Secur. 2017, 14, 23–30. [Google Scholar] [CrossRef]
- Huang, A.S.; Tanudjaja, L.; Lum, D. Content of Alpha-, Beta-Carotene, and Dietary Fiber in 18 Sweetpotato Varieties Grown in Hawaii. J. Food Compos. Anal. 1999, 12, 147–151. [Google Scholar] [CrossRef]
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Masawi, G.N.; Baingana, R.; Carriquiry, A.; de Brauw Meenakshi, A.; Gilligan, D.O. Introduction of β-Carotene-Rich orange sweet potato in rural Uganda resulted in increased vitamin a intakes among children and women and improved vitamin a status among children. J. Nutr. 2012, 142, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Loechl, C.; De Brauw, A.; Eozenou, P.; Gilligan, D.; Moursi, M.; Munhaua, B.; Van Jaarsveld, P.; Carriquiry, A.; Meenakshi, J.V. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 2012, 108, 163–176. [Google Scholar] [CrossRef]
- Van Jaarsveld, P.J.; Faber, M.; Tanumihardjo, S.A.; Nestel, P.; Lombard, C.J.; Benadé, A.J.S. β-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am. J. Clin. Nutr. 2005, 81, 1080–1087. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D.; Htut, H.; Graham, R.D. Breeding for trace minerals in rice. Food Nutr. Bull. 2000, 21, 382–396. [Google Scholar] [CrossRef]
- Haas, J.D.; Beard, J.L.; Murray-Kolb, L.E.; Del Mundo, A.M.; Felix, A.; Gregorio, G.B. Iron-biofortified rice improves the iron stores of nonanemic filipino women. J. Nutr. 2005, 135, 2823–2830. [Google Scholar] [CrossRef]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The Molecular Genetics of Crop Domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Meyer, R.S.; Duval, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Meenakshi, J.V.; Johnson, N.L.; Manyong, V.M.; De Groote, H.; Javelosa, J.; Yanggen, D.R.; Naher, F.; Gonzalez, C.; García, J.; Meng, E. How Cost-Effective is Biofortification in Combating Micronutrient Malnutrition? An Ex ante Assessment. World Dev. 2010, 38, 64–75. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agricultural Commodities Production. Available online: http://www.faostat.fao.org (accessed on 12 January 2021).
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef]
- Welch, R.M. Breeding strategies for biofortified staple plant foods to reduce micronutrient malnutrition globally. J. Nutr. 2002, 132, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Brodt, S.; Six, J.; Feenstra, G.; Ingels, C.; Campbell, D. Sustainable Agriculture. Nat. Educ. Knowl. 2011, 3, 1. [Google Scholar]
- Hart, M.R.; Quin, B.F.; Nguyen, M.L. Phosphorus Runoff from Agricultural Land and Direct Fertilizer Effects: A Review. J. Environ. Qual. 2004, 33, 1954–1972. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, A.; Mineau, P. The impact of agricultural practices on biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Warschefsky, E.; Penmetsa, R.V.; Cook, D.R.; Von Wettberg, E.J.B. Back to the wilds: Tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. Am. J. Bot. 2014, 101, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Von Wettberg, E.J.B.; Chang, P.L.; Başdemir, F.; Carrasquila-Garcia, N.; Korbu, L.B.; Moenga, S.M.; Bedada, G.; Greenlon, A.; Moriuchi, K.S.; Singh, V.; et al. Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nat. Commun. 2018, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.J.; Kumar, S.; Wettberg, E.J.B.; Marques, E.; Berger, J.D.; Redden, R.J.; Ellis, T.H.N.; Brus, J.; Zablatzká, L.; Smýkal, P. Potential and limits of exploitation of crop wild relatives for pea, lentil, and chickpea improvement. Legum. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Brozynska, M.; Furtado, A.; Henry, R.J. Genomics of crop wild relatives: Expanding the gene pool for crop improvement. Plant Biotechnol. J. 2016, 14, 1070–1085. [Google Scholar] [CrossRef]
- Sivasakthi, K.; Marques, E.; Kalungwana, N.; Cordeiro, M.; Sani, S.G.A.S.; Udupa, S.M.; Rather, I.A.; Mir, R.R.; Vadez, V.; Vandemark, G.J.; et al. Functional Dissection of the Chickpea (Cicer arietinum L.) Stay-Green Phenotype Associated with Molecular Variation at an Ortholog of Mendel’s I Gene for Cotyledon Color: Implications for Crop Production and Carotenoid Biofortification. Int. J. Mol. Sci. 2019, 20, 5562. [Google Scholar] [CrossRef] [PubMed]
- Fernández-marín, B.; Milla, R.; Martín-robles, N.; Arc, E.; Kranner, I.; Becerril, J.M.; García-plazaola, J.I. Side-effects of domestication: Cultivated legume seeds contain similar tocopherols and fatty acids but less carotenoids than their wild counterparts. BMC Plant Biol. 2014, 14, 1599. [Google Scholar] [CrossRef]
- Fernández-Ruiz, V.; Olives, A.I.; Cámara, M.; Sánchez-Mata, M.D.C.; Torija, M.E. Mineral and trace elements content in 30 accessions of tomato fruits (Solanum lycopersicum L.) and wild relatives (Solanum pimpinellifolium L., Solanum cheesmaniae L. Riley, and Solanum habrochaites S. Knapp & D.M. Spooner). Biol. Trace Elem. Res. 2011, 141, 329–339. [Google Scholar] [CrossRef]
- Zhang, H.; Yasmin, F.; Song, B.H. Neglected treasures in the wild—Legume wild relatives in food security and human health. Curr. Opin. Plant Biol. 2019, 49, 17–26. [Google Scholar] [CrossRef]
- Schier, H.E.; Eliot, K.A.; Herron, S.A.; Landfried, L.K.; Migicovsky, Z.; Rubin, M.J.; Miller, A.J. Comparative analysis of perennial and annual Phaseolus seed nutrient concentrations. Sustainability 2019, 11, 2787. [Google Scholar] [CrossRef]
- Jewell, C.P.; Zhang, S.V.; Gibson, M.J.S.; Tovar-Méndez, A.; Mcclure, B.; Moyle, L.C. Intraspecific Genetic Variation Underlying Postmating Reproductive Barriers between Species in the Wild Tomato Clade (Solanum sect. Lycopersicon). J. Hered. 2020, 111, 216–226. [Google Scholar] [CrossRef]
- Dempewolf, H.; Hodgins, K.A.; Rummell, S.E.; Ellstrand, N.C.; Rieseberg, L.H. Reproductive isolation during domestication. Plant Cell 2012, 24, 2710–2717. [Google Scholar] [CrossRef]
- Munguía-Rosas, M.A.; Jácome-Flores, M.E. Reproductive isolation between wild and domesticated chaya (Cnidoscolus aconitifolius) in sympatry. Plant Biol. 2020, 22, 932–938. [Google Scholar] [CrossRef]
- Greene, S.L.; Williams, K.A.; Khoury, C.K.; Kantar, M.B.; Marek, L.F. North American crop wild relatives: Conservation strategies. North Am. Crop Wild Relat. Conserv. Strateg. 2018, 1, 1–346. [Google Scholar] [CrossRef]
- Varshney, R.K.; Terauchi, R.; McCouch, S.R. Harvesting the Promising Fruits of Genomics: Applying Genome Sequencing Technologies to Crop Breeding. PLoS Biol. 2014, 12, e1001883. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pfeiffer, T.W.; Cornelius, P.L. Soybean QTL for yield and yield components associated with Glycine soja alleles. Crop Sci. 2008, 48, 571–581. [Google Scholar] [CrossRef]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Glenn, K.C.; Alsop, B.; Bell, E.; Goley, M.; Jenkinson, J.; Liu, B.; Martin, C.; Parrott, W.; Souder, C.; Sparks, O.; et al. Bringing new plant varieties to market: Plant breeding and selection practices advance beneficial characteristics while minimizing unintended changes. Crop Sci. 2017, 57, 2906–2921. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. bioRxiv 2018, 13, 369512. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Heffner, E.L.; Lorenz, A.J.; Jannink, J.L.; Sorrells, M.E. Plant breeding with Genomic selection: Gain per unit time and cost. Crop Sci. 2010, 50, 1681–1690. [Google Scholar] [CrossRef]
- Gorjanc, G.; Battagin, M.; Dumasy, J.F.; Antolin, R.; Gaynor, R.C.; Hickey, J.M. Prospects for cost-effective genomic selection via accurate within-family imputation. Crop Sci. 2017, 57, 216–228. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Commun. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Mabesa, R.L.; Impa, S.M.; Grewal, D.; Johnson-Beebout, S.E. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. Field Crop. Res. 2013, 149, 223–233. [Google Scholar] [CrossRef]
- Bressan, E.A.; de Carvalho, I.A.S.; Borges, M.T.M.R.; da Carneiro, M.S.; Silva, E.F.; Gazaffi, R.; Shirasuna, R.T.; Abreu, V.; Popin, R.V.; Figueira, A.; et al. Assessment of Gene Flow to Wild Relatives and Nutritional Composition of Sugarcane in Brazil. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- World Health Organization. Bifortification of Staple Crops. Available online: http://www.who.int/elena/titles/complementary_feeding/en/ (accessed on 12 January 2021).
- Hirschi, K.D. Nutrient biofortification of food crops. Annual Rev. Nutr. 2009, 29, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Pang, L.L.; Yan, P.; Liu, D.Y.; Zhang, W.; Yost, R.; Zhang, F.S.; Zou, C.Q. Zinc fertilizer placement affects zinc content in maize plant. Plant Soil 2013, 372, 81–92. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Shan, D.; Chen, Y.; Deng, N.; Khan, F.; Wu, C.; Wu, W.; et al. Grain Cadmium and Zinc Concentrations in Maize Influenced by Genotypic Variations and Zinc Fertilization. Clean Soil Air Water 2015, 43, 1433–1440. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir, A.R. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in Loess Plateau, China. Field Crop. Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Yang, X.; Tian, X.; Gale, W.; Cao, Y.; Lu, X.; Zhao, A. Effect of soil and foliar zinc application on zinc concentration and bioavailability in wheat grain grown on potentially zinc-deficient soil. Cereal Res. Commun. 2011, 39, 535–543. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous Biofortification of Wheat with Zinc, Iodine, Selenium, and Iron through Foliar Treatment of a Micronutrient Cocktail in Six Countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Soil and foliar zinc biofortification in field pea (Pisum sativum L.): Grain accumulation and bioavailability in raw and cooked grains. Food Chem. 2016, 212, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Shivay, Y.S.; Singh, U.; Prasad, R.; Kaur, R. Agronomic interventions for micronutrient biofortification of pulses. Indian J. Agron. 2016, 61, 161–172. [Google Scholar]
- White, P.J.; Broadley, M.R.; Hammond, J.P.; Ramsay, G.; Subramanian, N.K.; Thompson, J.; Wright, G. Bio-fortification of potato tubers using foliar zinc-fertiliser. J. Hortic. Sci. Biotechnol. 2012, 87, 123–129. [Google Scholar] [CrossRef]
- White, P.J.; Thompson, J.A.; Wright, G.; Rasmussen, S.K. Biofortifying Scottish potatoes with zinc. Plant Soil 2017, 411, 151–165. [Google Scholar] [CrossRef]
- Kromann, P.; Valverde, F.; Alvarado, S.; Vélez, R.; Pisuña, J.; Potosí, B.; Taipe, A.; Caballero, D.; Cabezas, A.; Devaux, A. Can Andean potatoes be agronomically biofortified with iron and zinc fertilizers? Plant Soil 2017, 411, 121–138. [Google Scholar] [CrossRef]
- Wei, Y.; Shohag, M.J.I.; Yang, X. Biofortification and Bioavailability of Rice Grain Zinc as Affected by Different Forms of Foliar Zinc Fertilization. PLoS ONE 2012, 7, e45428. [Google Scholar] [CrossRef]
- Guo, J.X.; Feng, X.M.; Hu, X.Y.; Tian, G.L.; Ling, N.; Wang, J.H.; Shen, Q.R.; Guo, S.W. Effects of soil zinc availability, nitrogen fertilizer rate and zinc fertilizer application method on zinc biofortification of rice. J. Agric. Sci. 2016, 154, 584–597. [Google Scholar] [CrossRef]
- Boonchuay, P.; Cakmak, I.; Rerkasem, B.; Prom-U-Thai, C. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci. Plant Nutr. 2013, 59, 180–188. [Google Scholar] [CrossRef]
- Ram, H.; Rashid, A.; Zhang, W.; Duarte, A.P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R.S.; et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil 2016, 403, 389–401. [Google Scholar] [CrossRef]
- Nakandalage, N.; Nicolas, M.; Norton, R.M.; Hirotsu, N.; Milham, P.J.; Seneweera, S. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Treftz, C.; Omaye, S.T. Hydroponics: Potential for augmenting sustainable food production in non-arable regions. Nutr. Food Sci. 2016, 46, 672–684. [Google Scholar] [CrossRef]
- Wiesner-Reinhold, M.; Schreiner, M.; Baldermann, S.; Schwarz, D.; Hanschen, F.S.; Kipp, A.P.; Rowan, D.D.; Bentley-Hewitt, K.L.; McKenzie, M.J. Mechanisms of selenium enrichment and measurement in brassicaceous vegetables, and their application to human health. Front. Plant Sci. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Dalla, L.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. Trends in Food Science & Technology New ‘solutions’ for floating cultivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohtani, Y.; Tatsukami, Y.; Aoki, W.; Amemiya, T.; Sukekiyo, Y.; Kubokawa, S.; Ueda, M. Folate Biofortification in Hydroponically Cultivated Spinach by the Addition of Phenylalanine. J. Agric. Food Chem. 2017, 65, 4605–4610. [Google Scholar] [CrossRef]
- Montesano, F.F.; Imp, M.D.; Parente, A.; Car, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O. Combined biofortification of soilless grown lettuce with iodine, selenium and zinc and its effect on essential and non-essential elemental composition. J. Plant Nutr. 2020, 44, 673–678. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc biofortification improves phytochemicals and amino-acidic profile in Brassica oleracea cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Zahirul, M.; Park, B.; Kang, H.; Lee, Y. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.J. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 2012, 361, 131–141. [Google Scholar] [CrossRef]
- Rodrigo, S.; Santamaria, O.; Poblaciones, M.J. Selenium Application Timing: Influence in Wheat Grain and Flour Selenium Accumulation under Mediterranean Conditions. J. Agric. Sci. 2014, 6, 6. [Google Scholar] [CrossRef]
- Signore, A.; Renna, M.; D’Imperio, M.; Serio, F.; Santamaria, P. Preliminary evidences of biofortification with iodine of “carota di polignano”, an Italian carrot landrace. Front. Plant Sci. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Rosa, A.T.; Diaz, D.A.R.; Hansel, F.D.; Sebastian, J.S.V.; Adee, E.A. Genotypic Variation on Root Growth and Nutrient Uptake in Corn and Soybean. Agrosyst. Geosci. Environ. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Clark, R.B. Plant genotype differences in the uptake, translocation, accumulation, and use of mineral elements required for plant growth. Plant Soil 1983, 72, 175–196. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.C.; Mandal, B.; Bell, R.W. Agronomic biofortification of zinc in rice: Influence of cultivars and zinc application methods on grain yield and zinc bioavailability. Field Crop. Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Irum, A.; Kausar, S. Iron Biofortification of Cereals Grown under Calcareous Soils: Problems and Solutions. In Soil Science: Agricultural and Environmental Prospectives; Springer: Cham, Switzerland, 2016; pp. 232–258. ISBN 9783319344515. [Google Scholar]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. Conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Bhardwaj, D.; Tuteja, N. Biofertilizers: A Sustainable Eco-Friendly Agricultural Approach to Crop Improvement. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2013; pp. 403–432. ISBN 9781461450016. [Google Scholar]
- Mishra, D.J.; Rajvir, S.; Mishra, U.K.; Kumar, S.S. Role of Bio-Fertilizer in Organic Agriculture: A Review. Res. J. Recent Sci. ISSN 2020, 2, 39–41. [Google Scholar]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crop. Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Hazra, G. Different Types of Eco-Friendly Fertilizers: An Overview. Sustain. Environ. 2016, 1, 54. [Google Scholar] [CrossRef]
- Naguib, N.Y.M. Organic vs. chemical fertilization of medicinal plants: A concise review of researches. Adv. Environ. Biol. 2011, 5, 394–400. [Google Scholar]
- Li, B.Y.; Zhou, D.M.; Cang, L.; Zhang, H.L.; Fan, X.H.; Qin, S.W. Soil micronutrient availability to crops as affected by long-term inorganic and organic fertilizer applications. Soil Tillage Res. 2007, 96, 166–173. [Google Scholar] [CrossRef]
- Maleki, F.S.; Chaichi, M.R.; Mazaheri, D.; Tavakkol, A.R.; Savaghebi, G. Barley Grain Mineral Analysis as Affected by Different Fertilizing Systems and by Drought Stress. J. Agric. Sci. Technol. 2011, 13, 315–326. [Google Scholar]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Ahmad, R.; Shahid, M. Iron biofortification of wheat grains through integrated use of organic and chemical fertilizers in pH affected calcareous soil. Plant Physiol. Biochem. 2016, 104, 284–293. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Khalid, M.; Anjum, S.; Ali, S.; Hannan, F.; Iqbal, M. Cost-effective enhanced iron bioavailability in rice grain grown on calcareous soil by sulfur mediation and its effect on heavy metals mineralization. Environ. Sci. Pollut. Res. 2017, 24, 1219–1228. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Basamba, T.A. How safe is chicken litter for land application as an organic fertilizer? A review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hussain, N. A critical study on the use, application and effectiveness of organic and inorganic fertilizers. J. Ind. Pollut. Control 2014, 30, 191–193. [Google Scholar]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food Agric. 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K.H.M. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Raj, A.B.; Raj, S.K. Seed priming: An approach towards agricultural sustainability. J. Appl. Nat. Sci. 2019, 11, 227–234. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Intech Open: London, UK, 2016. [Google Scholar]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming; Springer Nature: Gateway East, Singapore, 2019; ISBN 9789811386251. [Google Scholar]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Yunas, M. “On-farm” seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil 2008, 306, 3–10. [Google Scholar] [CrossRef]
- Praharaj, S.; Singh, R.; Singh, V.K.; Chandra, R. Yield and grain zinc concentration of wheat as affected by nutri priming and foliar application of zinc. J. Pharmacogn. Phytochem. 2019, 8, 503–505. [Google Scholar]
- Holub, B.J.; Nagpurkar, A. Method of Fortifying Seeds with an Essential Fatty Acid, Fortified Seed and Food Product. U.S. Patent 7416752, 26 August 2008. [Google Scholar]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.; Deeba, F.; Ali, I.; Hussain, H. Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; ISBN 9789811386251. [Google Scholar]
| Approach | Benefits | Limitations | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cost-Effective | Simple | Eco-Friendly | Timely | High-Cost | Labor Intensive | Unsustainable | Time Consuming | Reliant on Variation 1 | Environment- Dependent 2 | |
| Conventional Breeding |  |  |  |  |  |  |  | |||
| Agronomic | ||||||||||
| Inorganic Fertilizers |  |  |  |  |  |  |  | |||
| Organic Fertilizers |  |  |  |  | ||||||
| Biofertilizers |  |  |  |  |  |  |  | |||
| Nutri-Priming |  |  |  |  |  |  | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, E.; Darby, H.M.; Kraft, J. Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches. Agronomy 2021, 11, 464. https://doi.org/10.3390/agronomy11030464
Marques E, Darby HM, Kraft J. Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches. Agronomy. 2021; 11(3):464. https://doi.org/10.3390/agronomy11030464
Chicago/Turabian StyleMarques, Edward, Heather M. Darby, and Jana Kraft. 2021. "Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches" Agronomy 11, no. 3: 464. https://doi.org/10.3390/agronomy11030464
APA StyleMarques, E., Darby, H. M., & Kraft, J. (2021). Benefits and Limitations of Non-Transgenic Micronutrient Biofortification Approaches. Agronomy, 11(3), 464. https://doi.org/10.3390/agronomy11030464






