Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Large-Scale Cultivation of D. deltoidea Cell Suspension in Bioreactors
2.3. Assessment of Growth and Physiological Characteristics of Cell Suspension Culture
- growth index: I = (Xmax − Xo)/Xo,
- specific growth rate at exponential growth phase: μ = (ΔlnXmax/Xo)/Δt, [day−1]
- productivity on dry biomass: P = (Xmax − Xo)/Δtmax, [g/(L day)]
2.4. Content of Macro and Microelements in Cell Biomass
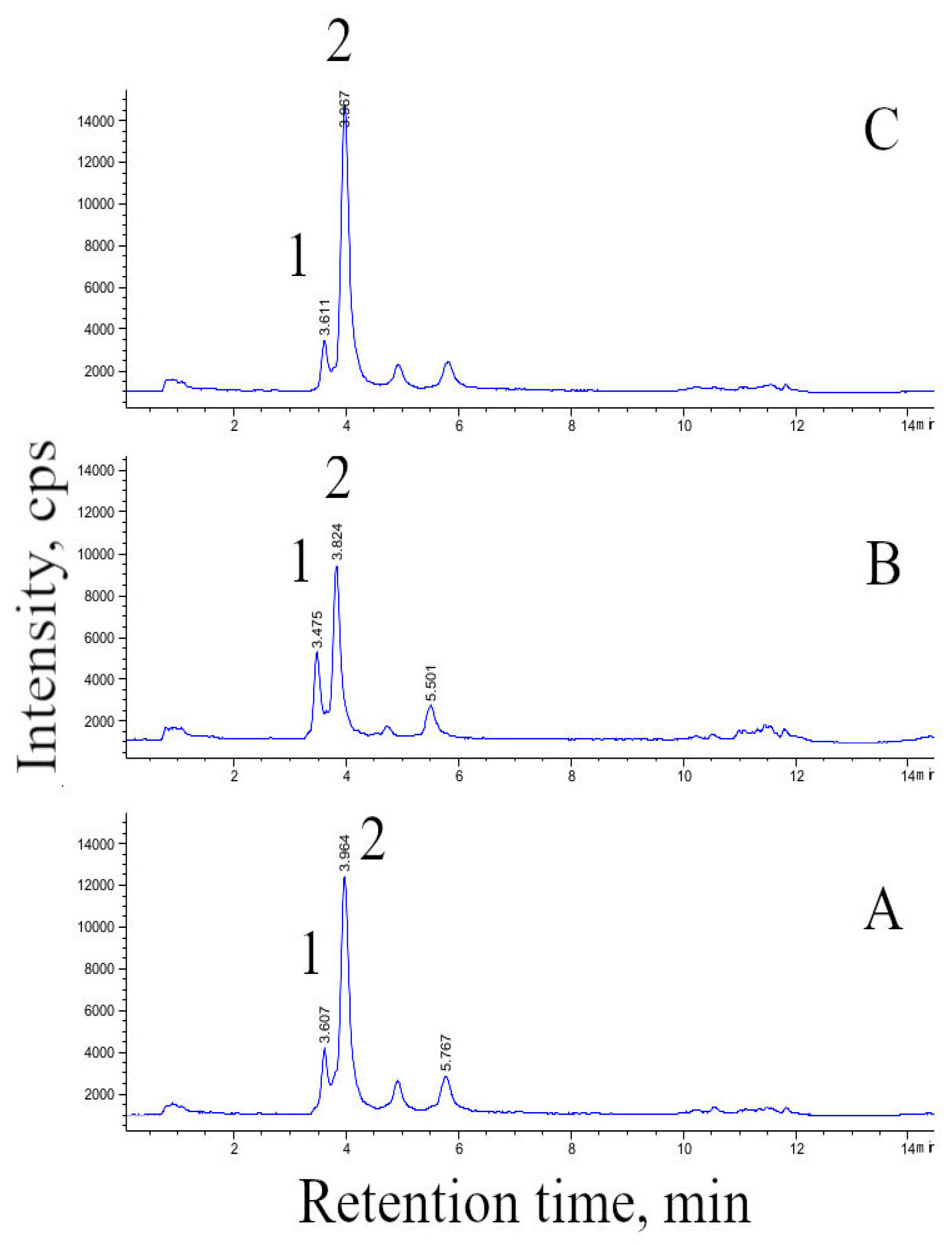
2.5. HPLC and HPLC–MS Analysis of Furostanol Glycosides (FG) in Cell Biomass
2.6. Acute Toxicity Test of Dioscorea deltoidea Cell Biomass on Rats
2.7. Statistical Analysis
3. Results
3.1. Growth and Furostanol Glycoside Content of Dioscorea deltoidea Cell Suspension Culture in Bioreactors
3.2. Content of Macro and Microelements in Cell Biomass
3.3. Acute Toxicity Test of Dioscorea deltoidea Cell Biomass
4. Discussion
4.1. Bioreactor Culture of Dioscorea deltoidea Suspension Cells
4.2. Inorganic Elements in Cell Culture of Dioscorea deldoidea
4.3. Acute Toxicity Test of Dioscorea deltoidea Cell Biomass in Rats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obidiegwu, J.E.; Lyons, J.B.; Chilaka, C.A. The Dioscorea genus (yam)—An appraisal of nutritional and therapeutic potentials. Foods 2020, 9, 1304. [Google Scholar] [CrossRef]
- Hooker, E. Final report of the amended safety assessment of Dioscorea villosa (wild yam) root extract. Int. J. Toxicol. 2004, 23 (Suppl. 2), 49–54. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, L.; Li, M.; Zhang, B.; Zhou, N.; Ke, Y.; Weisheng, F.; Zheng, X. Estrogenic effects of the extracts from the Chinese Yam (Dioscorea opposita Thunb.) and its effective compounds in vitro and in vivo. Molecules 2018, 23, 11. [Google Scholar] [CrossRef]
- Araghiniknam, M.; Chung, S.; Nelson-White, T.; Eskelson, C.; Watson, R. Antioxidant activity of dioscorea and dehydroepiandrosterone (DHEA) in older humans. Life Sci. 1996, 59, PL147–PL157. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Iemitsu, K.; Uchida, M.; Hamaoka, T.; Iemitsu, M. Effects of Dioscorea esculenta intake with resistance training on muscle hypertrophy and strength in sprint athletes. J. Clin. Biochem. Nutr. 2020, 67, 338–343. [Google Scholar] [CrossRef]
- Son, I.S.; Kim, J.H.; Sohn, H.Y.; Son, K.H.; Kim, J.-S.; Kwon, C.-S. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol fed rats. Biosci. Biotechnol. Biochem. 2007, 71, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Dubinskaya, V.A.; Strelkova, L.B.; Vasil’eva, I.S.; Nikolaeva, S.S.; Rebrov, L.B.; Paseshnichenko, V.A. Anabolic properties of Dioscorea deltoidea Wall furostanol glycosides. Bull. Exp. Biol. Med. 1998, 126, 800–802. [Google Scholar] [CrossRef]
- Liang, X.-J.; Guo, Y.-C.; Sun, T.-Y.; Song, H.-R.; Gao, Y.-X. Anti-angiogenic effect of total saponins of Rhizoma Dioscorea nipponica on collagen induced-arthritis in rats. Exp. Ther. Med. 2016, 12, 2155–2160. [Google Scholar] [CrossRef]
- Qin, J.; Kang, Y.; Xu, Z.; Zang, C.; Fang, B.; Liu, X. Dioscin prevents the mitochondrial apoptosis and attenuates oxidative stress in cardiac H9c2 cells. Drug Res. 2013, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Sun, X.; Yin, L.; Han, X.; Xu, L.; Qi, Y.; Xua, Y.; Lia, H.; Lina, Y.; Liu, K.; et al. Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radic. Biol. Med. 2015, 84, 103–115. [Google Scholar] [CrossRef]
- Jayachandran, S.K.; Vasanthi, R.H.; Rajamanickam, V.G. Flavonoid rich fraction of Dioscorea bulbifera Linn. (yam) enhances mitochondrial enzymes and antioxidant status, thereby protects heart from isoproterenol induced myocardial infarction. Curr. Pharm. Biotechnol. 2010, 11, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-N.; He, X.-C.; Ye, M.; Huang, H.; Chen, H.-L.; Peng, W.-L.; Zhao, Z.-Z.; Tao, Y.; Chen, H.-B. Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. J. Ethnopharmacol. 2015, 175, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Vasil’eva, I.S.; Paseshnichenko, V.A. Steroid glycosides from suspension cultures of Dioscorea deltoidea cells and their biological activity. In Saponins Used in Traditional and Modern Medicine. Advances in Experimental Medicine and Biology; Waller, G.R., Yamasaki, K., Eds.; Springer: Boston, MA, USA, 1996; Volume 404, pp. 15–22. [Google Scholar] [CrossRef]
- Galani, V.J.; Patel, D.M. A comprehensive phytopharmacological review of Dioscorea bulbifera Linn. Int. J. Environ. Sci. Nat. Res. 2017, 4, 555650. [Google Scholar] [CrossRef]
- Galakatu Sameer, S.; Surve Pankaj, P.; Ghotankar Aparna, M.; Kharat Ravindra, S. Review of Varahikanda (Dioscorea bulbifera) for its pharmacological properties. World J. Pharm. Res. 2016, 5, 1738–1746. [Google Scholar] [CrossRef]
- Korokin, M.; Gudyrev, O.; Gureev, V.; Korokina, L.; Peresypkina, A.; Pokrovskaia, T.; Pokrovskii, M. Studies to elucidate the effects of eurostanol glycosides from Dioscorea deltoidea cell culture in a rat model of endothelial dysfunction. Molecules 2019, 25, 169. [Google Scholar] [CrossRef]
- Cui, H.; Li, T.; Wang, L.; Su, Y.; Xian, C.J. Dioscorea bulbifera polysaccharide and cyclophosphamide combination enhances anti-cervical cancer effect and attenuates immunosuppression and oxidative stress in mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sener, B.; Kilic, M.; Sharifi-Rad, J.; Naz, R.; Yousaf, Z.; Mudau, F.N.; Fokou, P.V.T.; Ezzat, S.M.; El Bishbishy, M.H.; et al. Dioscorea plants: A genus rich in vital nutra-pharmaceuticals-a review. Iran. J. Pharm. Res. 2019, 18, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Bibi, Y.; Iqbal, M.; Hussain, M.; Laraib, S.; Safdar, I.; Bibi, G. Overview of Dioscorea deltoidea Wall. Ex Griseb: An endangered medicinal plant from Himalaya region. J. Biodivers. Environ. Sci. 2016, 9, 13–24. [Google Scholar]
- Avula, B.; Wang, Y.-H.; Ali, Z.; Smillie, T.J.; Khan, I.A. Chemical fingerprint analysis and quantitative determination of steroidal compounds from Dioscorea villosa, Dioscorea species and dietary supplements using UHPLC-ELSD. Biomed. Chromatogr. 2013, 28, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Mitaine-Offer, A.-C.; Lacaille-Dubois, M.-A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Luo, L.; Hu, H.; Meng, X.; Li, X.; Chen, S. Predicting the potential global distribution of diosgenin-contained Dioscorea species. Chin. Med. 2018, 13. [Google Scholar] [CrossRef]
- Khandy, M.T.; Titova, M.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Nosov, A.M. Formation of protodioscin and deltoside isomers in suspension cultures of Nepal yam (Dioscorea deltoidea Wall.) cells. Appl. Biochem. Microbiol. 2016, 52, 657–662. [Google Scholar] [CrossRef]
- Titova, M.V.; Khandy, M.T.; Konstantinova, S.V.; Kulichenko, I.E.; Sukhanova, E.S.; Kochkin, D.V.; Nosov, A.M. Effect of inhibitors of two isoprenoid biosynthetic pathways on physiological and biosynthetic characteristics of Dioscorea deltoidea cell suspension culture. Russ. J. Plant Physiol. 2016, 63, 894–900. [Google Scholar] [CrossRef]
- Titova, M.V.; Shumilo, N.A.; Kulichenko, I.E.; Ivanov, I.M.; Sukhanova, E.S.; Nosov, A.M. Features of respiration and formation of steroidal glycosides in Dioscorea deltoidea cell suspension culture grown in flasks and bioreactors. Russ. J. Plant Physiol. 2015, 62, 557–563. [Google Scholar] [CrossRef]
- Nosov, A.M. Aspects of Steroid Metabolism in Cultured Cells of Dioscorea deltoidea as the Base for Biotechnological Production of Furostanol Glycosides. Ph.D. Thesis, All-Russian Scientific Institute of Applied Biochemistry, Moscow, Russia, 1992. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Kaul, B.; Staba, J. Biosynthesis and isolation of diosgenin from Dioscorea deltoidea callus and suspension cells. Lloydia 1968, 31, 171–179. [Google Scholar]
- Baker, J.C.; Mock, N.M. An improved method for monitoring cell death in cell suspension and leaf disc assays using evans blue. Plant Cell Tiss Organ Cult. 1994, 39, 7–12. [Google Scholar] [CrossRef]
- Nosov, A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl. Biochem. Microbiol. 2012, 48, 609. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş.; Dokan, F.K.; Köprü, S. ICP-MS multi-element analysis for determining the origin by multivariate analysis of red pepper flakes from three different regions of Turkey. LWT 2019. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Babos, D.V.; Barros, A.I.; Pereira-Filho, E.R.; Nóbrega, J.A. Microwave-assisted digestion using dilute nitric acid solution and investigation of calibration strategies for determination of As, Cd, Hg and Pb in dietary supplements using ICP-MS. J. Pharm. Biomed. Anal. 2019, 174, 471–478. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Khandy, M.T.; Zaitsev, G.P.; Tolkacheva, N.V.; Shashkov, A.S.; Titova, M.V.; Chirva, V.Y.; Nosov, A.M. Protodioscin in Dioscorea deltoidea suspension cell culture. Chem. Nat. Compd. 2016, 52, 664–668. [Google Scholar] [CrossRef]
- Evans, G.O. (Ed.) Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 2009; 368p. [Google Scholar]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Largest Global Supplier of Paclitaxel and Docetaxel via PCF®. Available online: https://phytonbiotech.com (accessed on 15 January 2021).
- Nordlund, E.; Lille, M.; Silventoinen, P.; Nygren, H.; Seppänen-Laakso, T.; Mikkelson, A.; Aura, A.M.; Heiniö, R.L.; Nohynek, L.; Puupponen-Pimiä, R.; et al. Plant cells as food—A concept taking shape. Food Res. Int. 2018, 107, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kotin, A.M.; Bichevaya, N.K. Anti-Teratogenic. Agent. Patent No. WO1996002266A1, 1 February 1996. [Google Scholar]
- Murthy, H.K.; Georgiev, M.I.; Park, S.-Y.; Dandin, V.S.; Paek, K.-Y. The safety assessment of food ingredients derived from plant cell, tissue and organ cultures: A review. Food Chem. 2015, 176, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, E.S.; Chernyak, N.D.; Nosov, A.M. Obtaining and characteristics of callus and suspension cultures of Polyscias filicifolia and Polyscias fruticose. Biotekhnologiya 2010, 4, 44–50. [Google Scholar]
- Khandy, M.T.; Kochkin, D.V.; Tomilova, S.V.; Galishev, B.A.; Sukhanova, E.S.; Klyushin, A.G.; Ivanov, I.M.; Nosov, A.M. Obtaining and study of callus and suspension plant cell cultures of Tribulus terrestris L., a producer of steroidal glycosides. Appl. Biochem. Microbiol. 2017, 53, 800–806. [Google Scholar] [CrossRef]
- Kochkin, D.V.; Sukhanova, E.S.; Nosov, A.M. The accumulation of triterpene glycosides in the growing cycle cell suspension cultures Polyscias fruticosa. Vestn. Volga State Univ. Technol. Ser. Forest Ecol. Nat. Manag. 2014, 24, 67–73. [Google Scholar]
- Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Tal, B.; Goldberg, I. Growth and diosgenin production by Dioscorea deltoidea cells in batch and continuous cultures. Planta Med. 1982, 44, 107–110. [Google Scholar] [CrossRef]
- Drapeau, D.; Blanch, H.W.; Wilke, C.R. Growth kinetics of Dioscorea deltoidea and Catharanthus roseus in batch culture. Biotechnol. Bioeng. 1986, 28, 1555–1563. [Google Scholar] [CrossRef]
- Zhang, R.; Li, P.; Xu, L.; Chen, Y.; Sui, P.; Zhou, L.; Li, J. Enhancement of diosgenin production in Dioscorea zingiberensis cell culture by oligosaccharide elicitor from its endophytic fungus Fusarium oxysporum Dzf17. Nat. Prod. Commun. 2009, 4, 1459–1462. [Google Scholar] [PubMed]
- Nosov, A.M.; Popova, E.V.; Kochkin, D.V. Isoprenoid Production via Plant Cell Cultures: Biosynthesis, Accumulation and Scaling-Up to Bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 563–623. [Google Scholar]
- Zhao, X.; Wei, J.; Shu, X.; Kong, W.; Yang, M. Multi-elements determination in medical and edible Alpinia oxyphylla and Morinda officinalis and their decoctions by ICP-MS. Chemosphere 2016, 164, 430–435. [Google Scholar] [CrossRef]
- Dico, G.M.L.; Galvano, F.; Dugo, G.; D’Ascenzi, C.; Macaluso, A.; Vella, A.; Giangrosso, G.; Cammilleri, G.; Ferrantelli, V. Toxic metal levels in cocoa powder and chocolate by ICP-MS method after microwave-assisted digestion. Food Chem. 2018, 245, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Marycz, K.; Basińska, K.; Chojnacka, K. Using SEM-EDX and ICP-OES to investigate the elemental composition of green macroalga Vaucheria sessilis. Sci. World J. 2014, 1–8. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 15 January 2021). [CrossRef]
- Ushiyama, K. Regulations for plant cell culture derived products in Japan. In Plant Cell and Tissue Culture for the Production of Food Ingredients; Fu, T.J., Singh, G., Curtis, W.R., Eds.; Springer: Boston, MA, USA, 1999; pp. 281–285. [Google Scholar] [CrossRef]
- Paek, K.Y. (Cheongju National University, Korea). Personal communication, 2020.
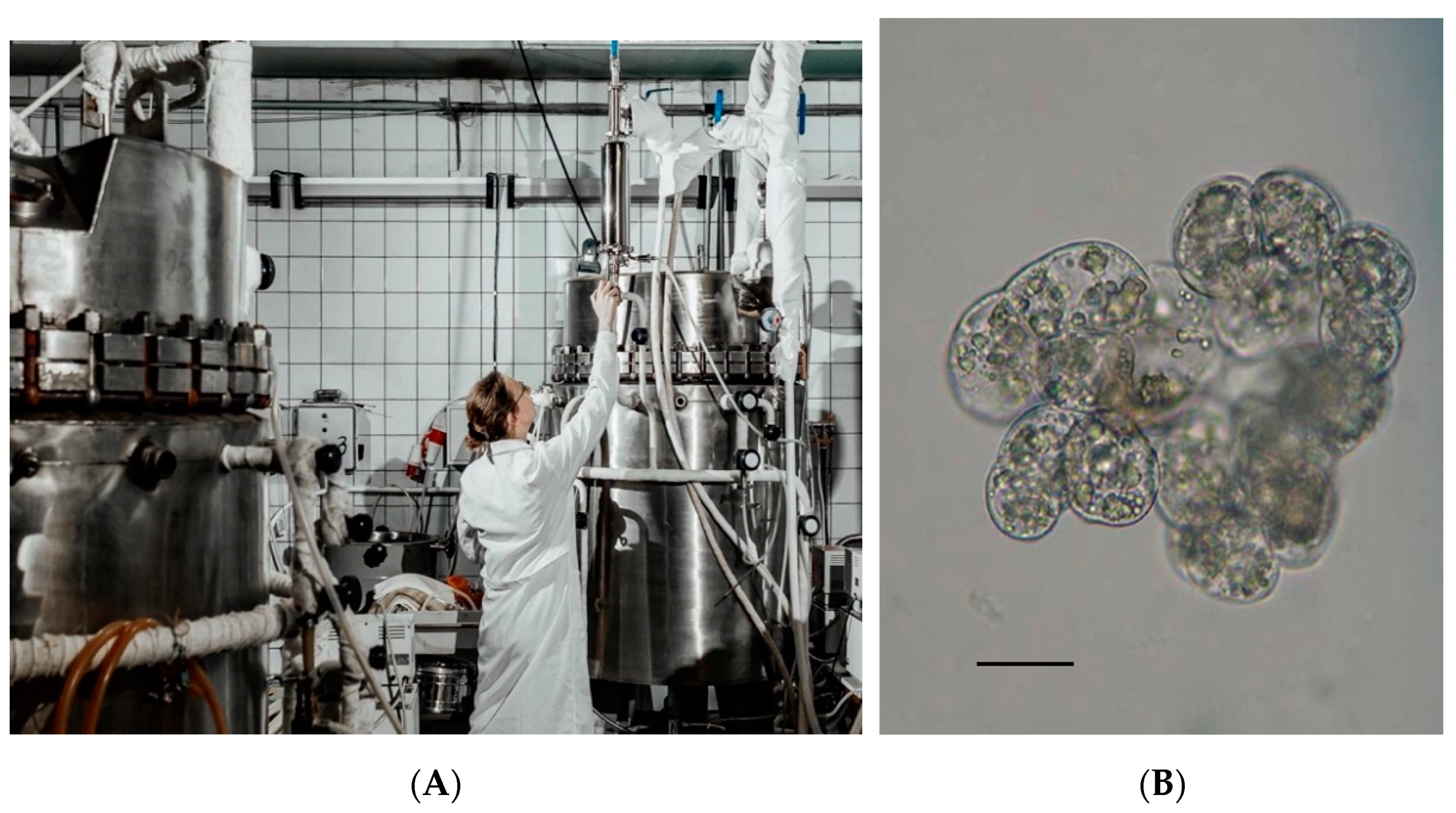
| Cultivation System | Viability (%) | Maximum Dry Weight Accumulation, Xmax (g/L) | Productivity, P [g/(L day)] | Specific Growth Rate, µ (day−1) | Growth Index, I | Content of Furostanol Glycosides (mg/g DW) 1 |
|---|---|---|---|---|---|---|
| 250 mL flasks | 90.4 ± 5.5 a | 9.5 ± 2.6 a | 0.40 ± 0.11 a | 0.16 ± 0.04 a | 4.22 ± 0.79 a | 76.3 ± 22.5 a |
| 20 L bioreactors | 79.0 ± 8.1 a | 8.5 ± 2.1 a | 0.37 ± 0.12 a | 0.13 ± 0.03 a | 3.76 ± 0.72 a | 47.7 ± 16.2 a |
| 630 L bioreactors | 83.5 ± 4.5 a | 8.8 ± 2.3 a | 0.33 ± 0.10 a | 0.12 ± 0.01 a | 3.51 ± 0.62 a | 57.4 ± 19.8 a |
| Element | Content, µg/g of Dry Biomass |
|---|---|
| Microelements | |
| Zinc (Zn) | (0.37 ± 0.05) × 103 |
| Manganese (Mn) | (0.33 ± 0.08) × 103 |
| Iron (Fe) | (0.10 ± 0.01) × 103 |
| Boron (B) | 15.42 ± 1.41 |
| Aluminum (Al) | 2.69 ± 0.50 |
| Copper (Cu) | 1.44 ± 0.62 |
| Chromium (Cr) | 0.24 ± 0.07 |
| Selenium (Se) | 0.24 ± 0.04 |
| Cobalt (Co) | 0.18 ± 0.10 |
| Nickel (Ni) | 0.14 ± 0.01 |
| Macroelements | |
| Potassium (K) | (11.39 ± 4.21) × 103 |
| Calcium (Ca) | (1.12 ± 0.33) × 103 |
| Magnesium (Mg) | (0.74 ± 0.55) × 103 |
| Sodium (Na) | (0.40 ± 0.27) × 103 |
| Animals | Organ Weight (g) | ||||
|---|---|---|---|---|---|
| Heart | Liver | Kidney | Spleen | Thymus | |
| Biomass dose 2000 mg/kg | 0.35 ± 0.02 a | 3.8 ± 0.24 a | 0.37 ± 0.02 a | 0.23 ± 0.02 a | 0.16 ± 0.01 a |
| Biomass dose 5000 mg/kg | 0.35 ± 0.02 a | 3.8 ± 0.24 a | 0.37 ± 0.02 a | 0.23 ± 0.02 a | 0.16 ± 0.01 a |
| Intact animals | 0.33 ± 0.03 a | 3.79 ± 0.18 a | 0.37 ± 0.02 a | 0.20 ± 0.01 a | 0.13 ± 0.02 a |
| Indicators | Norm | Animal Group | ||
|---|---|---|---|---|
| Intact | Dose 2000 mg/kg | Dose 5000 mg/kg | ||
| Leukocytes, 109/L | 6.6–12.6 | 4.35 ± 0.12 | 4.62 ± 1.16 | 9.74 ± 0.69 * |
| Lymphocytes, 109/L | 4.78–9.12 | 3.38 ± 0.12 | 3.42 ± 0.77 | 7.83 ± 0.46 * |
| Content of monocytes, eosinophils, basophils and blast cells mixture, 109/L | 0.02–0.15 | 0.11 ± 0.02 | 0.20 ± 0.06 | 0.36 ± 0.03 * |
| Granulocytes, 109/L | 1.77–3.38 | 0.84 ± 0.04 | 1.00 ± 0.35 | 1.56 ± 0.24 |
| Lymphocytes, % | 57.5–83.6 | 79.92 ± 1.60 | 76.08 ± 2.10 | 80.73 ± 1.42 |
| Monocytes, % | 2.16–2.9 | 2.48 ± 0.42 | 4.05 ± 0.99 | 3.67 ± 0.13 |
| Relative content of granulocytes, % | 20–28 | 16.50 ± 2.33 | 19.88 ± 2.70 | 15.60 ± 1.44 |
| Erythrocytes, 1012/L | 7.07–9.03 | 8.87 ± 0.03 | 8.60 ± 0.25 | 9.05 ± 0.18 |
| Haemoglobin, g/L | 129–161 | 150.00 ± 1.50 | 151.70 ± 3.20 | 158.30 ± 2.30 * |
| Hematocrit, % | 34–44 | 43.16 ± 0.57 | 43.94 ± 0.71 | 45.49 ± 1.05 * |
| Mean erythrocyte volume, mcm3 | 50–59 | 48.67 ± 0.49 | 51.17 ± 0.91 * | 50.33 ± 0.42 |
| Mean haemoglobin content in erythrocyte, pg | 17.8–20.9 | 16.92 ± 0.12 | 17.67 ± 0.29 * | 17.48 ± 0.12 * |
| Mean haemoglobin concentration in erythrocyte, g/L | 332–379 | 347.50 ± 1.50 | 345.00 ± 4.10 | 348.50 ± 3.80 |
| Erythrocyte distribution, % | 10.5–14.9 | 16.27 ± 0.12 | 17.37 ± 0.39 * | 15.93 ± 0.12 |
| Thrombocytes, 109/L | 680–1200 | 1007.80 ± 42.30 | 962.50 ± 47.30 | 1069.20 ± 29.60 |
| Thrombocyte, % | - | 0.70 ± 0.02 | 0.67 ± 0.04 | 0.76 ± 0.01 |
| Mean thrombocyte volume, mcm3 | 6.2–9.8 | 6.95 ± 0.08 | 7.12 ± 0.11 | 7.07 ± 0.16 |
| Thrombocyte distribution, % | - | 33.18 ± 0.29 | 33.23 ± 0.31 | 34.28 ± 0.54 |
| Indicators | Norm | Animal Group | ||
|---|---|---|---|---|
| Intact | Dose 2000 mg/kg | Dose 5000 mg/kg | ||
| Total protein, g/L | 52.0–77.0 | 63.60 ± 0.72 | 60.93 ± 2.37 | 65.90 ± 0.42 |
| Albumin, g/L | 34.0–50.0 | 47.47 ± 0.49 | 45.83 ± 1.11 | 47.73 ± 0.67 |
| Creatinine, μmol/L | 9.0–70.0 | 43.67 ± 0.88 | 42.67 ± 0.44 | 40.67 ± 1.64 |
| Urea, mmol/L | 4.28–8.57 | 8.24 ± 0.08 | 8.36 ± 0.09 | 8.19 ± 0.09 |
| Bilirubin (total), µmol/L | 0–8.5 | 3.07 ± 0.13 | 3.07 ± 0.12 | 2.63 ± 0.34 |
| AspAT, U/L | 47–143 | 75.14 ± 0.40 | 98.68 ± 21.10 | 79.65 ± 0.68 |
| ALAT, U/L | 18.0–80.0 | 39.33 ± 1.20 | 34.00 ± 1.53 | 34.67 ± 3.53 |
| Alkaline phosphatase, U/L | 62.0–450.0 | 190.10 ± 16.60 | 185.00 ±9.90 | 199.40 ± 4.30 |
| GGT, U/L | 0–4.0 | 2.31 ± 0.68 | 2.25 ± 0.41 | 1.87 ± 0.24 |
| Potassium, mmol/L | 3.82–6.00 | 6.37 ± 0.06 | 6.49 ± 0.21 | 6.39 ± 0.33 |
| Cholesterol, mmol/L | 0.51–2.85 | 1.69 ± 0.28 | 2.08 ± 0.01 | 1.88 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titova, M.V.; Popova, E.V.; Konstantinova, S.V.; Kochkin, D.V.; Ivanov, I.M.; Klyushin, A.G.; Titova, E.G.; Nebera, E.A.; Vasilevskaya, E.R.; Tolmacheva, G.S.; et al. Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry. Agronomy 2021, 11, 394. https://doi.org/10.3390/agronomy11020394
Titova MV, Popova EV, Konstantinova SV, Kochkin DV, Ivanov IM, Klyushin AG, Titova EG, Nebera EA, Vasilevskaya ER, Tolmacheva GS, et al. Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry. Agronomy. 2021; 11(2):394. https://doi.org/10.3390/agronomy11020394
Chicago/Turabian StyleTitova, Maria V., Elena V. Popova, Svetlana V. Konstantinova, Dmitry V. Kochkin, Igor M. Ivanov, Andrey G. Klyushin, Elena G. Titova, Elena A. Nebera, Ekaterina R. Vasilevskaya, Galina S. Tolmacheva, and et al. 2021. "Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry" Agronomy 11, no. 2: 394. https://doi.org/10.3390/agronomy11020394
APA StyleTitova, M. V., Popova, E. V., Konstantinova, S. V., Kochkin, D. V., Ivanov, I. M., Klyushin, A. G., Titova, E. G., Nebera, E. A., Vasilevskaya, E. R., Tolmacheva, G. S., Kotenkova, E. A., Nosov, A. M., & Paek, K.-Y. (2021). Suspension Cell Culture of Dioscorea deltoidea—A Renewable Source of Biomass and Furostanol Glycosides for Food and Pharmaceutical Industry. Agronomy, 11(2), 394. https://doi.org/10.3390/agronomy11020394







