Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Soil Analysis
2.3. Experimental Design
Soil and Plant Analysis after Harvesting
2.4. Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Nutrient Uptake by Crops
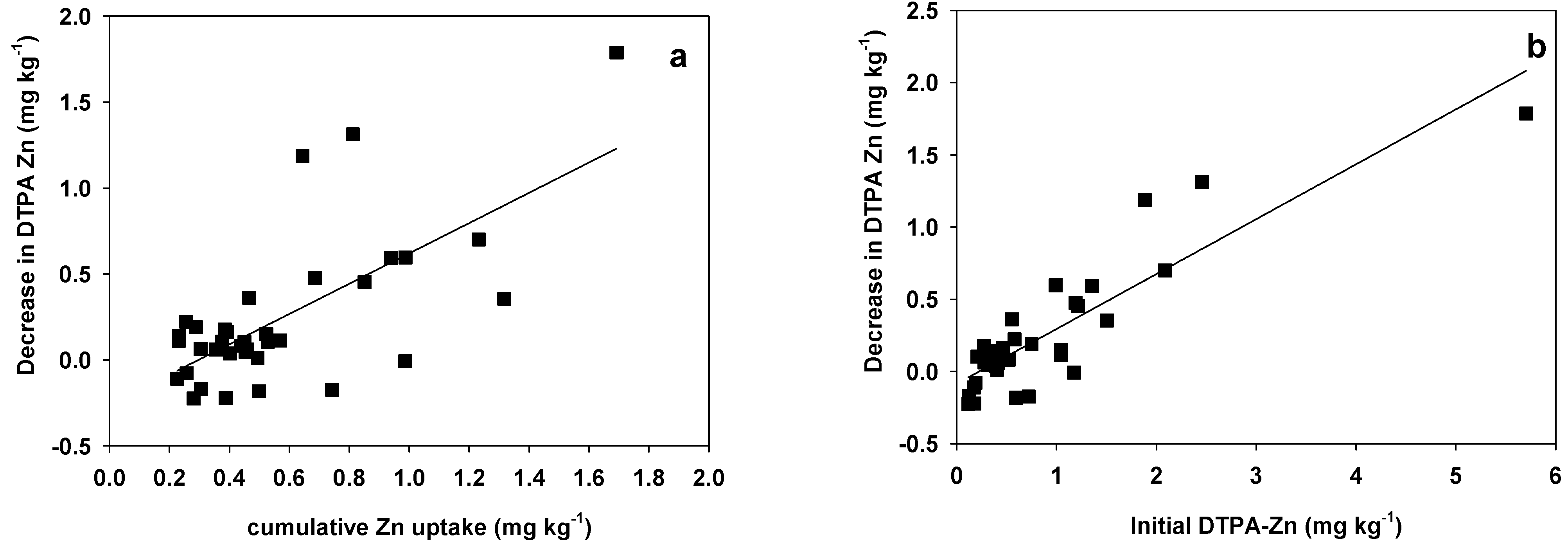
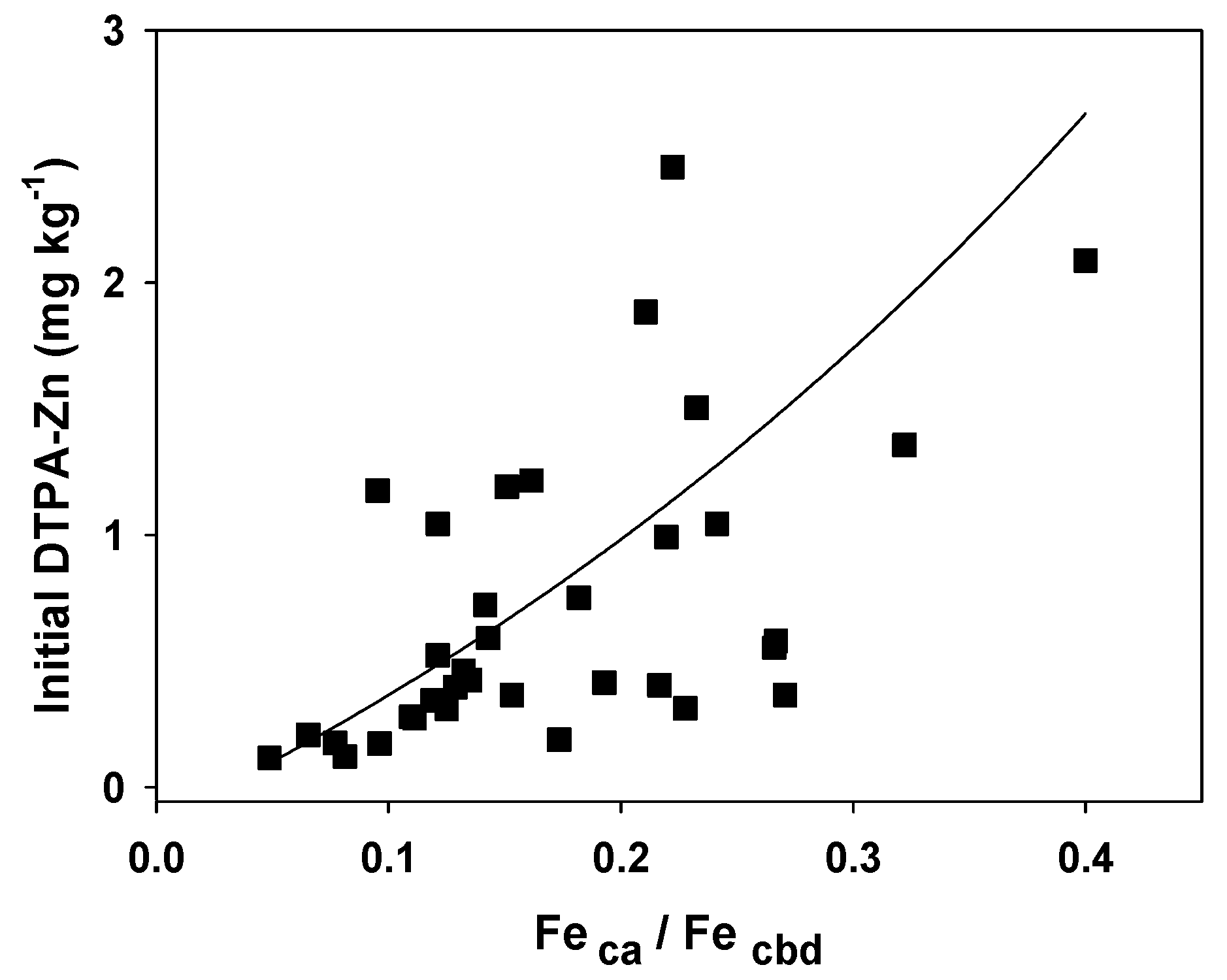
3.3. Relationship between the Zn Availability Index and Soil Properties
3.4. Dry Matter Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.E.; Chen, W.R.; Feng, Y. Improving human micronutrient nutrition through biofortification in the soil–plant system: China as a case study. Environ. Geochem. Health 2007, 29, 413–428. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2008; p. 130. Available online: www.zinc-crops.org (accessed on 14 March 2019).
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Recena, R.; Delgado, A. Bacillus subtilis QST713 and cellulose amendment enhance phosphorus uptake while improving zinc biofortification in wheat. Appl. Soil Ecol. 2019, 142, 81–89. [Google Scholar] [CrossRef]
- Graham, R.D.; Senadhira, D.; Ortiz-Monasterio, I. A strategy for breeding staple-food crops with high micronutrient density. J Soil Sci. Plant Nutr. 1997, 43, 1153–1157. [Google Scholar] [CrossRef]
- Cakmak, I. Plants nutrition research priorities to meet human needs food in sustainable ways. Plant Sci. 2002, 247, 3–24. [Google Scholar]
- Watts-Williams, S.J.; Turney, T.W.; Patti, A.F.; Cavagnaro, T.R. Uptake of zinc and phosphorus by plants is affected by zinc fertiliser material and arbuscular mycorrhizas. Plant Soil 2014, 376, 165–175. [Google Scholar] [CrossRef]
- Sacristán, D.; González–Guzmán, A.; Barrón, V.; Torrent, J.; Del Campillo, M.C. Phosphorus-induced zinc deficiency in wheat pot-grown on noncalcareous and calcareous soils of different properties. Arch. Agron. Soil Sci. 2019, 65, 208–223. [Google Scholar] [CrossRef]
- Fageria, N.K. Zinc. In The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2010; pp. 241–271. [Google Scholar]
- Ryan, J.; Rashid, A.; Torrent, J.; Yau, S.K.; Ibrikci, H.; Sommer, R.; Erenoglu, E.B. Micronutrient constraints to crop production in the middle east-west Asia region: Significance, research, and management. In Advances in Agronomy, 1st ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar] [CrossRef]
- Roholla, S. Zinc in Crop Production and Interaction with Phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Wang, Q.; Kong, X.-P.; Zhang, B.-H.; Wang, J. Adsorption of Zn (II) on the kaolinite(001) surfaces in aqueous environment: A combined DFT and molecular dynamics study. Appl. Surf. Sci. 2017, 414, 405–412. [Google Scholar] [CrossRef]
- Montilla, I.; Parra, M.A.; Torrent, J. Zinc phytotoxicity to oilseed rape grown on zinc loaded substrates consisting of Fe oxide-coated and calcite sand. Plant Soil 2003, 257, 227–236. [Google Scholar] [CrossRef]
- Komárek, M.; Antelo, J.; Králová, M.; Veselská, V.; Číhalová, S.; Chrastný, V.; Ettler, V.; Filip, J.; Yu, Q.; Fein, J.B.; et al. Revisiting models of Cd, Cu, Pb and Zn adsorption onto Fe(III) oxides. Chem. Geol. 2018, 493, 189–198. [Google Scholar]
- Moreno-Lora, A.; Delgado, A. Factors determining Zn availability and uptake by plants in soils developed under Mediterranean climate. Geoderma 2020, 376, 114509. [Google Scholar] [CrossRef]
- Buekers, J.; Van Laer, L.; Amery, F.; Van Buggenhout, S.; Maes, A.; Smolders, E. Role of soil constituents in fixation of soluble Zn, Cu, Ni and Cd added to soils. Eur. J. Soil Sci. 2007, 58, 1514–1524. [Google Scholar] [CrossRef]
- Buekers, J.; Amery, F.; Maes, A.; Smolders, E. Long-term reactions of Ni, Zn and Cd with iron oxyhydroxides depend on crystallinity and structure and on metal concentrations. Eur. J. Soil Sci. 2008, 59, 706–715. [Google Scholar] [CrossRef]
- Buekers, J.; Degryse, F.; Maes, A.; Smolders, E. Modelling the effects of ageing on Cd, Zn, Ni and Cu solubility in soils using an assemblage model. Eur. J. Soil Sci. 2008, 59, 1160–1170. [Google Scholar] [CrossRef]
- Donner, E.; McLaughlin, M.J.; Hodson, M.E.; Heemsbergen, D.; Warne, M.S.J.; Nortcliff, S.; Broos, K. Ageing of zinc in highly-weathered iron-rich soils. Plant Soil 2012, 361, 83–95. [Google Scholar] [CrossRef]
- Brümmer, G.; Tiller, K.G.; Herms, U.; Clayton, P.M. Adsorption—Esorption and/or precipitation—Dissolution processes of zinc in soils. Geoderma 1983, 31, 337–354. [Google Scholar] [CrossRef]
- Uygur, V.; Rimmer, D.L. Reactions of zinc with iron-oxide coated calcite surfaces at alkaline pH. Eur. J. Soil Sci. 2000, 51, 511–516. [Google Scholar] [CrossRef]
- Duffner, A.; Weng, L.; Hoffland, E.; van der Zee, S.E.A.T.M. Multi-surface Modeling To Predict Free Zinc Ion Concentrations in Low-Zinc Soils. Environ. Sci. Technol. 2014, 48, 5700–5708. [Google Scholar] [CrossRef]
- Obrador, A.; Novillo, J.; Alvarez, J.M. Mobility and availability to plants of two zinc sources applied to a calcareous soil. Soil Sci. Soc. Am. J. 2003, 67, 564–572. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef]
- Amer, F.; Rezk, A.I.; Khalid, H.M. Fertilizer efficiency in flooded calcareous soils. Soil Sci. Soc. Am. J. 1980, 44, 1025–1030. [Google Scholar] [CrossRef]
- Srivastava, P.C.; Singh, A.P.; Kumar, S.; Ramachandran, V.; Shrivastava, M.; D’souza, S.F. Comparative study of a Zn-enriched post-methanation bio-sludge and Zn sulfate as Zn sources for a rice–wheat crop rotation. Nutr. Cycl. Agroecosyst. 2009, 85, 195–202. [Google Scholar] [CrossRef]
- Lu, X.C.; Cui, J.; Tian, X.H.; Ogunniyi, J.E.; Gale, W.J.; Zhao, A.Q. Effects of zinc fertilization on zinc dynamics in potentially zinc-deficient calcareous soil. Agron. J. 2012, 104, 963–969. [Google Scholar] [CrossRef]
- Tye, A.M.; Young, S.D.; Crout, N.M.J.; Zhang, H.; Preston, S.; Barbosa-Jefferson, V.L.; Davison, W.; McGrath, S.P.; Paton, G.I.; Kilham, K.; et al. Predicting the activity of Cd2+ and Zn2+ in soil pore water from the radio-labile metal fraction. Geochim. Cosmochim. Acta 2003, 67, 375–385. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Tian, X.H.; Cao, Y.X.; Lu, X.C.; Liu, T. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 2014, 94, 2016–2022. [Google Scholar] [CrossRef]
- Joy, E.J.; Stein, A.J.; Young, S.D.; Ander, E.L.; Watts, M.J.; Broadley, M.R. Zinc-enriched fertilisers as a potential public health intervention in Africa. Plant Soil 2015, 389, 1–24. [Google Scholar] [CrossRef]
- Loneragan, J.F.; Grove, T.S.; Robson, A.D.; Snowball, K. Phosphorus Toxicity as a Factor in Zinc-Phosphorus Interactions in Plants. Soil Sci. Soc. Am. J. 1979, 43, 966–972. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Deng, Y.; Chen, R.Y.; Cui, Z.L.; Chen, X.P.; Yost, R.; Zhang, F.S.; Zou, C.Q. The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant Soil 2012, 361, 143–152. [Google Scholar] [CrossRef]
- Thompson, J.P. Soil sterilization methods to show VA-mycorrhizae aid P and Zn nutrition of wheat in vertisols. Soil Biol. Biochem. 1990, 22, 229–240. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Smith, S.E.; Smith, F.A. Zinc (Zn)-phosphorus (P) interactions in two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. Ann Bot. 2001, 88, 941–945. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J. Phosphorus reduces the zinc concentration in cereals pot-grown on calcareous Vertisols from southern Spain. J. Sci. Food Agric. 2017, 97, 3427–3432. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Chen, X.; Zou, C. Overuse of phosphorus fertilizer reduces the grain and flour protein contents and zinc bioavailability of winter wheat (Triticum aestivum L.). J. Agric. Food Chem. 2017, 65, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Recena, R.; García-López, A.M.; Torrent, J.; del Campillo, M.C. Phosphorus and Zinc uptake by sugar beet as affected by soil P status. In Proceedings of the XIV European Society for Agronomy Congress, Edinburgh, UK, 5–9 September 2020. [Google Scholar]
- Delgado, A.; Scalenghe, R. Aspects of phosphorus transfer from soils in Europe. J. Plant Nutr. Soil Sci. 2008, 171, 552–575. [Google Scholar] [CrossRef]
- Torrent, J. Phosphorus dynamics and uptake by wheat in a model calcite ferrihydrite system. Soil Sci. 2000, 165, 803–812. [Google Scholar]
- Pérez-Novo, C.; Bermúdez-Couso, A.; López-Periago, E.; Fernández-Calviño, D.; Arias-Estévez, M. Zinc adsorption in acid soils: Influence of phosphate. Geoderma 2011, 162, 358–364. [Google Scholar] [CrossRef]
- Zhao, K.; Selim, H.M. Adsorption-Desorption Kinetics of Zn in Soils: Influence of Phosphate. Soil Sci. 2010, 175, 145–153. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, R.; Xu, T.; Xu, Y.; Ge, F.; Xi, Y.; Zhu, J.; He, H. Co-adsorption of phosphate and zinc (II) on the surface of ferrihydrite. Chemosphere 2016, 144, 1148–1155. [Google Scholar] [CrossRef]
- Recena, R.; Fernández-Cabanás, V.M.; Delgado, A. Soil fertility assessment by Vis-NIR spectroscopy: Predicting soil functioning rather than availability indices. Geoderma 2019, 337, 368–374. [Google Scholar] [CrossRef]
- Staff, S.S. Keys to Soil Taxonomy; United States Department of Agriculture: Washington, DC, USA, 2014.
- Gee, G.W.; Bauder, J. Particle-size analysis 1. In Methods of Soil Analysis: Part 1-Physical and Mineralogical Methods; Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- De Santiago, A.; Delgado, A. Predicting iron chlorosis of lupin in calcareous Spanish soils from iron extracts. Soil Sci. Soc. Am. J. 2006, 70, 1945–1950. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circula 939; US Government Printing Office: Washington, DC, USA, 1954.
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chem. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- StatPoint Technologies. Statgraphics Centurion XVI; StatPoint Technologies: Warrenton, VA, USA, 2013. [Google Scholar]
- Cate, R.B.; Nelson, L.A. A simple statistical procedure for partitioning soil test correlation data into two classes. Soil Sci. Soc. Am. Proc. 1971, 35, 658–660. [Google Scholar] [CrossRef]
- Geng, X.; Guillard, K.; Morris, T.F. Relating turfgrass growth and quality to frequently measured soil nitrate. Crop Sci. 2014, 54, 366–382. [Google Scholar] [CrossRef]
- Recena, R.; Díaz, I.; Del Campillo, M.C.; Torrent, J.; Delgado, A. Calculation of threshold Olsen P values for fertilizer response from soil properties. Agron. Sustain. Dev. 2016, 36, 54. [Google Scholar] [CrossRef]
- Peng, S.; Wang, P.; Peng, L.; Cheng, T.; Sun, W.; Shi, Z. Predicting Heavy Metal Partition Equilibrium in Soils: Roles of Soil Components and Binding Sites. Soil Sci. Soc. Am. J. 2018, 82, 839–849. [Google Scholar] [CrossRef]
- Adriano, D.C.; Murphy, L.S. Effects of Ammonium Polyphosphates on Yield1 and Chemical Composition of Irrigated Corn. Agron. J. 1970, 62, 561–567. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Su, R.; Zhang, Z.; Chang, C.; Peng, Q.; Dong, Z.; Pang, J.; Lambers, H. Strong phosphorus (P)-zinc (Zn) interactions in a calcareous soil-alfalfa system suggest that rational P fertilization should be considered for Zn biofortification on Zn-deficient soils and phytoremediation of Zn-contaminated soils. Plant Soil 2021. [Google Scholar] [CrossRef]
- Pérez-Novo, C.; Fernández-Calviño, D.; Bermúdez-Couso, A.; López-Periago, J.E.; Arias-Estévez, M. Phosphorus effect on Zn adsorption–desorption kinetics in acid soils. Chemosphere 2011, 83, 1028–1034. [Google Scholar] [CrossRef]
- Wang, J.J.; Harrell, D.L. Effect of ammonium, potasium, and sodium cations and phosphate, nitrate, and chloride anions on zinc sorption and lability in selected acid and calcareous soils. Soil Sci. Soc. Am. J. 2005, 69, 1036–1046. [Google Scholar] [CrossRef]
- Saeed, M. Phosphate fertilization reduces zinc adsorption by calcareous soils. Plant Soil 1977, 48, 641–664. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Ternary Complex Formation of Phosphate with Ca and Mg Ions Binding to Ferrihydrite: Experiments and Mechanisms. ACS Earth Space Chem. 2020, 4, 545–557. [Google Scholar] [CrossRef]
- Sebei, H.; Minh, D.P.; Nzihou, A.; Sharrock, P. Sorption behavior of Zn(II) ions on synthetic apatitic calcium phosphates. Appl. Surf. Sci. 2015, 357, 1958–1966. [Google Scholar] [CrossRef][Green Version]



| Clay | CCE | Feca | Fecbd | pH | SOC | Olsen P | Initial DTPA–Zn | Final DTPA–Zn | Cumulative Zn Uptake | |
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | mg kg−1 | ||||||||
| Non-calcareous soil | ||||||||||
| Mean | 90.7 | 0 | 1.47 | 5.2 | 7.0 | 9.85 | 24.2 | 2.00 | 1.32 | 1.09 |
| SD | 52.1 | 0 | 0.50 | 1.8 | 0.4 | 4.04 | 16.3 | 1.90 | 1.34 | 0.45 |
| Range | 48–157 | 0 | 0.8–2.0 | 2.0–6.8 | 6.5–7.7 | 6.1–15 | 4.8–49.1 | 0.37–5.7 | 0.31–3.9 | 0.36–1.69 |
| Median | 67 | 0 | 1.60 | 5.5 | 7.0 | 7.80 | 19.6 | 1.43 | 0.96 | 1.11 |
| Calcareous soil | ||||||||||
| Mean | 334.1 | 311.7 | 0.89 | 5.66 | 7.99 | 10.9 | 14.76 | 0.62 | 0.47 | 0.46 |
| SD | 133.18 | 128.8 | 0.64 | 2.93 | 0.15 | 3.75 | 8.65 | 0.54 | 0.31 | 0.20 |
| Range | 175–640 | 28–723 | 0.2–2.4 | 2.1–13.2 | 7.7–8.3 | 5.5–20 | 5.0–32.8 | 0.12–2.46 | 0.10-1.18 | 0.23–0.99 |
| Median | 326.5 | 262 | 0.8 | 4.9 | 8.0 | 10.15 | 11.9 | 0.42 | 0.36 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recena, R.; García-López, A.M.; Delgado, A. Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties. Agronomy 2021, 11, 390. https://doi.org/10.3390/agronomy11020390
Recena R, García-López AM, Delgado A. Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties. Agronomy. 2021; 11(2):390. https://doi.org/10.3390/agronomy11020390
Chicago/Turabian StyleRecena, Ramiro, Ana M. García-López, and Antonio Delgado. 2021. "Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties" Agronomy 11, no. 2: 390. https://doi.org/10.3390/agronomy11020390
APA StyleRecena, R., García-López, A. M., & Delgado, A. (2021). Zinc Uptake by Plants as Affected by Fertilization with Zn Sulfate, Phosphorus Availability, and Soil Properties. Agronomy, 11(2), 390. https://doi.org/10.3390/agronomy11020390






