The Multiple Activities and the Plant Beneficial Potential of Bacillus spp. Derived from the Permafrost of Western Siberia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation Conditions
2.2. Screening and Selection of Active Isolates
2.2.1. Antifungal Activity
2.2.2. Determination of Chitinase Activity
2.2.3. Determination of Indole-3-Acetic acid Production
2.2.4. Design and Set Up of the Greenhouse Experiments
2.2.5. Design and Set Up of the Field Experiments
2.2.6. Assessment of Wheat Yield
2.2.7. Statistical Processing
3. Results
3.1. Effect of Cultivation Temperature on Antifungal Activity of the Permafrost-Derived Bacillus spp.
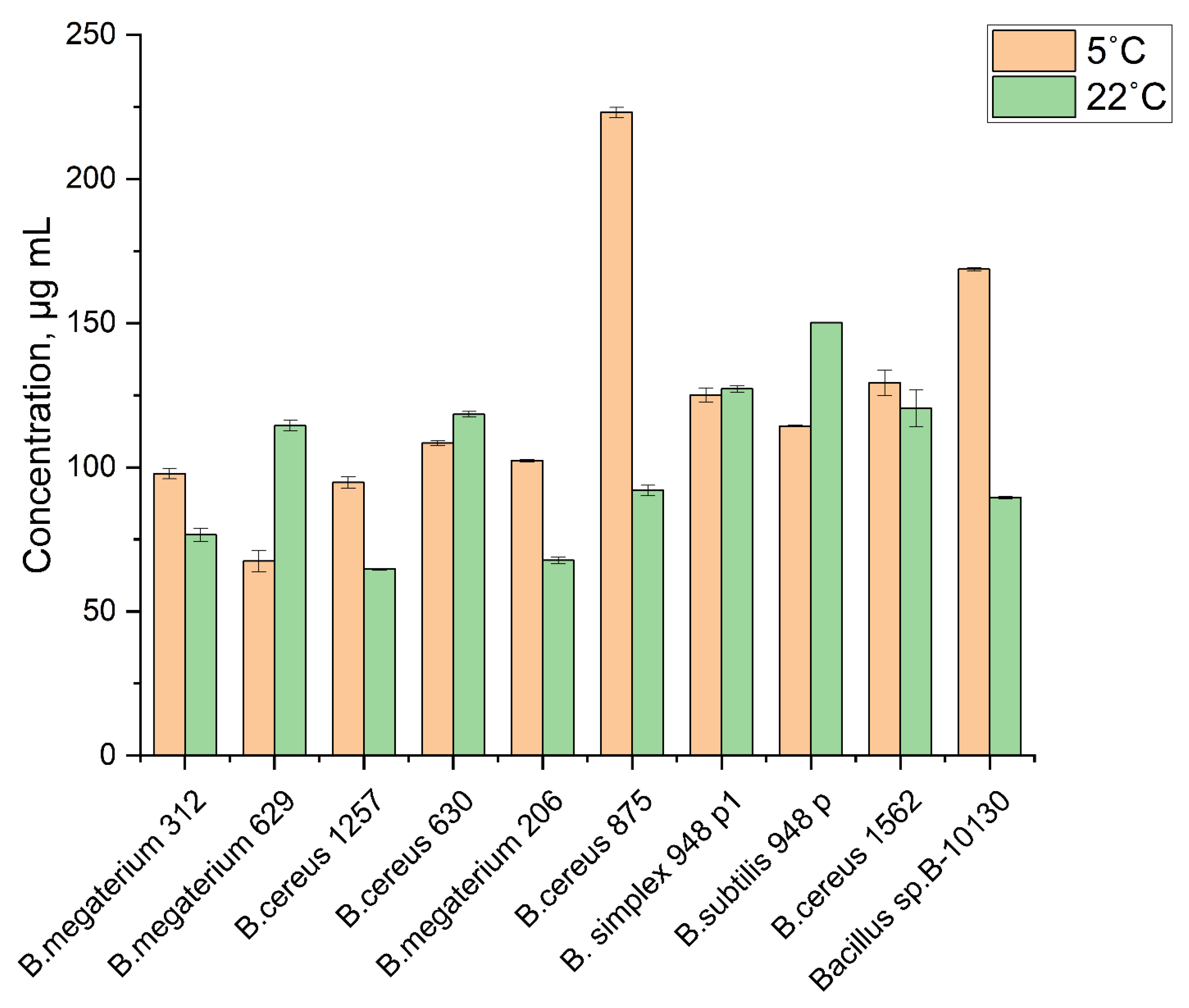
3.2. Chitinolytic Activity of Psychrotolerant Bacillus spp.
3.3. Auxin-Producing Activity in the Permafrost-Derived Bacillus spp.
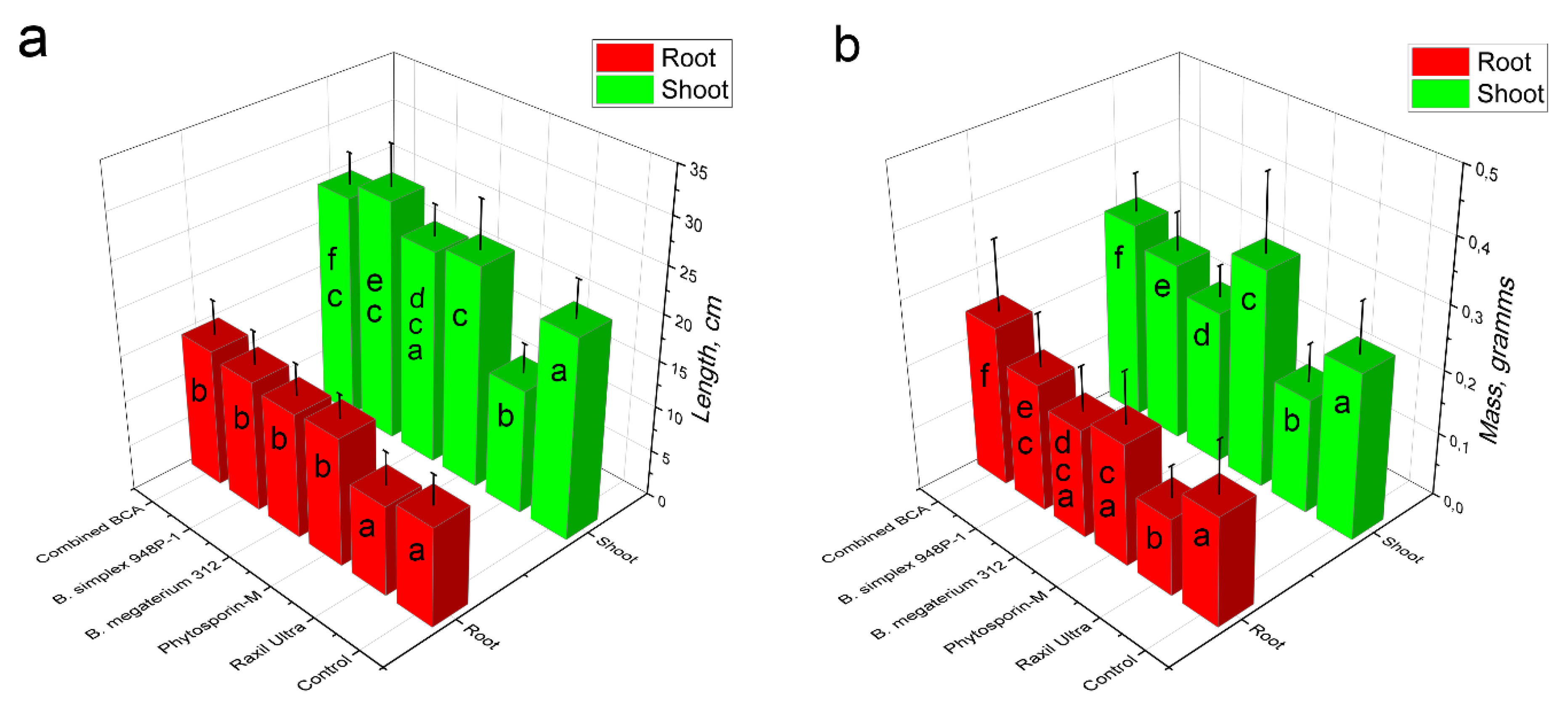
3.4. Growth-Stimulating Activity of Psychrotolerant Bacillus spp.
3.5. Assessment of Wheat Yield
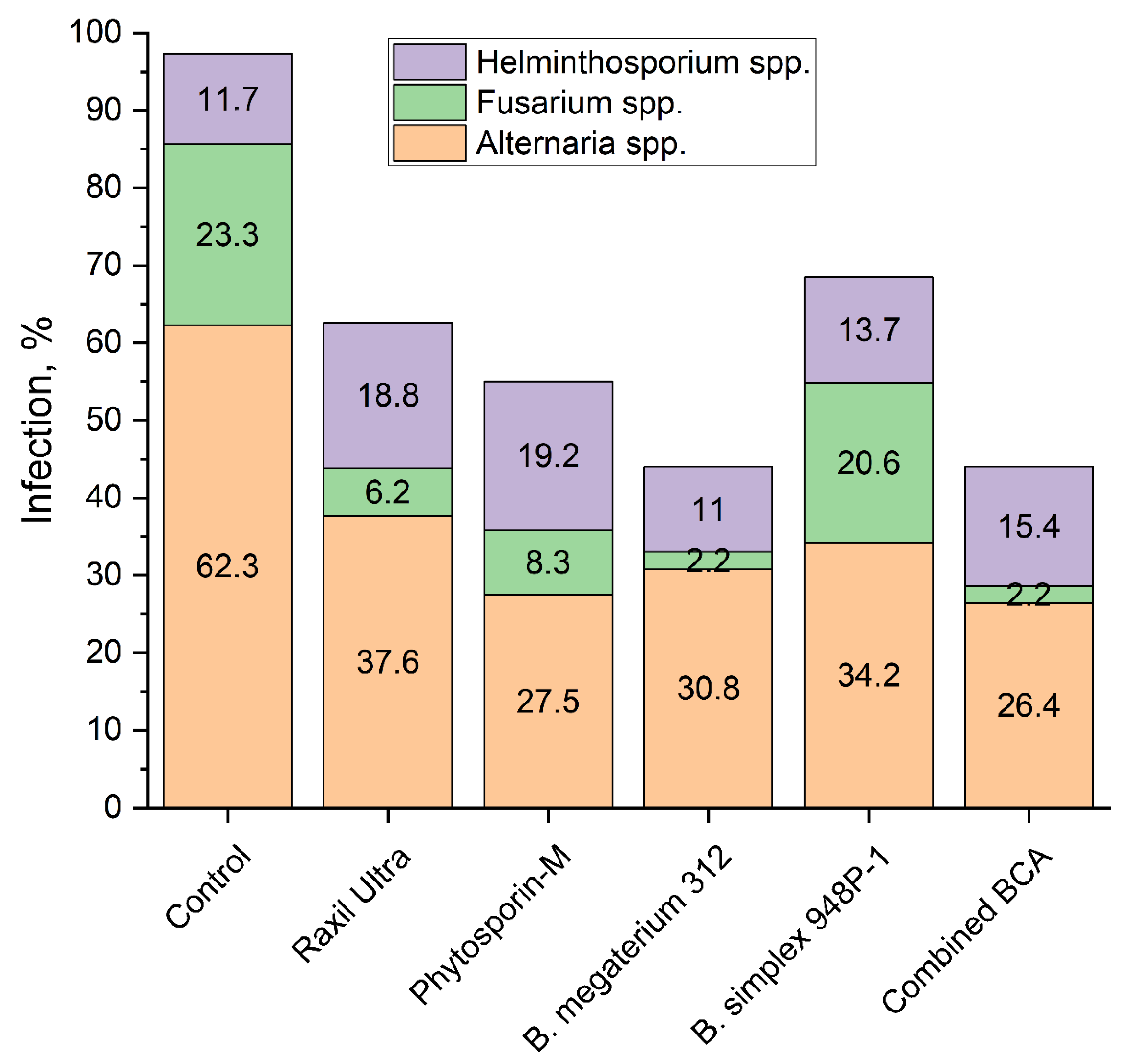
3.6. Phyto-Examination of the First Generation Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ewert, F.; Rounsevell, M.; Reginster, I.; Metzger, M.; Leemans, R. Future scenarios of European agricultural land use: I. Estimating changes in crop productivity. Agric. Ecosyst. Environ. 2005, 107, 101–116. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Hannukkala, A.; Huusela-Veistola, E.; Voutila, L.; Niemi, J.; Valaja, J.; Jauhiainen, L.; Hakala, K. Potential and realities of enhancing rapeseed- and grain legume-based protein production in a northern climate. J. Agric. Sci. 2013, 151, 303–321. [Google Scholar] [CrossRef]
- Ruelland, E.; Collin, S. Chilling stress. In Plant Stress Physiology; Shabala, S., Ed.; CABI Publishing: Wallingford, UK, 2011; pp. 94–118. [Google Scholar]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Mageswari, A.; Selvakumar, G.; Sa, T. Cold Stress Tolerance in Psychrotolerant Soil Bacteria and Their Conferred Chilling Resistance in Tomato (Solanum lycopersicum Mill.) under Low Temperatures. PLoS ONE 2016, 11, e0161592. [Google Scholar] [CrossRef]
- Tibbett, M.; Sanders, F.; Cairney, J. Low-temperature-induced changes in trehalose, mannitol and arabitol associated with enhanced tolerance to freezing in ectomycorrhizal basidiomycetes (Hebeloma spp.). Mycorrhiza 2002, 12, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Küdela, V. Potential impact of climate change on geographic distribution of plant pathogenic bacteria in Central Europe. Plant Prot. Sci. 2010, 45, S27–S32. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, J.P.; Lee, R.E. Supercooling and ice nucleation in vertebrate ectotherms. In Biological Ice Nucleation and Its Applications; American Phytopathological Society: St. Paul, MN, USA, 1995. [Google Scholar]
- Barford, E. Crop pests advancing with global warming. Nature 2013. [Google Scholar] [CrossRef]
- Prank, M.; Kenaley, S.C.; Bergstrom, G.; Acevedo, M.; Mahowald, N.M. Climate change impacts the spread potential of wheat stem rust, a significant crop disease. Environ. Res. Lett. 2019, 14, 124053. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.; Kalam, S.; Sayyed, R.; Reddy, M.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Fu, Y.; Kang, W.; Li, H.; Gao, H.; Vitalievitch, K.S.; Liu, H. Isolation and identification of a cold-adapted bacterium and its characterization for biocontrol and plant growth-promoting activity. Ecol. Eng. 2017, 105, 362–369. [Google Scholar] [CrossRef]
- Pandey, A.; Yarzábal, L.A. Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments. Appl. Microbiol. Biotechnol. 2019, 103, 643–657. [Google Scholar] [CrossRef]
- Wu, H.; Gu, Q.; Xie, Y.; Lou, Z.; Xue, P.; Fang, L.; Yu, C.; Jia, D.; Huang, G.; Zhu, B.; et al. Cold-adaptedBacilliisolated from the Qinghai–Tibetan Plateau are able to promote plant growth in extreme environments. Environ. Microbiol. 2019, 21, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Cortesão, M.; Fuchs, F.M.; Commichau, F.; Eichenberger, P.; Schuerger, A.C.; Nicholson, W.L.; Setlow, P.; Moeller, R. Bacillus subtilis Spore Resistance to Simulated Mars Surface Conditions. Front. Microbiol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domanskaya, O.V.; Melnikov, V.P.; Ogurtsova, L.V.; Soromotin, A.V.; Domanskii, V.O.; Polyakova, N.V. Some features of enzyme activity in different strains of the Bacillus genus isolated from permafrost. Earth’s Cryosphere 2017, XXI, 53–59. [Google Scholar] [CrossRef]
- Valgas, C.; De Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Kabana, R.; Godoy, G.; Morgan-Jones, G.; Shelby, R.A. The determination of soil chitinase activity: Conditions for assay and ecological studies. Plant Soil 1983, 75, 95–106. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric Estimation of Indoleacetic Acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, S.C.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Characterisation of a psychrotolerant plant growth promoting Pseudomonas sp. strain PGERs17 (MTCC 9000) isolated from North Western Indian Himalayas. Ann. Microbiol. 2008, 58, 561–568. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, R.; Ning, H.; Li, W.; Bai, Y.; Li, Y. Evaluation and management of fungal-infected carrot seeds. Sci. Rep. 2020, 10, 10808. [Google Scholar] [CrossRef]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. Introductory Mycology, 4th ed.; John Willey and Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Houwenhuyse, S.; Macke, E.; Reyserhove, L.; Bulteel, L.; Decaestecker, E. Back to the future in a petri dish: Origin and impact of resurrected microbes in natural populations. Evol. Appl. 2018, 11, 29–41. [Google Scholar] [CrossRef]
- Yarzábal, L.A.; Salazar, L.M.B.; Batista-García, R.A. Climate change, melting cryosphere and frozen pathogens: Should we worry…? Environ. Sustain. 2021, 4, 489–501. [Google Scholar] [CrossRef]
- Hough, M.; McClure, A.; Bolduc, B.; Dorrepaal, E.; Saleska, S.; Klepac-Ceraj, V.; Rich, V. Biotic and Environmental Drivers of Plant Microbiomes Across a Permafrost Thaw Gradient. Front. Microbiol. 2020, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Brouchkov, A.; Melnikov, V.; Kalenova, L.; Fursova, O.; Pogorelko, G.; Potapov, V.; Fursova, N.; Ignatov, S.; Brenner, E.; Bezrukov, V.; et al. Permafrost Bacteria in Biotechnology: Biomedical Applications. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Singapore, 2017; pp. 541–554. [Google Scholar]
- Mishra, P.K.; Joshi, P.; Bisht, S.C.; Bisht, J.K.; Selvakumar, G. Cold-Tolerant Agriculturally Important Microorganisms. In Plant Growth and Health Promoting Bacteria; Springer: Singapore, 2010; pp. 273–296. [Google Scholar]
- Jallouli, W.; Driss, F.; Fillaudeau, L.; Rouis, S. Review on biopesticide production by Bacillus thuringiensis subsp. kurstaki since 1990: Focus on bioprocess parameters. Process. Biochem. 2020, 98, 224–232. [Google Scholar] [CrossRef]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.; DAS, A.; Samadder, S.; Rajan, S.S. Biosynthesis and characterization of a thermostable, alkali-tolerant chitinase from Bacillus pumilus JUBCH08 displaying antagonism against phytopathogenic Fusarium oxysporum. 3 Biotech 2016, 6, 87. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Zavala, S.A.; Barboza-Perez, U.E.; Hernández-Guzmán, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, Modular Structure, and Applied Potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef] [Green Version]
- Senol, M.; Nadaroglu, H.; Dikbas, N.; Kotan, R. Purification of Chitinase enzymes from Bacillus subtilis bacteria TV-125, investigation of kinetic properties and antifungal activity against Fusarium culmorum. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Honda, S.; Kunii, T.; Nohara, K.; Wakita, S.; Sugahara, Y.; Kawakita, M.; Oyama, F.; Sakaguchi, M. Characterization of a Bacillus thuringiensis chitinase that binds to cellulose and chitin. AMB Express 2017, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, E. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.-X.; Wang, W.-S.; Gong, W.; Liu, W.-C.; Chen, H.-G.; Xu, H.-H.; Lu, Y.-T. Low Temperature Inhibits Root Growth by Reducing Auxin Accumulation via ARR1/12. Plant Cell Physiol. 2014, 56, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, W.; Östin, A.; Sandberg, G.; Romano, C.P.; Estelle, M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7197–7202. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, G.; Mohan, M.; Kundu, S.; Gupta, A.; Joshi, P.; Nazim, S.; Gupta, H. Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo). Lett. Appl. Microbiol. 2007, 46, 171–175. [Google Scholar] [CrossRef]
- Luziatelli, F.; Melini, F.; Bonini, P.; Melini, V.; Cirino, V.; Ruzzi, M. Production of Indole Auxins by Enterobacter sp. Strain P-36 under Submerged Conditions. Fermentation 2021, 7, 138. [Google Scholar] [CrossRef]
- Chagas Junior, A.F.; De Oliveira, A.G.; De Oliveira, L.A.; Dos Santos, G.; Chagas, L.F.B.; da Silva, A.L.; Costa, J.D.L. Production of indole-3-acetic acid by Bacillus isolated from different soils. Bulg. J. Agric. Sci. 2015, 21, 282–287. [Google Scholar]
- Swain, M.; Naskar, S.; Ramesh, R. Indole-3acetic acid production and effect on sprouting of yam (Dioscorea rotundata L.) minisetts by Bacillus subtilis isolated from culturable cowdung microflora. Pol. J. Microbiol. 2007, 56, 103–110. [Google Scholar] [PubMed]
- Deising, H.B.; Gase, I.; Kubo, Y. The unpredictable risk imposed by microbial secondary metabolites: How safe is biological control of plant diseases? J. Plant Dis. Prot. 2017, 124, 413–419. [Google Scholar] [CrossRef]
- Koch, E.; Becker, J.O.; Berg, G.; Hauschild, R.; Jehle, J.; Kohl, J.; Smalla, K. Biocontrol of plant diseases is not an unsafe technology! J. Plant Dis. Prot. 2018, 125, 121–125. [Google Scholar] [CrossRef]
- Kiewnick, S. Practicalities of developing and registering microbial biologicalcontrol agents. CAB Rev. 2007, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Camargo, A.F.; Venturin, B.; Bordin, E.R.; Scapini, T.; Stefanski, F.; Klanovicz, N.; Dalastra, C.; Kubeneck, S.; Preczeski, K.P.; Rossetto, V.; et al. A Low-Genotoxicity Bioherbicide Obtained from Trichoderma koningiopsis Fermentation in a Stirred-Tank Bioreactor. Ind. Biotechnol. 2020, 16, 176–181. [Google Scholar] [CrossRef]
- Curbelo, A.; Mancebo, A.; Molier, T.; Arteaga, M.E.; González, C.; Grandía, R.; Bada, A.M.; Rivero, Y.; García, A.; Legró, M.; et al. Assessment of the in vivo genotoxicity of a new formulation of Bacillus thuringiensis var israelensis SH-14. Toxicol. Environ. Chem. 2011, 93, 691–699. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Sharma, C.K.; Vishnoi, V.K.; Dubey, R.; Maheshwari, D. A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 2018, 5, 71–75. [Google Scholar] [CrossRef]
| Strains | t °C | Growth Inhibition Zones, mm | |||
|---|---|---|---|---|---|
| Alternaria sp. | F. avenaceum | F. graminearum | M. nivale | ||
| B. megaterium 312 | 5 | 21.0 ± 2.12 | 17.0 ± 1.41 | 13.0 ± 1.06 | 23.0 ± 0.14 |
| 22 | 0 | 0 | 0 | 0 | |
| B. cereus 875 | 5 | 0 | 0 | 0 | 0 |
| 22 | 0 | 5.0 ± 1.14 | 8.0 ± 2.8 | 0 | |
| Strains | Cultivation Temperature | |
|---|---|---|
| 5 °C | 22 °C | |
| B. simplex 948 P1 | 16.0 ± 3.6 | 95.2 ± 10.8 |
| B. cereus 1257 | 14.7 ± 2.4 | 4.0 ± 2.22 |
| B. cereus 875 | 11.5 ± 0.8 | 5.2 ± 0.53 |
| Bacillus sp.B-10130 | 8.8 ± 1.3 | 28.8 ± 5.8 |
| B. cereus 1562 | 8.5 ± 0.9 | 28.0 ± 4.1 |
| B. megaterium 206 | 7.8 ± 1.5 | 5.2 ± 0.7 |
| B. megaterium 312 | 7.7 ± 0.8 | 17.0 ± 2.9 |
| B. cereus 630 | 6.8 ± 0.6 | 58.0 ± 1.07 |
| B. subtilius 948P | 6.5 ± 0.9 | 4.5 ± 0.5 |
| B. megaterium 629 | 5.0 ± 0.8 | 27.5 ± 6.2 |
| Experimental Groups | The Number of Grains per Ear, pcs. | Weight of 1000 Grains, g | Yield, g/m2 |
|---|---|---|---|
| Control | 45.8 ± 11.70 | 38.31 | 395.23 |
| Raxil-Ultra | 42.73 ± 9.63 | 35.9 | 400.40 |
| Phytosporin-M | 42.67 ± 9.66 | 35.72 | 331.20 |
| B. megaterium 312 | 38.26 ± 9.74 | 38.93 | 357.86 |
| B. simplex 948P-1 | 41.27 ± 2.46 * | 34.51 | 285.12 |
| combined BCA | 46.13 ± 8.57 | 39.73 | 593.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domanskaya, O.V.; Bome, N.A.; Iashnikov, A.V.; Vasilchenko, A.V.; Vasilchenko, A.S. The Multiple Activities and the Plant Beneficial Potential of Bacillus spp. Derived from the Permafrost of Western Siberia. Agronomy 2021, 11, 2347. https://doi.org/10.3390/agronomy11112347
Domanskaya OV, Bome NA, Iashnikov AV, Vasilchenko AV, Vasilchenko AS. The Multiple Activities and the Plant Beneficial Potential of Bacillus spp. Derived from the Permafrost of Western Siberia. Agronomy. 2021; 11(11):2347. https://doi.org/10.3390/agronomy11112347
Chicago/Turabian StyleDomanskaya, Olga V., Nina A. Bome, Aleksandr V. Iashnikov, Anastasia V. Vasilchenko, and Alexey S. Vasilchenko. 2021. "The Multiple Activities and the Plant Beneficial Potential of Bacillus spp. Derived from the Permafrost of Western Siberia" Agronomy 11, no. 11: 2347. https://doi.org/10.3390/agronomy11112347
APA StyleDomanskaya, O. V., Bome, N. A., Iashnikov, A. V., Vasilchenko, A. V., & Vasilchenko, A. S. (2021). The Multiple Activities and the Plant Beneficial Potential of Bacillus spp. Derived from the Permafrost of Western Siberia. Agronomy, 11(11), 2347. https://doi.org/10.3390/agronomy11112347







