Effective Biocontrol of Rice Blast through Dipping Transplants and Foliar Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kenya
2.2. Tanzania
2.3. Data Analysis
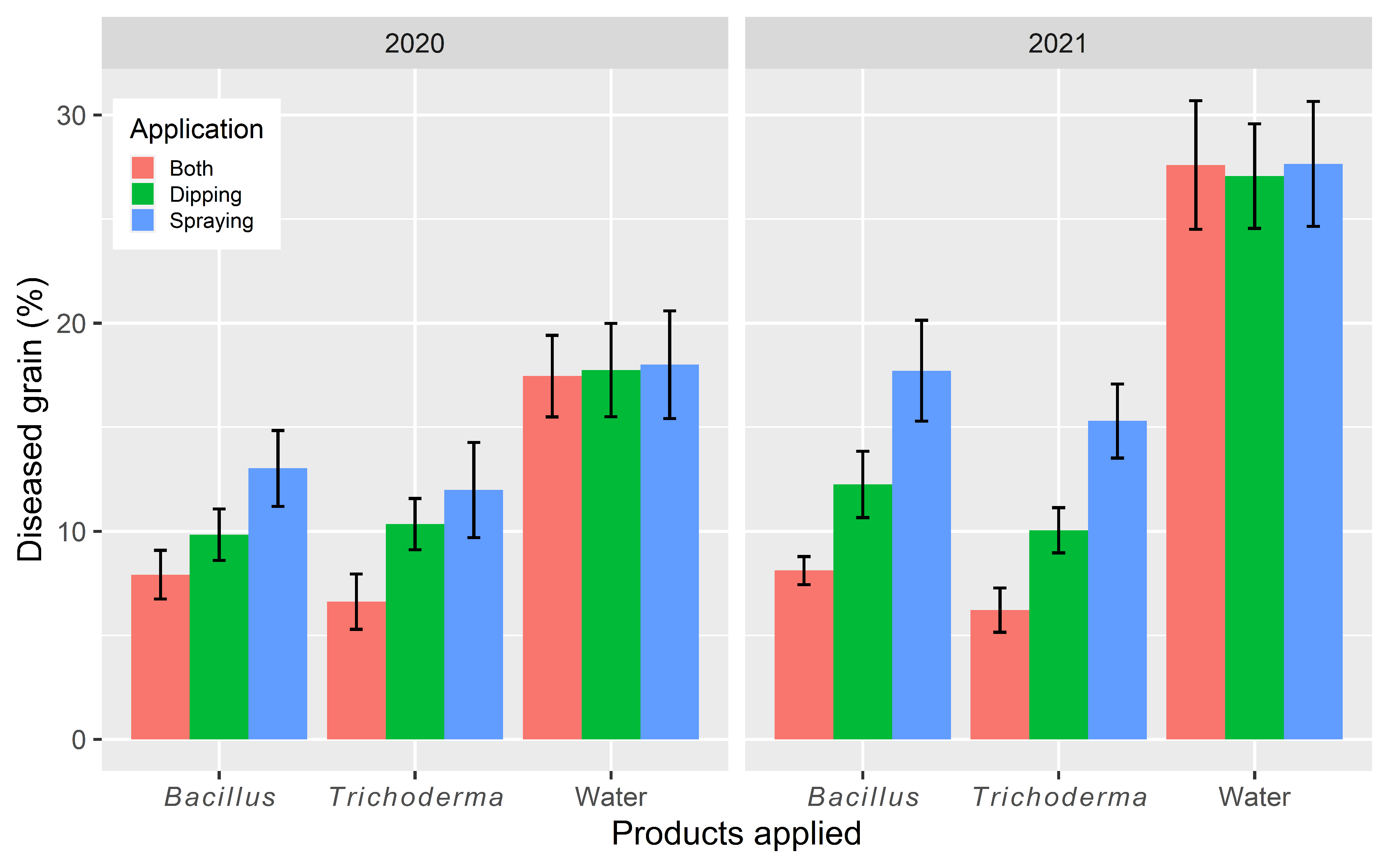
3. Results
3.1. Kenya Experiments
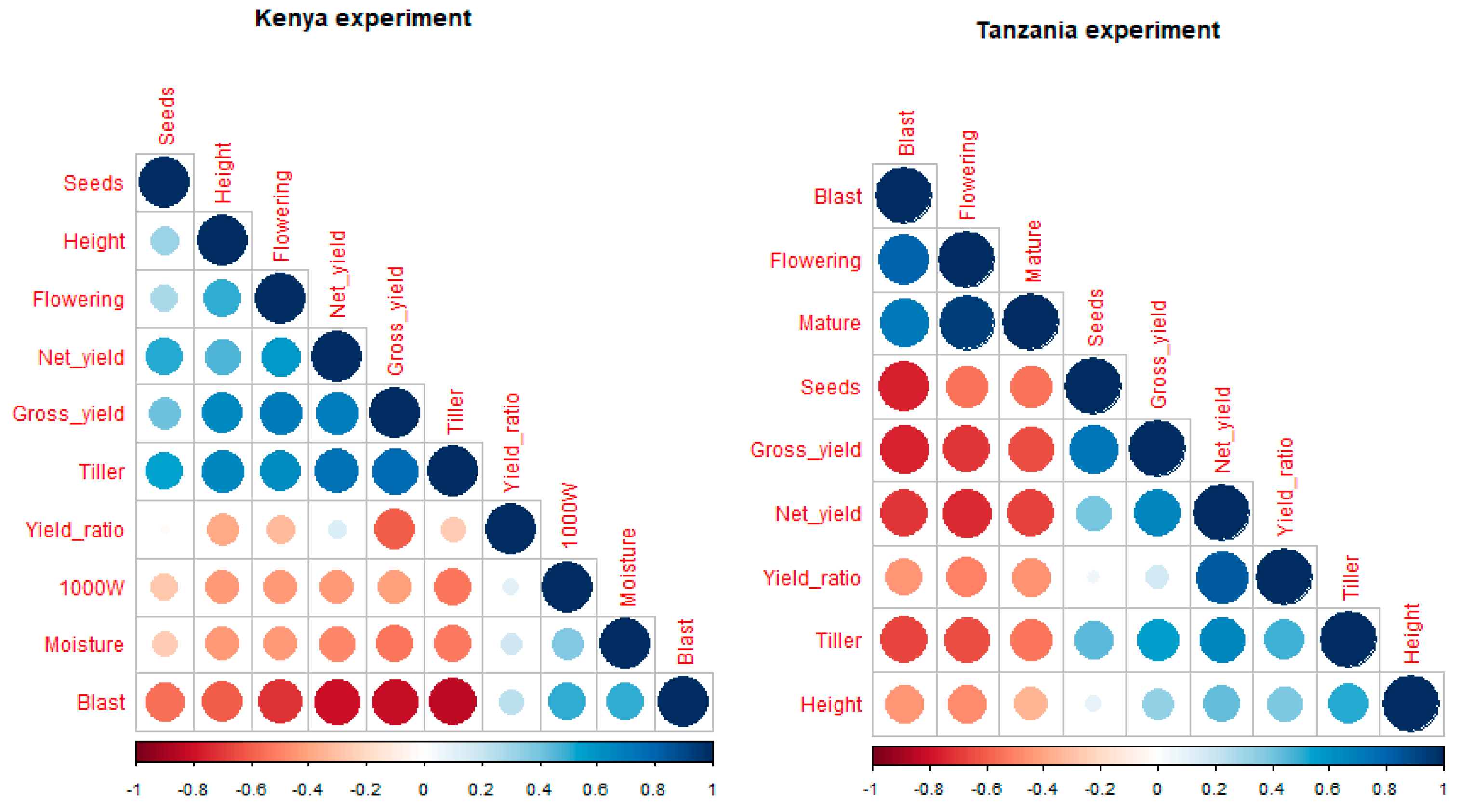
3.1.1. Overall Correlation among Variables
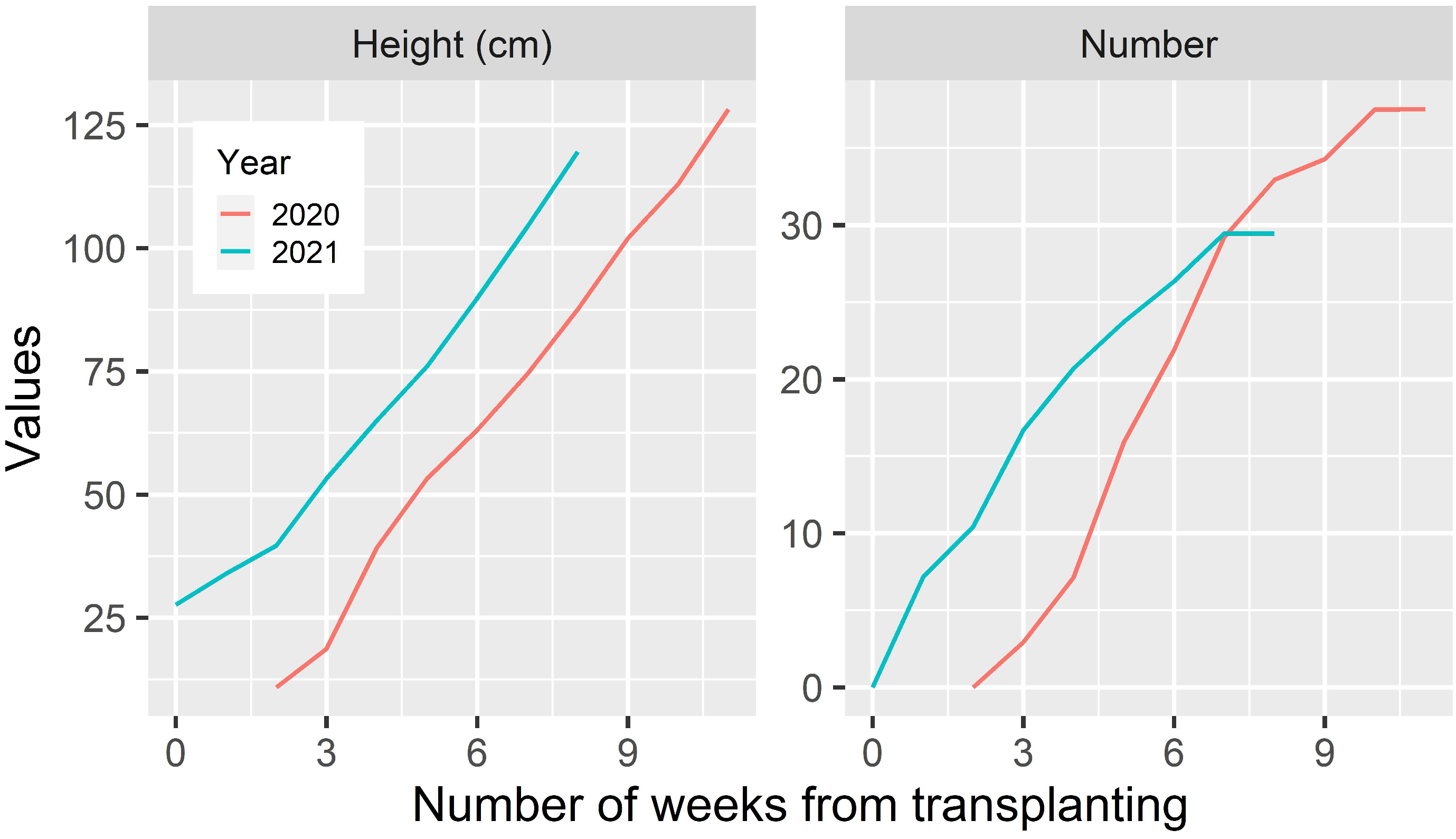
3.1.2. Tiller Number and Height
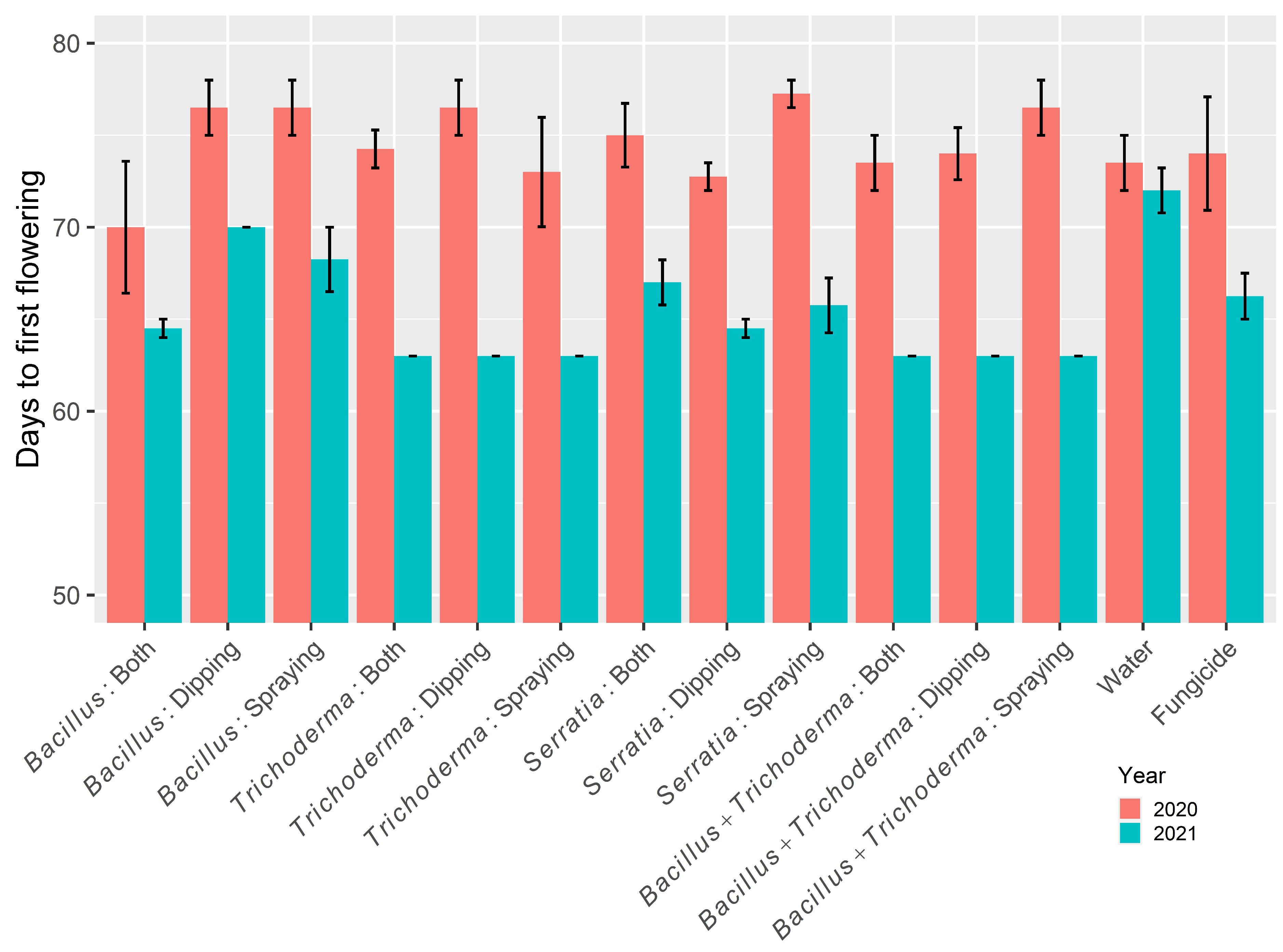
3.1.3. Days to First Flowering
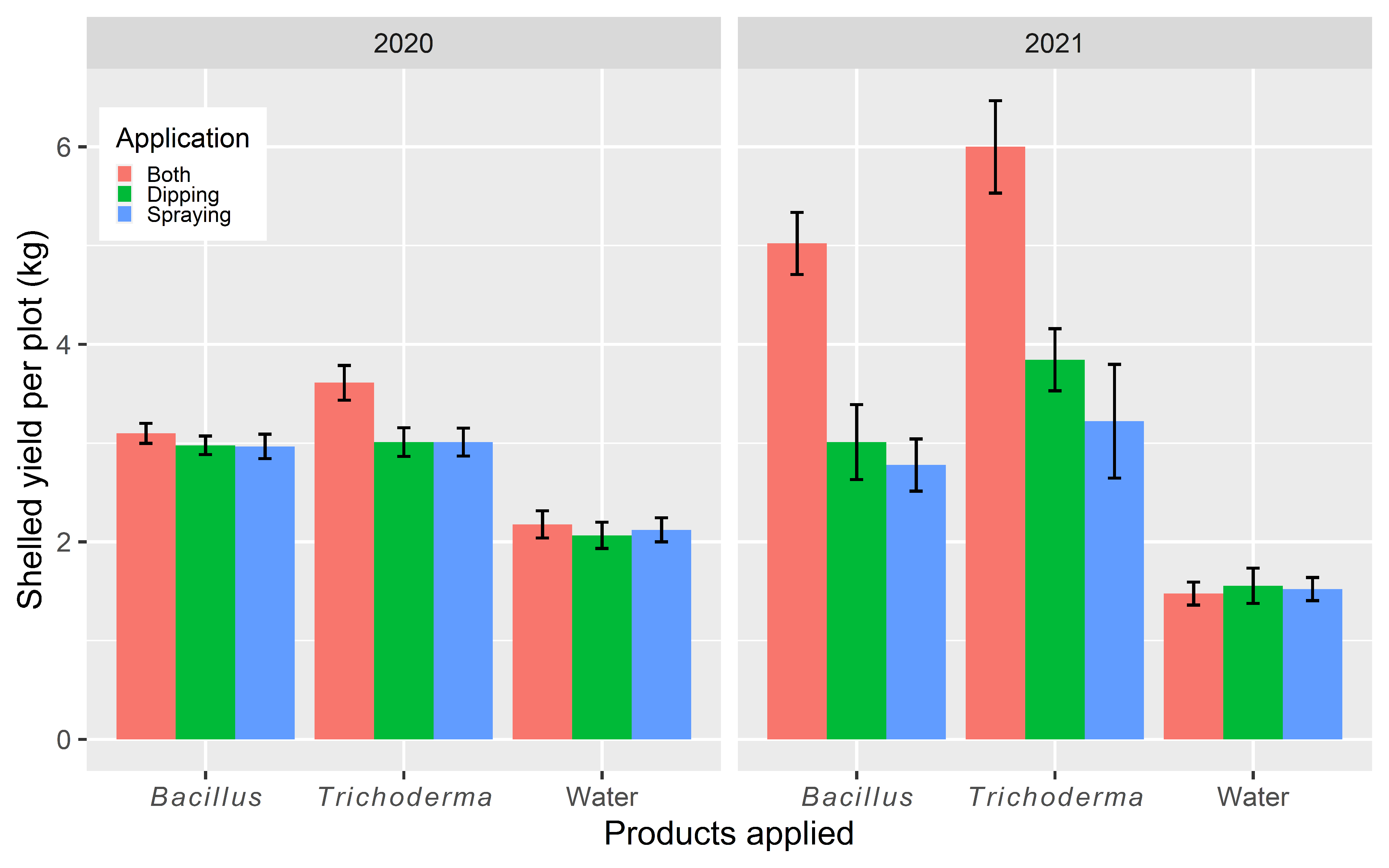
3.1.4. Yield Data
3.1.5. Blast Development
3.2. Tanzania Experiments
3.2.1. Overall Correlation among Variables
3.2.2. Tiller Number and Height
3.2.3. Time to 30% Flowering
3.2.4. Yield
3.2.5. Blast Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zenna, N.; Senthilkumar, K.; Sie, M. Rice Production in Africa. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 117–135. ISBN 978-3-319-47516-5. [Google Scholar]
- Seck, P.A.; Touré, A.A.; Coulibaly, J.Y.; Diagne, A.; Wopereis, M.C.S. Africa’s rice economy before and after the 2008 rice crisis. In Realizing Africa’s Rice Promise; Wompereis, M.C.S., Johnson, D.E., Ahmadi, N., Tollens, E., Jalloh, A., Eds.; CABI: Wallingford, UK, 2013; pp. 24–34. [Google Scholar] [CrossRef] [Green Version]
- International Rice Research Institute. Rice Blast Disease; Zeigler, R.S., Leong, S.A., Teng, P.S., Eds.; C.A.B. International: Wallingford, UK, 1994; p. 626. [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Rice Blast: A disease with implications for global food security. Agronomy 2019, 9, 451. [Google Scholar] [CrossRef] [Green Version]
- Besi, M.I.; Tucker, S.L.; Sesma, A. Magnaporthe and its relatives. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–9. [Google Scholar]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Spence, C.; Alff, E.; Johnson, C.; Ramos, C.; Donofrio, N.; Sundaresan, V.; Bais, H. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014, 14, 130. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Li, Y.; Zeng, X.; Yang, X.; Yang, Y.; Yuan, S.; Hu, X.; Zeng, J.; Wang, Z.; Liu, Q.; et al. Isolation and evaluation of endophytic Streptomyces endus OsiSh-2 with potential application for biocontrol of rice blast disease. J. Sci. Food Agric. 2017, 97, 1149–1157. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Dong, Y.; Zhao, L.; Rong, S.; Chen, W.; Lv, M.; Xu, H.; Gao, X.; Chen, R.; et al. Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae. PLoS ONE 2018, 13, e0203505. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-C.; Chiou, T.-Y.; Delgado, A.L.; Liao, C.-S. The control of rice blast disease by the novel biofungicide formulations. Sustainability 2019, 11, 3449. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Sharma, P. Biological control of rice blast disease with Trichoderma spp. under upland rice system. Oryza 2016, 53, 167–173. [Google Scholar]
- Soenens, A.; Imperial, J. Biocontrol capabilities of the genus Serratia. Phytochem. Rev. 2019, 19, 577–587. [Google Scholar] [CrossRef]
- Mahdi, I.; Hafidi, M.; Allaoui, A.; Biskri, L. Halotolerant endophytic bacterium Serratia rubidaea ED1 enhances phosphate solubilization and promotes seed germination. Agriculture 2021, 11, 224. [Google Scholar] [CrossRef]
- Vidhyasekaran, P.; Rabindran, R.; Muthamilan, M.; Nayar, K.; Rajappan, K.; Subramanian, N.; Vasumathi, K. Development of a powder formulation of Pseudomonas fluorescens for control of rice blast. Plant Pathol. 1997, 46, 291–297. [Google Scholar] [CrossRef]
- Chou, C.; Castilla, N.; Hadi, B.; Tanaka, T.; Chiba, S.; Sato, I. Rice blast management in Cambodian rice fields using Trichoderma harzianum and a resistant variety. Crop. Prot. 2020, 135, 104864. [Google Scholar] [CrossRef]
- Filippi, M.C.C.; da Silva, G.B.; Silva-Lobo, V.L.; Côrtes, M.V.C.B.; Moraes, A.J.G.; Prabhu, A.S. Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biol. Control 2011, 58, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Nadarajah, K. Evaluating the efficacy of Trichoderma spp and Bacillus substilis as biocontrol agents against Magnaporthe grisea in rice. AJCS 2014, 8, 1324–1335. [Google Scholar]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant. Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N.; Karaoglu, S.A.; Guzel, S.; Bozdeveci, A. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Bai, Y.; Souleimanov, A.; Smith, D.L. An inducible activator produced by a Serratia proteamaculans strain and its soybean growth-promoting activity under greenhouse conditions. J. Exp. Bot. 2002, 53, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 15 November 2021).
- Sesma, A.; Osbourn, A. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 2004, 431, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Kato, H. Dynamic simulation of blast epidemics using a multiple canopy spore dispersal model. In Proceedings of the International Rice Research Conference on the Rice Blast Modeling and Forecasting, Suweon, Korea, 27–31 August 1990; International Rice Research Institute: Manila, Philippines, 1991; pp. 75–88. [Google Scholar]
- Rijal, S.; Devkota, Y. A review on various management method of rice blast disease. Malays. J. Sustain. Agric. 2020, 4, 29–33. [Google Scholar] [CrossRef]




| Treatment | Days to First Flowering | Tiller on the Final Assessment Date | Yield (kg) | Seeds per Panicle | 1000 Seed Weight (g) | Seed Moisture (%) | Seeds with Blast (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Height (cm) | Gross | Net | Ratio (%) | ||||||
| Bacillus: Both | 67.3 ± 1.97 | 34.8 ± 1.53 | 123.5 ± 1.97 | 37.1 ± 2.42 | 9.5 ± 0.38 | 26.0 ± 1.33 | 106.5 ± 2.31 | 21.5 ± 0.53 | 13.7 ± 0.54 | 3.0 ± 0.72 |
| Bacillus: Dipping | 73.3 ± 1.41 | 32.2 ± 1.46 | 120.8 ± 2.82 | 35.9 ± 2.25 | 8.7 ± 0.51 | 24.4 ± 0.87 | 95.5 ± 3.45 | 21.4 ± 0.32 | 13.7 ± 0.38 | 3.7 ± 0.88 |
| Bacillus: Spraying | 72.4 ± 1.89 | 34.3 ± 0.85 | 127.2 ± 2.12 | 35.8 ± 2.66 | 9.0 ± 0.47 | 25.7 ± 1.32 | 104.0 ± 1.40 | 21.3 ± 0.51 | 13.9 ± 0.43 | 3.1 ± 0.63 |
| Trichoderma: Both | 68.6 ± 2.18 | 32.0 ± 2.11 | 123.1 ± 2.29 | 36.9 ± 2.28 | 8.9 ± 0.51 | 24.1 ± 0.62 | 100.6 ± 2.91 | 21.1 ± 0.44 | 13.3 ± 0.36 | 3.9 ± 1.07 |
| Trichoderma: Dipping | 69.8 ± 2.64 | 33.9 ± 2.01 | 125.1 ± 2.43 | 38.0 ± 1.94 | 9.0 ± 0.47 | 23.8 ± 0.80 | 97.7 ± 2.90 | 20.9 ± 0.38 | 13.7 ± 0.35 | 3.9 ± 1.10 |
| Trichoderma: Spraying | 68.0 ± 2.34 | 34.7 ± 2.01 | 124.9 ± 2.67 | 36.4 ± 2.56 | 8.4 ± 0.32 | 23.7 ± 1.42 | 102.2 ± 2.04 | 21.4 ± 0.32 | 13.0 ± 0.30 | 3.7 ± 1.04 |
| Serratia: Both | 71.0 ± 1.8 | 35.1 ± 1.17 | 123.8 ± 1.96 | 35.9 ± 2.55 | 9.8 ± 0.24 | 28.1 ± 1.56 | 107.0 ± 3.13 | 21.3 ± 0.47 | 14.1 ± 0.26 | 3.2 ± 0.74 |
| Serratia: Dipping | 68.6 ± 1.61 | 32.8 ± 1.46 | 123.0 ± 2.86 | 35.1 ± 3.02 | 9.7 ± 0.34 | 28.9 ± 2.59 | 101.2 ± 2.07 | 20.7 ± 0.52 | 13.0 ± 0.31 | 3.4 ± 0.89 |
| Serratia: Spraying | 71.5 ± 2.31 | 34.2 ± 1.37 | 125.3 ± 1.66 | 37.6 ± 2.66 | 9.3 ± 0.42 | 25.4 ± 1.70 | 104.0 ± 2.30 | 21.7 ± 0.27 | 13.5 ± 0.35 | 3.4 ± 0.90 |
| Bacillus + Trichoderma: Both | 68.3 ± 2.1 | 34.4 ± 2.35 | 123.4 ± 2.35 | 35.8 ± 2.43 | 8.9 ± 0.47 | 25.2 ± 0.80 | 101.7 ± 3.38 | 21.1 ± 0.38 | 13.6 ± 0.44 | 3.6 ± 0.88 |
| Bacillus + Trichoderma: Dipping | 68.5 ± 2.18 | 33.0 ± 1.81 | 125.4 ± 2.69 | 36.4 ± 2.39 | 8.4 ± 0.42 | 23.7 ± 1.69 | 99.9 ± 4.15 | 21.2 ± 0.37 | 13.0 ± 0.35 | 3.9 ± 1.09 |
| Bacillus + Trichoderma: Spraying | 69.8 ± 2.64 | 33.9 ± 2.22 | 122.8 ± 2.49 | 36.1 ± 2.71 | 9.1 ± 0.45 | 25.9 ± 1.71 | 101.6 ± 4.45 | 20.8 ± 0.69 | 13.6 ± 0.21 | 3.5 ± 0.86 |
| Water | 72.8 ± 0.94 | 30.0 ± 1.56 | 123.8 ± 2.25 | 35.8 ± 2.51 | 8.1 ± 0.60 | 22.8 ± 1.26 | 86.9 ± 5.45 | 21.7 ± 0.21 | 13.9 ± 0.35 | 6.0 ± 0.88 |
| Fungicide | 70.1 ± 2.13 | 33.6 ± 2.05 | 122.3 ± 2.97 | 38.4 ± 3.30 | 9.6 ± 0.71 | 25.4 ± 1.47 | 101.4 ± 2.98 | 21.2 ± 0.51 | 14.0 ± 0.65 | 2.2 ± 0.56 |
| Treatment 1 | Treatment 2 | Differences | Adjust p-Value |
|---|---|---|---|
| Tiller number on the final assessment date | |||
| Water | Bacillus: Both | −4.8 | 0.017 |
| Water | Bacillus + Trichoderma: Both | −4.4 | 0.045 |
| Water | Trichoderma: Spraying | −4.7 | 0.022 |
| Water | Serratia: Both | −5.1 | 0.008 |
| Number of seeds per panicle (on the natural logarithm scale) | |||
| Water | Bacillus: Both | −0.218 | <0.001 |
| Water | Bacillus: Spraying | −0.194 | 0.001 |
| Water | Trichoderma: Both | −0.158 | 0.022 |
| Water | Trichoderma: Spraying | −0.176 | 0.006 |
| Water | Serratia: Both | −0.220 | <0.001 |
| Water | Serratia: Dipping | −0.166 | 0.013 |
| Water | Serratia: Spraying | −0.193 | 0.001 |
| Water | Bacillus + Trichoderma: Both | −0.169 | 0.010 |
| Water | Bacillus + Trichoderma: Dipping | −0.149 | 0.044 |
| Water | Bacillus + Trichoderma: Spraying | −0.164 | 0.014 |
| Water | Fungicide | −0.166 | 0.012 |
| Net yield (kg, on the natural logarithm scale) | |||
| Serratia: Both | Trichoderma: Spraying | 0.160 | 0.026 |
| Bacillus + Trichoderma: Dipping | Serratia: Both | −0.159 | 0.028 |
| Water | Bacillus: Both | −0.172 | 0.011 |
| Water | Serratia: Both | −0.212 | <0.001 |
| Water | Serratia: Dipping | −0.192 | 0.002 |
| Water | Serratia: Spraying | −0.154 | 0.039 |
| Water | Fungicide | −0.170 | 0.012 |
| Flowering time (days, on the natural logarithm scale) | |||
| Bacillus: Dipping | Bacillus: Both | 0.087 | 0.023 |
| Water | Bacillus: Both | 0.081 | 0.049 |
| Incidence of grain with blast symptoms (on the logit scale) | |||
| Water | Bacillus: Both | 1.176 | <0.001 |
| Water | Bacillus: Dipping | 0.916 | <0.001 |
| Water | Bacillus: Spraying | 1.131 | <0.001 |
| Water | Trichoderma: Both | 0.874 | <0.001 |
| Water | Trichoderma: Dipping | 0.895 | <0.001 |
| Water | Trichoderma: Spraying | 0.932 | <0.001 |
| Water | Serratia: Both | 1.112 | <0.001 |
| Water | Serratia: Dipping | 1.051 | <0.001 |
| Water | Serratia: Spraying | 1.071 | <0.001 |
| Water | Bacillus + Trichoderma: Both | 0.951 | <0.001 |
| Water | Bacillus + Trichoderma: Dipping | 0.895 | <0.001 |
| Water | Bacillus + Trichoderma: Spraying | 0.989 | <0.001 |
| Water | Fungicide | 1.525 | <0.001 |
| Fungicide | Bacillus: Dipping | −0.608 | <0.001 |
| Fungicide | Trichoderma: Both | −0.651 | <0.001 |
| Fungicide | Trichoderma: Dipping | −0.629 | <0.001 |
| Fungicide | Trichoderma: Spraying | −0.593 | <0.001 |
| Fungicide | Serratia: Both | −0.413 | 0.040 |
| Fungicide | Serratia: Dipping | −0.474 | 0.008 |
| Fungicide | Serratia: Spraying | −0.454 | 0.013 |
| Fungicide | Bacillus + Trichoderma: Both | −0.574 | <0.001 |
| Fungicide | Bacillus + Trichoderma: Dipping | −0.630 | <0.001 |
| Fungicide | Bacillus + Trichoderma: Spraying | −0.535 | 0.001 |
| Application | Product | Tiller | Days to | Grain with Blast | Yield (kg) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Height (cm) | 30% Flowering | Maturity | Percent | Seeds/Head | Gross | Net | ||
| Both | Bacillus | 24.3 ± 0.52 | 46.8 ± 1.17 | 55.6 ± 2.00 | 89.4 ± 2.01 | 8.0 ± 0.66 | 126.3 ± 2.86 | 8.3 ± 0.41 | 4.1 ± 0.28 |
| Both | Trichoderma | 26.7 ± 0.51 | 48.9 ± 1.11 | 52.2 ± 1.74 | 85.0 ± 1.75 | 6.4 ± 0.83 | 130.3 ± 3.32 | 8.6 ± 0.28 | 4.8 ± 0.38 |
| Both | Water | 14.7 ± 0.37 | 33.3 ± 1.45 | 73.9 ± 2.14 | 101.6 ± 2.13 | 22.5 ± 2.16 | 113.0 ± 4.59 | 5.5 ± 0.33 | 1.8 ± 0.12 |
| Dipping | Bacillus | 19.2 ± 0.72 | 42.1 ± 0.80 | 59.4 ± 1.88 | 93.0 ± 2.16 | 11.0 ± 1.02 | 118.9 ± 4.61 | 6.8 ± 0.35 | 3.0 ± 0.19 |
| Dipping | Trichoderma | 24.2 ± 0.72 | 44.0 ± 0.96 | 61.1 ± 1.94 | 93.3 ± 2.27 | 10.2 ± 0.80 | 127.3 ± 2.77 | 7.6 ± 0.32 | 3.4 ± 0.20 |
| Dipping | Water | 15.4 ± 0.66 | 32.4 ± 1.24 | 74.4 ± 1.76 | 100.9 ± 1.89 | 22.4 ± 1.99 | 113.4 ± 5.37 | 5.6 ± 0.43 | 1.8 ± 0.12 |
| Spraying | Bacillus | 18.2 ± 0.72 | 39.3 ± 1.26 | 63.4 ± 1.40 | 95.5 ± 2.09 | 15.4 ± 1.58 | 118.3 ± 5.19 | 6.9 ± 0.42 | 2.9 ± 0.14 |
| Spraying | Trichoderma | 20.0 ± 0.63 | 44.7 ± 1.34 | 61.9 ± 2.49 | 92.4 ± 1.92 | 13.6 ± 1.46 | 121.9 ± 4.21 | 6.9 ± 0.41 | 3.1 ± 0.29 |
| Spraying | Water | 15.4 ± 0.52 | 33.8 ± 1.39 | 74.7 ± 1.86 | 101.6 ± 2.00 | 22.8 ± 2.25 | 112.6 ± 4.72 | 5.4 ± 0.34 | 1.8 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murunde, R.; Ringo, G.; Robinson-Boyer, L.; Xu, X. Effective Biocontrol of Rice Blast through Dipping Transplants and Foliar Applications. Agronomy 2022, 12, 592. https://doi.org/10.3390/agronomy12030592
Murunde R, Ringo G, Robinson-Boyer L, Xu X. Effective Biocontrol of Rice Blast through Dipping Transplants and Foliar Applications. Agronomy. 2022; 12(3):592. https://doi.org/10.3390/agronomy12030592
Chicago/Turabian StyleMurunde, Ruth, Gideon Ringo, Louisa Robinson-Boyer, and Xiangming Xu. 2022. "Effective Biocontrol of Rice Blast through Dipping Transplants and Foliar Applications" Agronomy 12, no. 3: 592. https://doi.org/10.3390/agronomy12030592
APA StyleMurunde, R., Ringo, G., Robinson-Boyer, L., & Xu, X. (2022). Effective Biocontrol of Rice Blast through Dipping Transplants and Foliar Applications. Agronomy, 12(3), 592. https://doi.org/10.3390/agronomy12030592







