Modeling of Flowering Time in Vigna radiata with Approximate Bayesian Computation
Abstract
1. Introduction
2. Materials and Methods
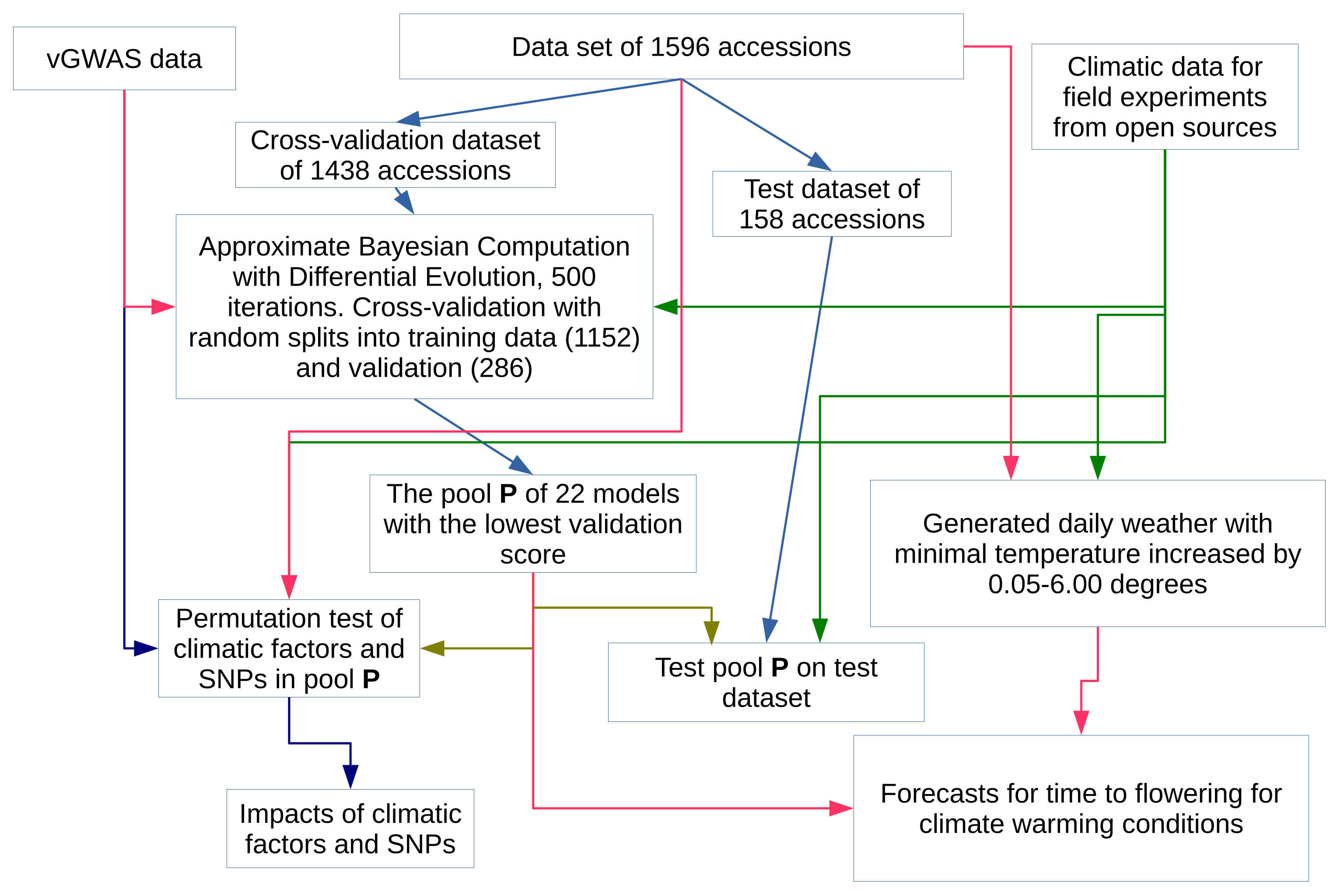
2.1. The Overview
2.2. Plant Material
2.3. Climate Data
- a day length D,
- a minimal temperature ,
- a maximal temperature ,
- a precipitation R,
- for field experiments were taken from a publicly available website https://rp5.ru/Weather_in_the_world (accessed on 26 January 2020) that provides historical weather archives for 172,500 locations, reported by weather stations. The data on solar radiation S were obtained from the NASA Langley Research Center (LaRC) POWER Project funded through the NASA Earth Science/Applied Science Program [47].
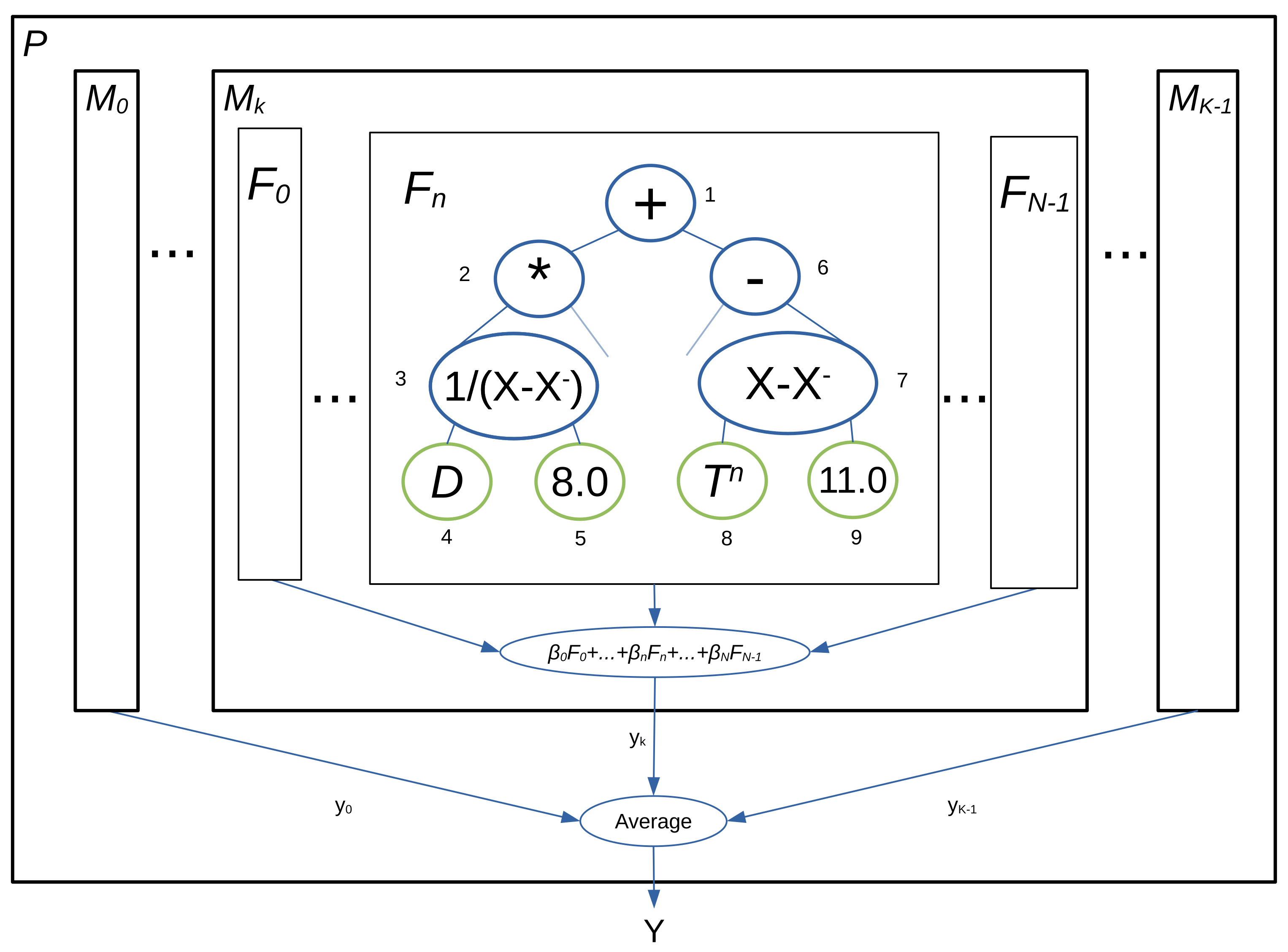
2.4. Prediction of Time to Flowering with a Pool of Models
2.5. Approximate Bayesian Computation
2.6. Construction of the Pool of Models
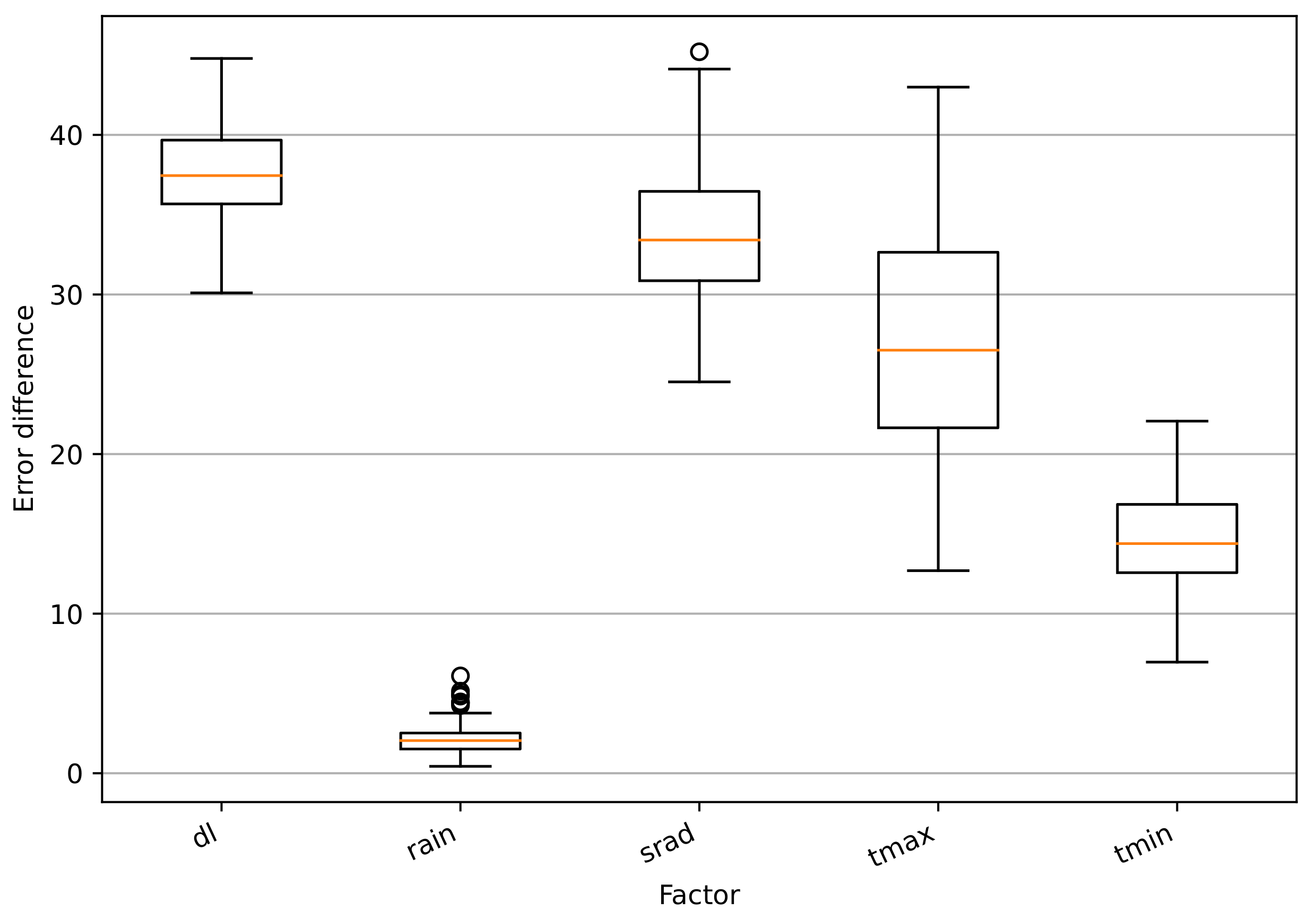
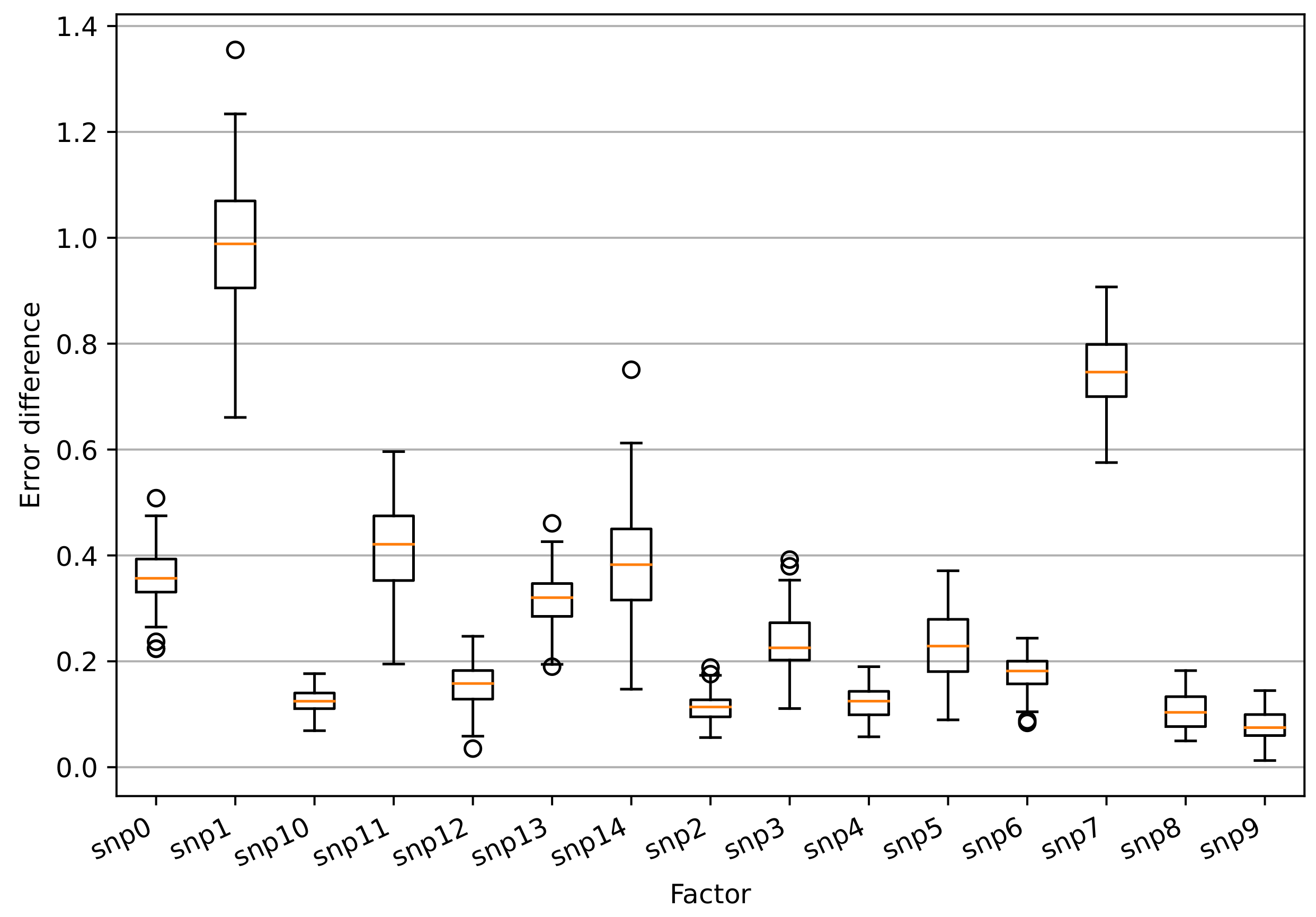
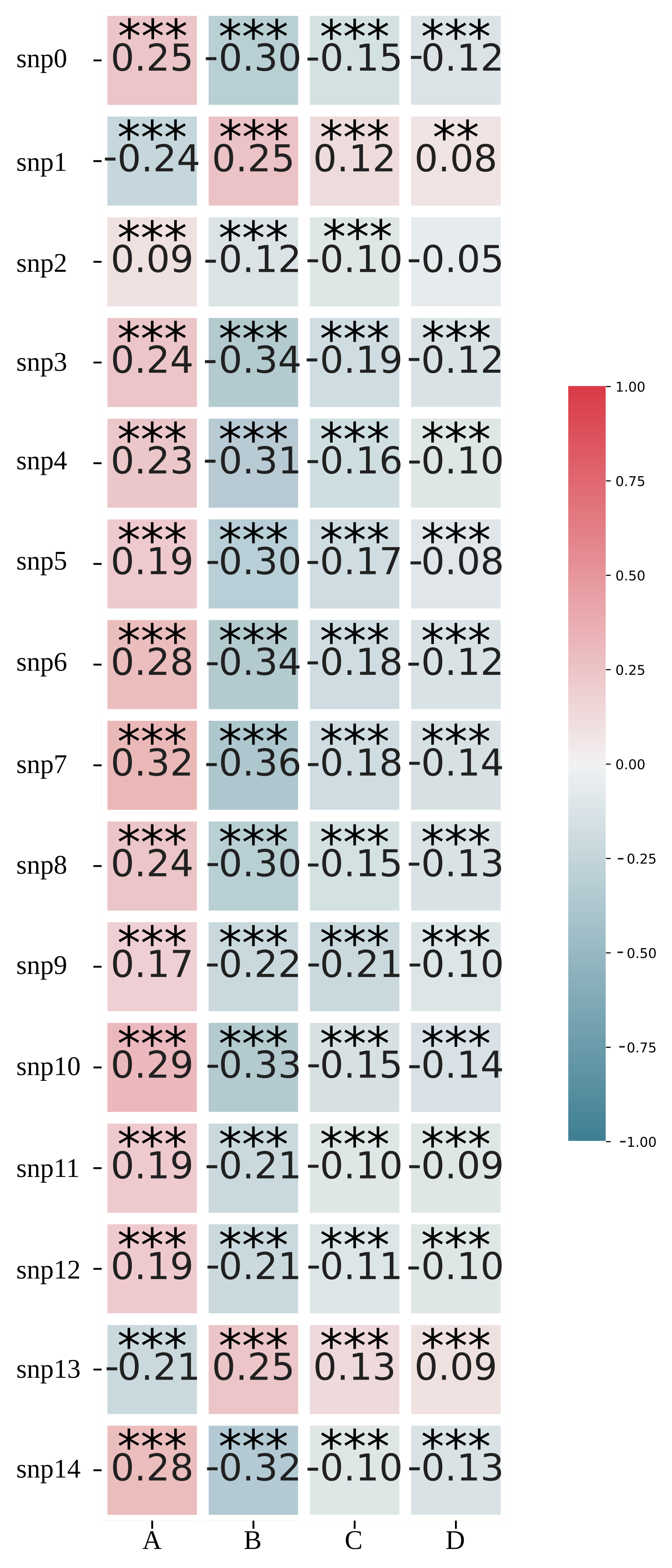
2.7. Estimation of Impacts of Climatic Factors and Genotype Information to the Model
2.8. Simulation of Climate Warming
3. Results
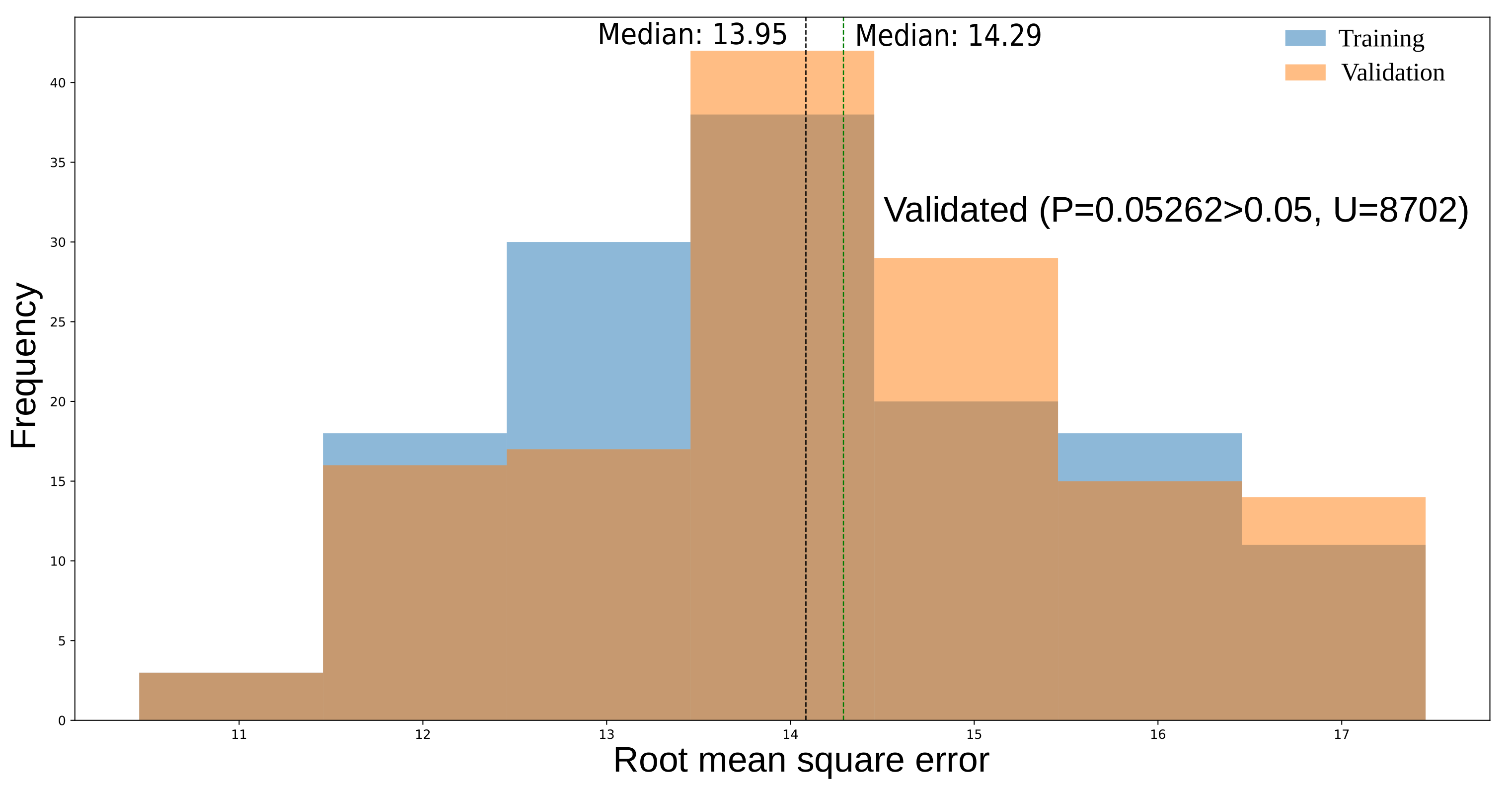
3.1. A Pool of Models for Time to Flowering
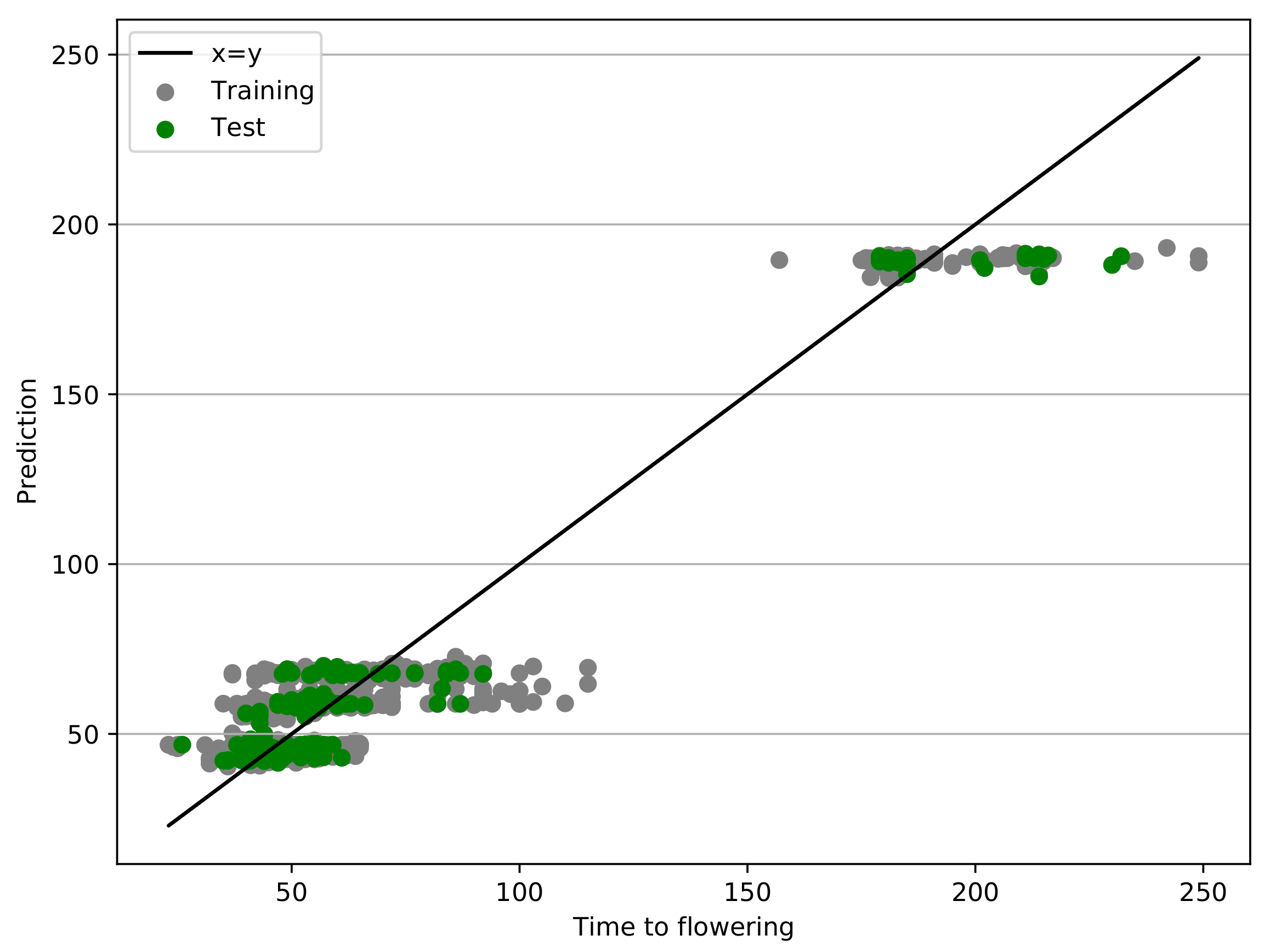
3.2. The Best Model in the Pool
3.3. Impacts of Climatic Factors and SNPs to the Model
3.4. Simulation of Climate Warming
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chivenge, P.; Mabhaudhi, T.; Modi, A.; Mafongoya, P. The Potential Role of Neglected and Underutilised Crop Species as Future Crops under Water Scarce Conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef] [PubMed]
- Vara-Ubol, S.; Chambers, E.; Chambers, D.H. Sensory characteristics of chemical compounds potentially associated with beany aroma in foods. J. Sens. Stud. 2004, 19, 15–26. [Google Scholar] [CrossRef]
- Vishnyakova, M.A.; Burlyaeva, M.O.; Samsonova, M.G. Green gram and black gram: Prospects of cultivation and breeding in Russian Federation. Vavilov J. Genet. Breed. 2018, 22, 957–966. [Google Scholar] [CrossRef]
- Burlyaeva, M.; Vishnyakova, M.; Gurkina, M.; Kozlov, K.; Lee, C.R.; Ting, C.T.; Schafleitner, R.; Nuzhdin, S.; Samsonova, M.; von Wettberg, E. Collections of Mungbean [Vigna radiata (L.) R. Wilczek] and Urdbean [V. mungo (L.) Hepper] in Vavilov Institute (VIR): Traits Diversity and Trends in the Breeding Process over the Last 100 Years. Genet. Resour. Crop. Evol. 2019, 66, 767–781. [Google Scholar] [CrossRef]
- Schafleitner, R.; Nair, R.M.; Rathore, A.; Wang, Y.w.; Lin, C.y.; Chu, S.h.; Lin, P.y.; Chang, J.C.; Ebert, A.W. The AVRDC—The World Vegetable Center Mungbean (Vigna radiata) Core and Mini Core Collections. BMC Genom. 2015, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.M.A.; Puteh, A.B.; Malek, M.A.; Ismail, M.R.; Rafii, M.Y.; Latif, M.A. Seed Yield of Mungbean (Vigna radiata (L.) Wilczek) in Relation to Growth and Developmental Aspects. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imrie, B.C.; Drake, D.W.; Delacy, I.H.; Byth, D.E. Analysis of Genotypic and Environmental Variation in International Mungbean Trials. Euphytica 1981, 30, 301–311. [Google Scholar] [CrossRef]
- Swindell, R.; Poehlman, J.M. Inheritance of photoperiod response (Vigna radiata [L.] wilczek). Euphytica 1978, 27, 325–333. [Google Scholar] [CrossRef]
- Ellis, R.H.; Lawn, R.J.; Summerfield, R.J.; Qi, A.; Roberts, E.H.; Chay, P.M.; Brouwer, J.B.; Rose, J.L.; Yeates, S.J.; Sandover, S. Towards the Reliable Prediction of Time to Flowering in Six Annual Crops. IV. Cultivated and Wild Mung Bean. Exp. Agric. 1994, 30, 31–43. [Google Scholar] [CrossRef]
- Nath, D.; Dasgupta, T. Genotype × Environment Interaction and Stability Analysis in Mungbean. IOSR J. Agric. Vet. Sci. 2013, 5, 62–70. [Google Scholar] [CrossRef]
- Godwin, I.D.; Rutkoski, J.; Varshney, R.K.; Hickey, L.T. Technological Perspectives for Plant Breeding. Theor. Appl. Genet. 2019, 132, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yadav, N.R.; Singh, J. Role of Genomic Tools for Mungbean [Vigna radiata (L.) Wilczek] Improvement. Legume Res. Int. J. 2017, 40, 601–608. [Google Scholar] [CrossRef][Green Version]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J.; et al. Genome Sequence of Mungbean and Insights into Evolution within Vigna Species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Nair, R.M.; Lee, J.; Lee, S.H. Genomic Resources in Mungbean for Future Breeding Programs. Front. Plant Sci. 2015, 6, 626. [Google Scholar] [CrossRef]
- Piekutowska, M.; Niedbała, G.; Piskier, T.; Lenartowicz, T.; Pilarski, K.; Wojciechowski, T.; Pilarska, A.A.; Czechowska-Kosacka, A. The Application of Multiple Linear Regression and Artificial Neural Network Models for Yield Prediction of Very Early Potato Cultivars before Harvest. Agronomy 2021, 11, 885. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.; Pickering, N. Potential Uses and Limitations of Crop Models. Agron. J. 1996, 88, 704–716. [Google Scholar] [CrossRef]
- Williams, J.R.; Jones, C.A.; Kiniry, J.R.; Spanel, D.A. The EPIC Crop Growth Model. Trans. ASAE 1989, 32, 497–511. [Google Scholar] [CrossRef]
- Sousa-Ortega, C.; Royo-Esnal, A.; Urbano, J.M. Predicting Seedling Emergence of Three Canarygrass (Phalaris) Species under Semi-Arid Conditions Using Parametric and Non-Parametric Models. Agronomy 2021, 11, 893. [Google Scholar] [CrossRef]
- Vadez, V.; Soltani, A.; Sinclair, T. Crop Simulation Analysis of Phenological Adaptation of Chickpea to Different Latitudes of India. Field Crop. Res. 2013, 146, 1–9. [Google Scholar] [CrossRef]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Toward a New Generation of Agricultural System Data, Models, and Knowledge Products: State of Agricultural Systems Science. Agric. Syst. 2017, 155, 269–288. [Google Scholar] [CrossRef]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Brief History of Agricultural Systems Modeling. Agric. Syst. 2016, 155, 240–254. [Google Scholar] [CrossRef]
- Jones, J.; Hoogenboom, G.; Porter, C.; Boote, K.; Batchelor, W.; Hunt, L.; Wilkens, P.; Singh, U.; Gijsman, A.; Ritchie, J. The DSSAT Cropping System Model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Boote, K.J.; Jones, J.W.; White, J.W.; Asseng, S.; Lizaso, J.I. Putting Mechanisms into Crop Production Models. Plant Cell Environ. 2013, 36, 1658–1672. [Google Scholar] [CrossRef]
- Wilkerson, G.; Jones, J.; Boote, K.; Ingram, K.; Mishoe, J. Modeling Soybean Growth for Crop Management. Trans. Am. Soc. Agric. Eng. 1983, 26, 63–73. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Rathore, A.; Das, R.R.; Singh, M.K.; Jain, A.; Srinivasan, S.; Gaur, P.M.; Chellapilla, B.; Tripathi, S.; Li, Y.; et al. Genome-Enabled Prediction Models for Yield Related Traits in Chickpea. Front. Plant Sci. 2016, 7, 1666. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, G.; White, J.; Jones, J.; Boote, K. BEANGRO: A Process-Oriented Dry Bean Model with a Versatile User Interface. Agon. J. 1994, 86, 186–190. [Google Scholar] [CrossRef]
- Jones, J.; Keating, B.; Porter, C. Approaches to Modular Model Development. Agric. Syst. 2001, 70, 421–443. [Google Scholar] [CrossRef]
- Wajid, A.; Rahman, M.H.U.; Ahmad, A.; Khaliq, T.; Mahmood, N.; Rasul, F.; Bashir, M.U.; Awais, M.; Hussain, J.; Hoogeboom, G. Simulating the Interactive Impact of Nitrogen and Promising Cultivars on Yield of Lentil (Lens culinaris) Using CROPGRO-Legume Model. Int. J. Agric. Biol. 2013, 15, 1331–1336. [Google Scholar]
- Ilkaee, M.N.; Paknejad, F.; Golzardi, F.; Tookalloo, M.R.; Habibi, D.; Tohidloo, G.; Pazoki, A.; Agayari, F.; Rezaee, M.; Rika, Z.F. Simulation of Some of Important Traits in Chickpea Cultivars under Different Sowing Date Using CROPGRO-Pea Model. Int. J. Biosci. 2014, 4, 84–92. [Google Scholar]
- Mabhaudhi, T.; Chibarabada, T.P.; Chimonyo, V.G.P.; Modi, A.T. Modelling Climate Change Impact: A Case of Bambara Groundnut (Vigna subterranea). Phys. Chem. Earth Parts A/B/C 2018, 105, 25–31. [Google Scholar] [CrossRef]
- Chapman, S.C.; Cooper, M.; Hammer, G.L.; Butler, D.G. Genotype by Environment Interactions Affecting Grain Sorghum. II. Frequencies of Different Seasonal Patterns of Drought Stress Are Related to Location Effects on Hybrid Yields. Aust. J. Agric. Res. 2000, 51, 209. [Google Scholar] [CrossRef]
- Chauhan, Y.S.; Douglas, C.; Rachaputi, R.C.N.; Agius, P.; Martin, W.; Skerman, A. Physiology of Mungbean and Development of the Mungbean Crop Model. In Proceedings of the 1st Australian Summer Grains Conference, Gold Coast, Australia, 21–24 June 2010; p. 11. [Google Scholar]
- Hwang, C.; Correll, M.; Gezan, S.; Zhang, L.; Bhakta, M.; Vallejos, C.; Boote, K.; Clavijo-Michelangeli, J.; Jones, J. Next Generation Crop Models: A Modular Approach to Model Early Vegetative and Reproductive Development of the Common Bean (Phaseolus vulgaris L.). Agric. Syst. 2017, 155, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.; Walthall, C. Meeting Global Food Needs: Realizing the Potential via Genetics x Environment x Management Interactions. Agron. J. 2015, 107, 1215–1226. [Google Scholar] [CrossRef]
- Tardieu, F.; Tuberosa, R. Dissection and Modelling of Abiotic Stress Tolerance in Plants. Curr. Opin. Plant Biol. 2010, 13, 206–212. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, X. Climate Change Impacts on Global Agricultural Land Availability. Environ. Res. Lett. 2011, 6, 014014. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Deb, P.; Shrestha, S.; Babel, M.S. Forecasting climate change impacts and evaluation of adaptation options for maize cropping in the hilly terrain of Himalayas: Sikkim, India. Theor. Appl. Climatol. 2015, 121, 649–667. [Google Scholar] [CrossRef]
- Shrestha, S.; Deb, P.; Bui, T.T.T. Adaptation strategies for rice cultivation under climate change in Central Vietnam. Mitig. Adapt. Strateg. Glob. Chang. 2016, 21, 15–37. [Google Scholar] [CrossRef]
- Ageev, A.; Aydogan, A.; Bishop-von Wettberg, E.; Nuzhdin, S.V.; Samsonova, M.; Kozlov, K. Simulation Model for Time to Flowering with Climatic and Genetic Inputs for Wild Chickpea. Agronomy 2021, 11, 1389. [Google Scholar] [CrossRef]
- Ageev, A.Y.; Bishop-von Wettberg, E.J.; Nuzhdin, S.V.; Samsonova, M.G.; Kozlov, K.N. Forecasting the Timing of Floral Initiation in Wild Chickpeas under Climate Change. Biophysics 2021, 66, 107–116. [Google Scholar] [CrossRef]
- Kozlov, K.; Singh, A.; Berger, J.; Wettberg, E.B.v.; Kahraman, A.; Aydogan, A.; Cook, D.; Nuzhdin, S.; Samsonova, M. Non-Linear Regression Models for Time to Flowering in Wild Chickpea Combine Genetic and Climatic Factors. BMC Plant Biol. 2019, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, K.; Sokolkova, A.; Lee, C.R.; Ting, C.T.; Schafleitner, R.; Bishop-von Wettberg, E.; Nuzhdin, S.; Samsonova, M. Dynamical climatic model for time to flowering in Vigna radiata. BMC Plant Biol. 2020, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M.; Van Zandt, T. A Tutorial on Approximate Bayesian Computation. J. Math. Psychol. 2012, 56, 69–85. [Google Scholar] [CrossRef]
- Turner, B.M.; Sederberg, P.B. Approximate Bayesian Computation with Differential Evolution. J. Math. Psychol. 2012, 56, 375–385. [Google Scholar] [CrossRef]
- Shen, X.; Pettersson, M.; Rönnegård, L.; Carlborg, O. Inheritance Beyond Plain Heritability: Variance-Controlling Genes in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1002839. [Google Scholar] [CrossRef]
- Stackhouse, P.W.; Perez, R.; Sengupta, M.; Knapp, K.; Mikovitz, J.C.; Schlemmer, J.; Scarino, B.; Zhang, T.; Cox, S.J. An Assessment of New Satellite Data Products for the Development of a Long-Term Global Solar Resource at 10–100 Km. In Proceedings of the Solar 2016 Conference, San Francisco, CA, USA, 10–14 July 2016; International Solar Energy Society: Freiburg, Germany, 2016; pp. 1–6. [Google Scholar]
- O’Neill, M.; Ryan, C. Grammatical Evolution. IEEE Trans. Evol. Comput. 2001, 5, 349–358. [Google Scholar] [CrossRef]
- Noorian, F.; de Silva, A.M.; Leong, P.H.W. gramEvol: Grammatical Evolution in R. J. Stat. Softw. 2016, 71, 1–26. [Google Scholar] [CrossRef]
- Kozlov, K.; Samsonov, A. DEEP—Differential Evolution Entirely Parallel Method for Gene Regulatory Networks. J. Supercomput. 2011, 57, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, K.; Samsonov, A.M.; Samsonova, M. A Software for Parameter Optimization with Differential Evolution Entirely Parallel Method. PeerJ Comput. Sci. 2016, 2, e74. [Google Scholar] [CrossRef][Green Version]
- Sokolkova, A. Genome-wide association study in accessions of the mini-core collection of mungbean (Vigna radiata) from the World Vegetable Gene Bank (Taiwan). BMC Plant Biol. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, M.; Zuccolo, A.; Tuna, M.; Gianfranceschi, L.; Pè, M. Targeting Environmental Adaptation in the Monocot Model Brachypodium Distachyon: A Multi-Faceted Approach. BMC Genom. 2014, 15, 801. [Google Scholar] [CrossRef] [PubMed]
- Westengen, O.T.; Berg, P.R.; Kent, M.P.; Brysting, A.K. Spatial Structure and Climatic Adaptation in African Maize Revealed by Surveying SNP Diversity in Relation to Global Breeding and Landrace Panels. PLoS ONE 2012, 7, e47832. [Google Scholar] [CrossRef]
- Marjoram, P.; Zubair, A.; Nuzhdin, S.V. Post-GWAS: Where next? More Samples, More SNPs or More Biology? Heredity 2014, 112, 79–88. [Google Scholar] [CrossRef]
- Vadez, V.; Berger, J.D.; Warkentin, T.; Asseng, S.; Ratnakumar, P.; Rao, K.P.C.; Gaur, P.M.; Munier-Jolain, N.; Larmure, A.; Voisin, A.S.; et al. Adaptation of grain legumes to climate change: A review. Agron. Sustain. Dev. 2012, 32, 31–44. [Google Scholar] [CrossRef]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.u.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef]
- Paudel, D.; Dareus, R.; Rosenwald, J.; Muñoz-Amatriaín, M.; Rios, E.F. Genome-Wide Association Study Reveals Candidate Genes for Flowering Time in Cowpea (Vigna unguiculata [L.] Walp.). Front. Genet. 2021, 12, 667038. [Google Scholar] [CrossRef] [PubMed]
- Angelotti, F.; Barbosa, L.G.; Barros, J.R.A.; Santos, C.A.F.d. Cowpea development under different temperatures and carbon dioxide concentrations. Pesqui. Agropecuária Trop. 2020, 50, e59377. [Google Scholar] [CrossRef]
- Li, Q.; Xu, L.; Pan, X.; Zhang, L.; Li, C.; Yang, N.; Qi, J. Modeling Phenological Responses of Inner Mongolia Grassland Species to Regional Climate Change. Environ. Res. Lett. 2016, 11, 015002. [Google Scholar] [CrossRef]
- Singh, P.; Nedumaran, S.; Boote, K.; Gaur, P.; Srinivas, K.; Bantilan, M. Climate change impacts and potential benefits of drought and heat tolerance in chickpea in South Asia and East Africa. Eur. J. Agron. 2014, 52, 123–137. [Google Scholar] [CrossRef]
- Craufurd, P.Q.; Wheeler, T.R. Climate change and the flowering time of annual crops. J. Exp. Bot. 2009, 60, 2529–2539. [Google Scholar] [CrossRef]


| Date of Sowing | Date of Harvest | Geographical Coordinates |
|---|---|---|
| 28/08/1984 | 24/10/1984 | N 23650 E 1201755 |
| 17/09/1985 | 03/10/1985 | N 23650 E 1201755 |
| 16/06/2016 | 22/08 to mid-September | N 173028 E 781610 |
| 21/09/2018 | from 24–28 December 2018 | N 23650 E 1201755 |
| 03/05/2018 | from mid-July | N 4518 E 4052 |
| 12/05/2018 | from mid-July | N 4614 E 4801 |
| Num | Chr | Pos | Major | Minor |
|---|---|---|---|---|
| 0 | NC_028351.1 | 10609109 | T | C |
| 1 | NC_028352.1 | 1756685 | A | T |
| 2 | NC_028353.1 | 4083774 | A | T |
| 3 | NC_028353.1 | 7715763 | T | C |
| 4 | NC_028354.1 | 19007443 | T | C |
| 5 | NC_028354.1 | 19193706 | A | T |
| 6 | NC_028356.1 | 5599756 | C | A |
| 7 | NC_028356.1 | 18228698 | C | T |
| 8 | NC_028357.1 | 36154036 | G | T |
| 9 | NC_028357.1 | 51548743 | T | C |
| 10 | NC_028358.1 | 42385899 | G | T |
| 11 | NC_028358.1 | 43289540 | T | C |
| 12 | NC_028358.1 | 43289553 | G | A |
| 13 | NC_028361.1 | 10688905 | A | C |
| 14 | NW_014541837.1 | 1074368 | C | T |
| CR PCC | CR MED | CR RMS | TE PCC | TE MED | TE RMS | |
|---|---|---|---|---|---|---|
| 0 | 0.97 | 7 | 11.91 | 0.98 | 8 | 12.19 |
| 1 | 0.97 | 6 | 11.55 | 0.98 | 7 | 11.75 |
| 2 | 0.97 | 7 | 12.09 | 0.98 | 7 | 12.71 |
| 3 | 0.97 | 8 | 12.60 | 0.97 | 8 | 12.20 |
| 4 | 0.97 | 6 | 12.26 | 0.97 | 7 | 12.44 |
| 5 | 0.97 | 7 | 12.65 | 0.97 | 7 | 13.90 |
| 6 | 0.97 | 7 | 12.73 | 0.97 | 8 | 13.46 |
| 7 | 0.97 | 6 | 12.32 | 0.94 | 7 | 19.19 |
| 8 | 0.97 | 6 | 12.00 | 0.98 | 7 | 12.14 |
| 9 | 0.97 | 6 | 11.23 | 0.98 | 7 | 11.64 |
| 10 | 0.97 | 7 | 12.67 | 0.98 | 7 | 13.54 |
| 11 | 0.97 | 8 | 12.93 | 0.96 | 9 | 14.62 |
| 12 | 0.97 | 7 | 12.30 | 0.98 | 7 | 11.84 |
| 13 | 0.97 | 8 | 12.48 | 0.97 | 9 | 12.96 |
| 14 | 0.97 | 8 | 12.77 | 0.97 | 8 | 12.78 |
| 15 | 0.97 | 8 | 13.13 | 0.97 | 9 | 13.06 |
| 16 | 0.96 | 6 | 13.60 | 0.96 | 7 | 15.79 |
| 17 | 0.97 | 10 | 13.11 | 0.96 | 10 | 14.66 |
| 18 | 0.97 | 8 | 13.35 | 0.97 | 9 | 13.62 |
| 19 | 0.97 | 10 | 13.19 | 0.97 | 10 | 12.66 |
| 20 | 0.97 | 7 | 13.47 | 0.98 | 7 | 12.74 |
| 21 | 0.96 | 11 | 14.51 | 0.97 | 11 | 14.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ageev, A.; Lee, C.-R.; Ting, C.-T.; Schafleitner, R.; Bishop-von Wettberg, E.; Nuzhdin, S.V.; Samsonova, M.; Kozlov, K. Modeling of Flowering Time in Vigna radiata with Approximate Bayesian Computation. Agronomy 2021, 11, 2317. https://doi.org/10.3390/agronomy11112317
Ageev A, Lee C-R, Ting C-T, Schafleitner R, Bishop-von Wettberg E, Nuzhdin SV, Samsonova M, Kozlov K. Modeling of Flowering Time in Vigna radiata with Approximate Bayesian Computation. Agronomy. 2021; 11(11):2317. https://doi.org/10.3390/agronomy11112317
Chicago/Turabian StyleAgeev, Andrey, Cheng-Ruei Lee, Chau-Ti Ting, Roland Schafleitner, Eric Bishop-von Wettberg, Sergey V. Nuzhdin, Maria Samsonova, and Konstantin Kozlov. 2021. "Modeling of Flowering Time in Vigna radiata with Approximate Bayesian Computation" Agronomy 11, no. 11: 2317. https://doi.org/10.3390/agronomy11112317
APA StyleAgeev, A., Lee, C.-R., Ting, C.-T., Schafleitner, R., Bishop-von Wettberg, E., Nuzhdin, S. V., Samsonova, M., & Kozlov, K. (2021). Modeling of Flowering Time in Vigna radiata with Approximate Bayesian Computation. Agronomy, 11(11), 2317. https://doi.org/10.3390/agronomy11112317







