Assessment of the Genetic Diversity of Chrysanthemum Cultivars Using SSR Markers
Abstract
:1. Introduction
2. Materials and Methods
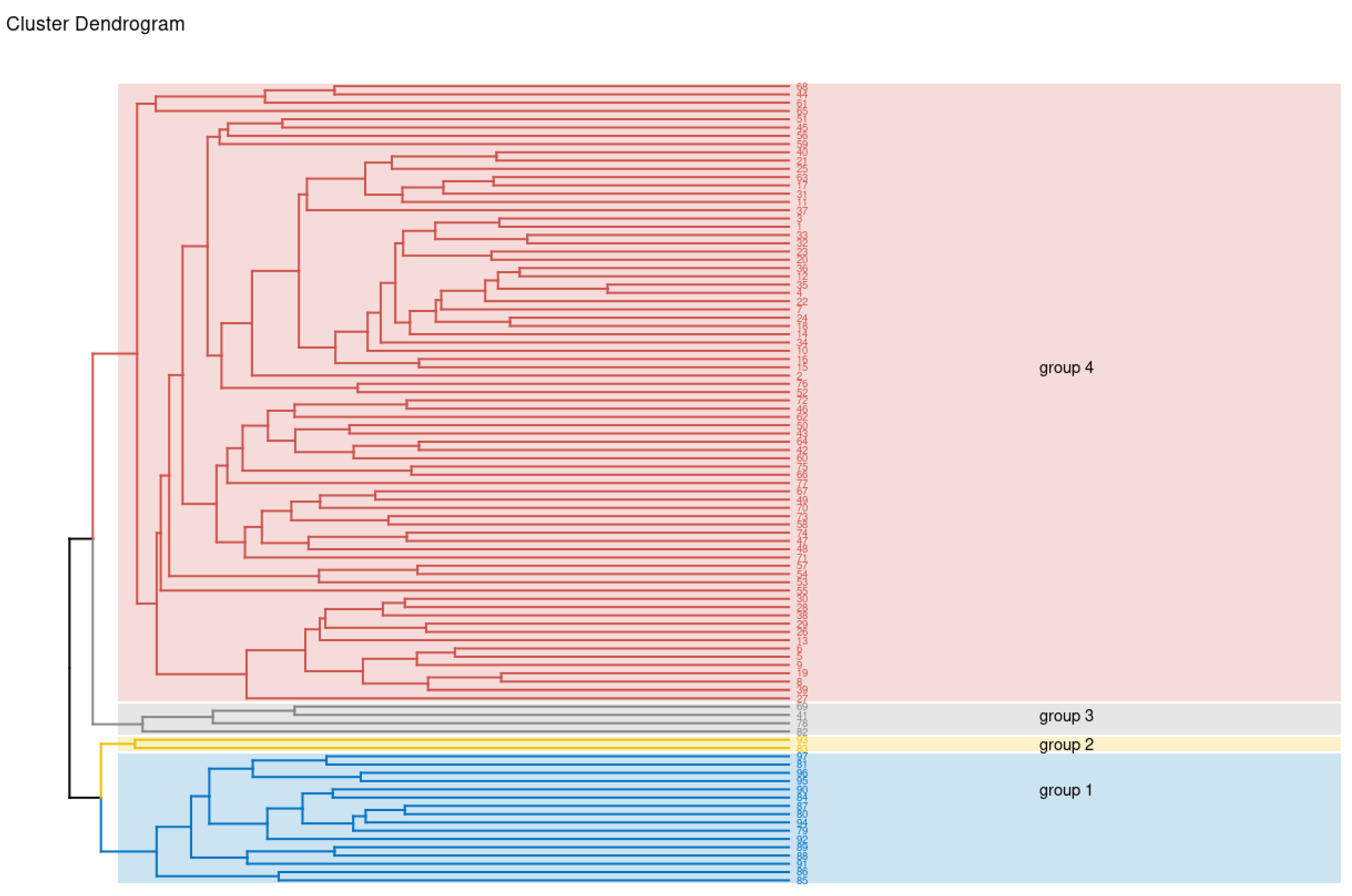
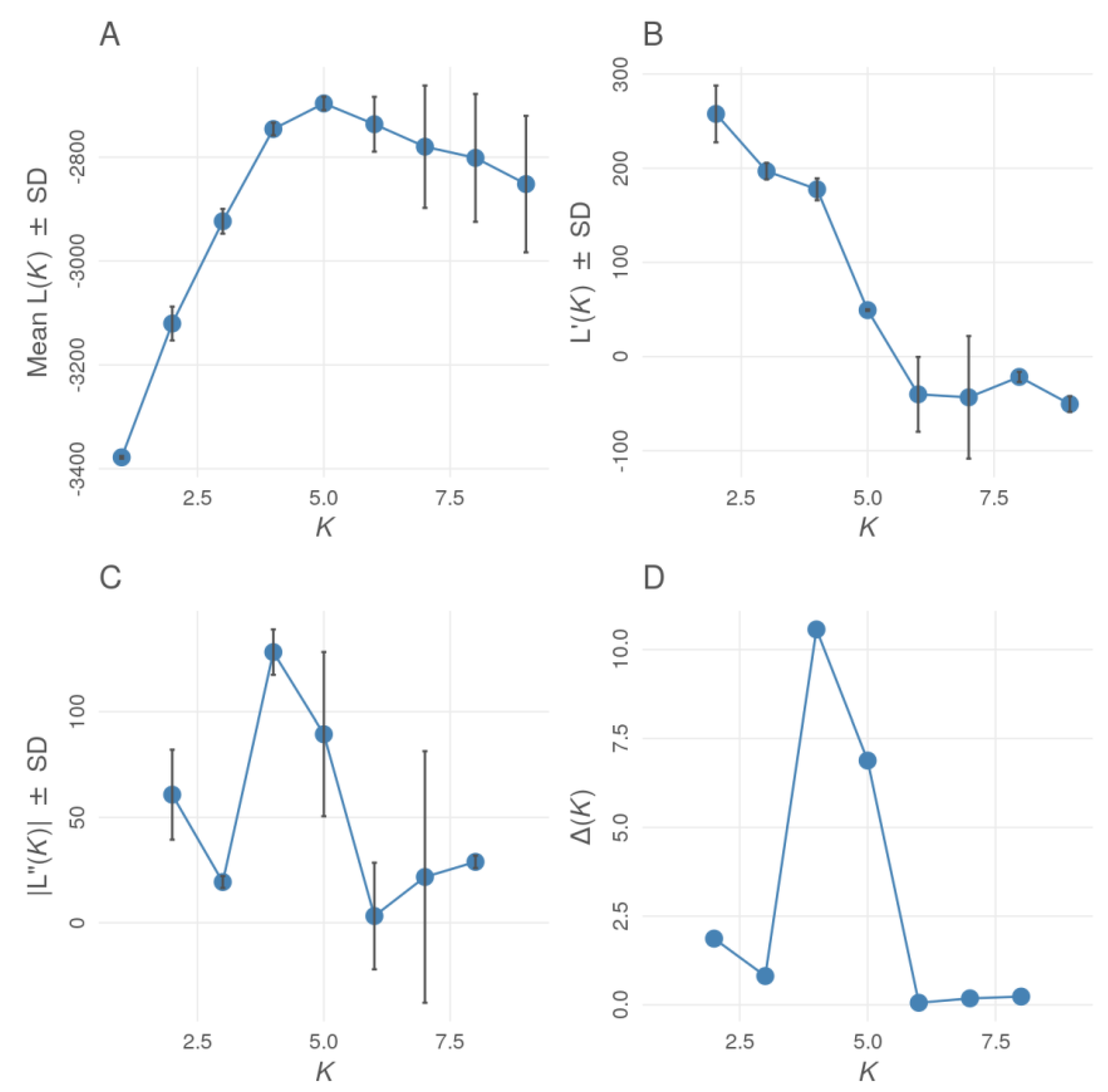
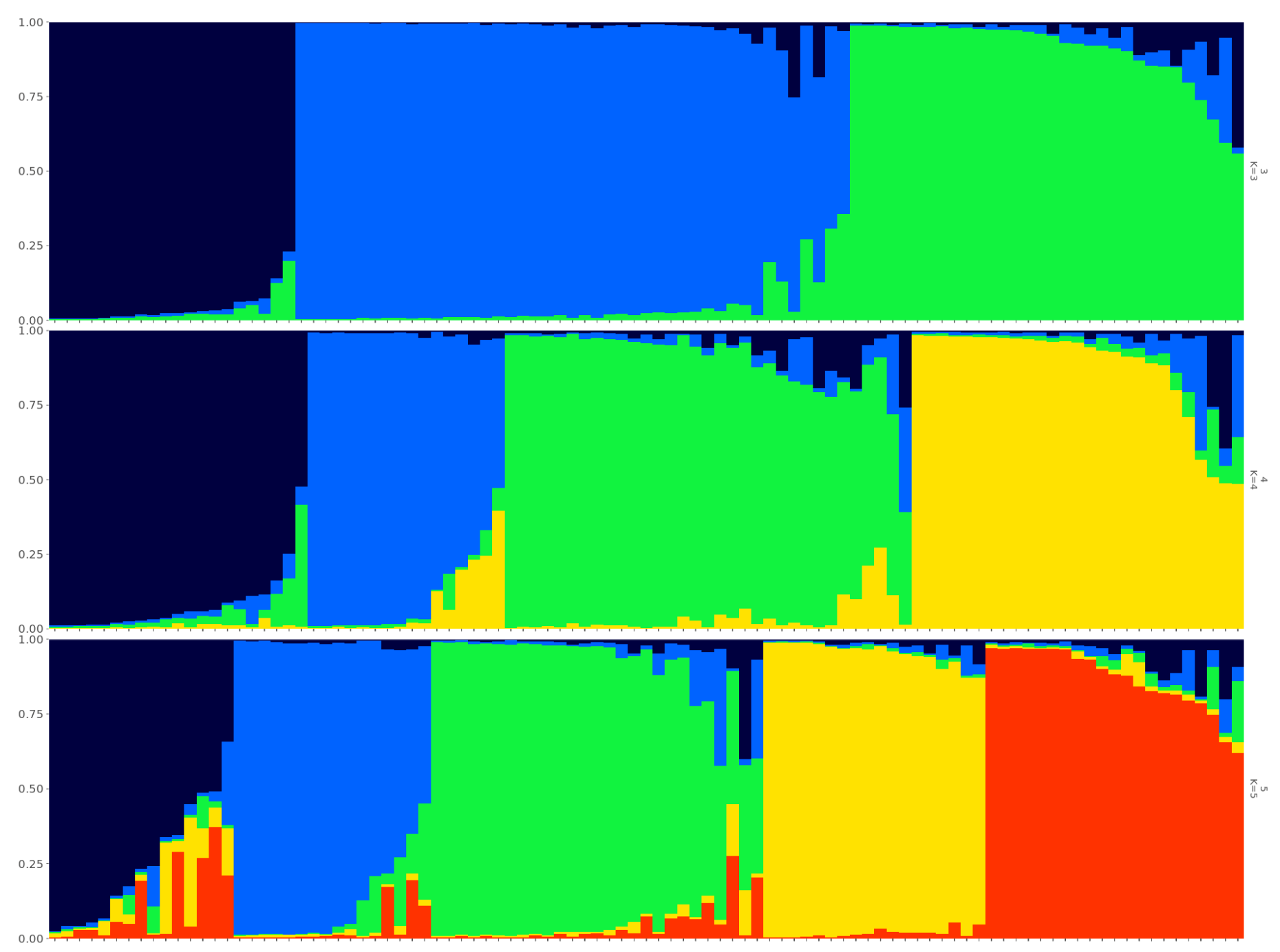
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, N.O. (Ed.) Chrysanthemum. In Flower Breeding and Genetics, 1st ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 389–437. [Google Scholar] [CrossRef]
- Padmadevi, K.; Jawaharlal, M. Molecular characterization of chrysanthemum (Dendranthema grandiflora tzvelev) mutants using RAPD analysis. Asian Australas. J. Plant Sci. Biotechnol. 2011, 5, 42–46. [Google Scholar]
- Shiroto, C.S.; Peres, N.V.; Sabbag, O.J. Economic viability of potted chrysanthemums production in Atibaia, São Paulo state. Ornam. Hortic. 2016, 22, 130–137. [Google Scholar] [CrossRef]
- Kuleung, C.; Baenziger, P.S.; Dweikat, I. Transferability of SSR markers among wheat, rye, and triticale. Theor. Appl. Genet. 2004, 108, 1147–1150. [Google Scholar] [CrossRef]
- Junghans, H.; Metzlaff, M. A simple and rapid method for the preparation of total plant DNA. Biotechniques 1990, 8, 176. [Google Scholar]
- Liu, H.; Zhang, Q.X.; Sun, M.; Pan, H.T.; Kong, Z.X. Development of expressed sequence tag-simple sequence repeat markers for Chrysanthemum morifolium and closely related species. Genet. Mol. Res. 2015, 14, 7578–7586. [Google Scholar] [CrossRef]
- Khaing, A.A.; Moe, K.T.; Hong, W.J.; Park, C.S.; Yeon, K.H.; Park, H.S.; Kim, D.C.; Choi, B.J.; Jung, J.Y.; Chae, S.C.; et al. Phylogenetic relationships of chrysanthemums in Korea based on novel SSR markers. Genet. Mol. Res. 2013, 12, 5335–5347. [Google Scholar] [CrossRef]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, J.; Mandal, A.K.A.; Ranade, S.A.; da Silva, J.A.T.; Datta, S.K. Molecular systematics in Chrysanthemum × grandiflorum (Ramat.) Kitamura. Sci. Hortic. 2006, 110, 373–378. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front. Genet. 2015, 6, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jombart, T. adegenet: Adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [Green Version]
- Schliep, K.P. phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, N.A.; Burke, T.; Elo, K.; Feldman, M.W.; Freidlin, P.J.; Groenen, M.; Hillel, J.; Mäki-Tanila, A.; Tixier-Boichard, M.; Vignal, A.; et al. Empirical Evaluation of Genetic Clustering Methods Using Multilocus Genotypes From 20 Chicken Breeds. Genetics 2001, 159, 699–713. [Google Scholar] [CrossRef]
- Francis, R.M. pophelper: An R package and web app to analyse and visualize population structure. Mol. Ecol. Resour. 2017, 17, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Kukus, D. Wybrane zagadnienia z zakresu biologii, hodowli i reprodukcji chryzantemy wielkokwiatowej. Zesz. Probl. Postępów Nauk Rol. 2017, 588. [Google Scholar] [CrossRef]
- Moe, K.-T.; Ra, S.-B.; Lee, G.-A.; Lee, M.-C.; Park, H.-S.; Kim, D.-C.; Lee, C.-H.; Choi, H.-G.; Jeon, N.-B.; Jung, J.-Y.; et al. Distribution and frequency of SSR motifs in the Chrysanthemum SSR- enriched library through 454 pyrosequencing technology. Korean J. Int. Agric. 2011, 23, 546–551. [Google Scholar]
- Tymoszuk, A. Hodowla chryzantemy wielkokwiatowej na drodze mutagenezy indukowanej promieniowaniem X i gamma. Zesz. Probl. Postępów Nauk Rol. 2015, 582, 101–113. [Google Scholar]
- Wolff, K.; Zietkiewicz, E.; Hofstra, H. Identification of chrysanthemum cultivars and stability of DNA fingerprint patterns. Theor. Appl. Genet. 1995, 91, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Tsai, C.C.; Sheu, C.S. Genetic analysis of Chrysanthemum hybrids based on RAPD molecular markers. Bot. Bull. Acad. Sin. 2000, 41, 257–262. [Google Scholar]
- Martí:n, C.; Uberhuaga, E.; Pérez, C. Application of RAPD markers in the characterisation of Chrysanthemum varieties and the assessment of somaclonal variation. Euphytica 2002, 127, 247–253. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Chen, F.; Fang, W.; Li, F. A preliminary genetic linkage map of chrysanthemum (Chrysanthemum morifolium) cultivars using RAPD, ISSR and AFLP markers. Sci. Hortic. 2010, 125, 422–428. [Google Scholar] [CrossRef]
- Wolff, K.; Rijn, J.P.-V.; Hofstra, H. RFLP analysis in chrysanthemum. I. Probe and primer development. Theor. Appl. Genet. 1994, 88, 472–478. [Google Scholar] [CrossRef]
- Klie, M.; Menz, I.; Linde, M.; Debener, T. Lack of structure in the gene pool of the highly polyploid ornamental chrysanthemum. MoI 2013, 32, 339–348. [Google Scholar] [CrossRef]
- Martín, C.; Cervera, M.T.; González-Benito, M.E. Genetic stability analysis of chrysanthemum (Chrysanthemum × morifolium Ramat) after different stages of an encapsulation–dehydration cryopreservation protocol. J. Plant Physiol. 2011, 168, 158–166. [Google Scholar] [CrossRef]
- Ouyang, C.H.; He, Q.; Qin, G.X.; Guo, Q.G.; Han, G.H.; Wang, Y.; Bing, Q.; Liang, G.L. Genetic diversity of 25 Chrysanthemum [Dendranthema× grandiflora (Ramat.) Kitamura] cultivars revealed by ISSR analysis. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2010, 3, 126–132. [Google Scholar]
- Shao, Q.-S.; Guo, Q.-S.; Deng, Y.-M.; Guo, H.-P. A comparative analysis of genetic diversity in medicinal Chrysanthemum morifolium based on morphology, ISSR and SRAP markers. Biochem. Syst. Ecol. 2010, 38, 1160–1169. [Google Scholar] [CrossRef]
- Lu, L.; Qin, M.; He, D.; Gu, Y. Analyses of ISSR molecular marker and genetic relationship of different provenances of Dendranthema morifolium, D. indicum and D. nankingese. J. Plant Resour. Environ. 2008, 17, 7–12. [Google Scholar]
- Wang, H.; Jiang, J.; Chen, S.; Qi, X.; Peng, H.; Li, P.; Song, A.; Guan, Z.; Fang, W.; Liao, Y.; et al. Next-generation sequencing of the Chrysanthemum nankingense (Asteraceae) transcriptome permits large scale unigene assembly and SSR marker discovery. PLoS ONE 2013, 8, e62293. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Oligo Name | Sequence | Annealing Temperature (°C) |
|---|---|---|
| KNUCRY-35 | F CCTCGCACTACTTCCAAATGA R CCTCGCACTACTTCCAAATGA | 50 |
| KNUCRY-76 | F TTGAGGTTGTGGAAATGCAG R CGCGTTAACTTTGGTGTTTTT | 55 |
| CM01 | F TCAAAACCTCAGAAACCCAAA R ATTTGTAGGAACTGGTGCGG | 56 |
| CM02 | F AAAGTAACCCAAACCCCCAT R ATCGCCGGTAATGGTTGTAA | 56 |
| CM03 | F TTCCTTCAATCTCACACACACT R AACCTAACAACGACAACGCC | 56 |
| CM04 | F GCACATTTCCTTCATGGGTT R TCCACGGTTTCAGATGATGA | 56 |
| CM05 | F ACAAAAGGCGACACACACAA R ATCTTCTTCCAACAGGCGAA | 56 |
| CM06 | F TCTTGGATCACTCCAATCCA R GTGAATTTGTTGCGGTGTTG | 56 |
| CM08 | F ATGGCTGCTGTCTCCTCAGT R TCCATAAATGTAGCAAGAACCAGA | 56 |
| CM09 | F AACCATGGGTTATGGGTCAA R TCATTTTAACGAAACAAATAAAATACG | 56 |
| CM10 | F CCCGTTGTTTGCACTATCCT R CCTGATCGAGCCTTCTTTTG | 56 |
| CM11 | F TCGCCATATCCACGTACAAA R TGGGTCAACTCATCAGACCA | 56 |
| CM12 | F TTGTCGGATTCTTTGGAAGG R GGGCACGATCGACTCATAAT | 56 |
| CM15 | F AGGTGGTGGCACTTGAGACT R AATGAGGAGCGGTCTTTCAA | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, A.; Parkitna, K.; Kozak, B.; Florczak, S.; Matkowski, J.; Nowosad, K. Assessment of the Genetic Diversity of Chrysanthemum Cultivars Using SSR Markers. Agronomy 2021, 11, 2318. https://doi.org/10.3390/agronomy11112318
Olejnik A, Parkitna K, Kozak B, Florczak S, Matkowski J, Nowosad K. Assessment of the Genetic Diversity of Chrysanthemum Cultivars Using SSR Markers. Agronomy. 2021; 11(11):2318. https://doi.org/10.3390/agronomy11112318
Chicago/Turabian StyleOlejnik, Agata, Katarzyna Parkitna, Bartosz Kozak, Szymon Florczak, Jakub Matkowski, and Kamila Nowosad. 2021. "Assessment of the Genetic Diversity of Chrysanthemum Cultivars Using SSR Markers" Agronomy 11, no. 11: 2318. https://doi.org/10.3390/agronomy11112318
APA StyleOlejnik, A., Parkitna, K., Kozak, B., Florczak, S., Matkowski, J., & Nowosad, K. (2021). Assessment of the Genetic Diversity of Chrysanthemum Cultivars Using SSR Markers. Agronomy, 11(11), 2318. https://doi.org/10.3390/agronomy11112318







