The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production
Abstract
:1. Introduction
2. The Use of Forage Legumes for Enteric Methane Abatement in Forage-Based Beef Production Systems
3. Constraints to the Use of Forage Legumes in Beef Cattle Grazing Systems
4. Tannin-Containing Legumes in Forage-Based Livestock Systems
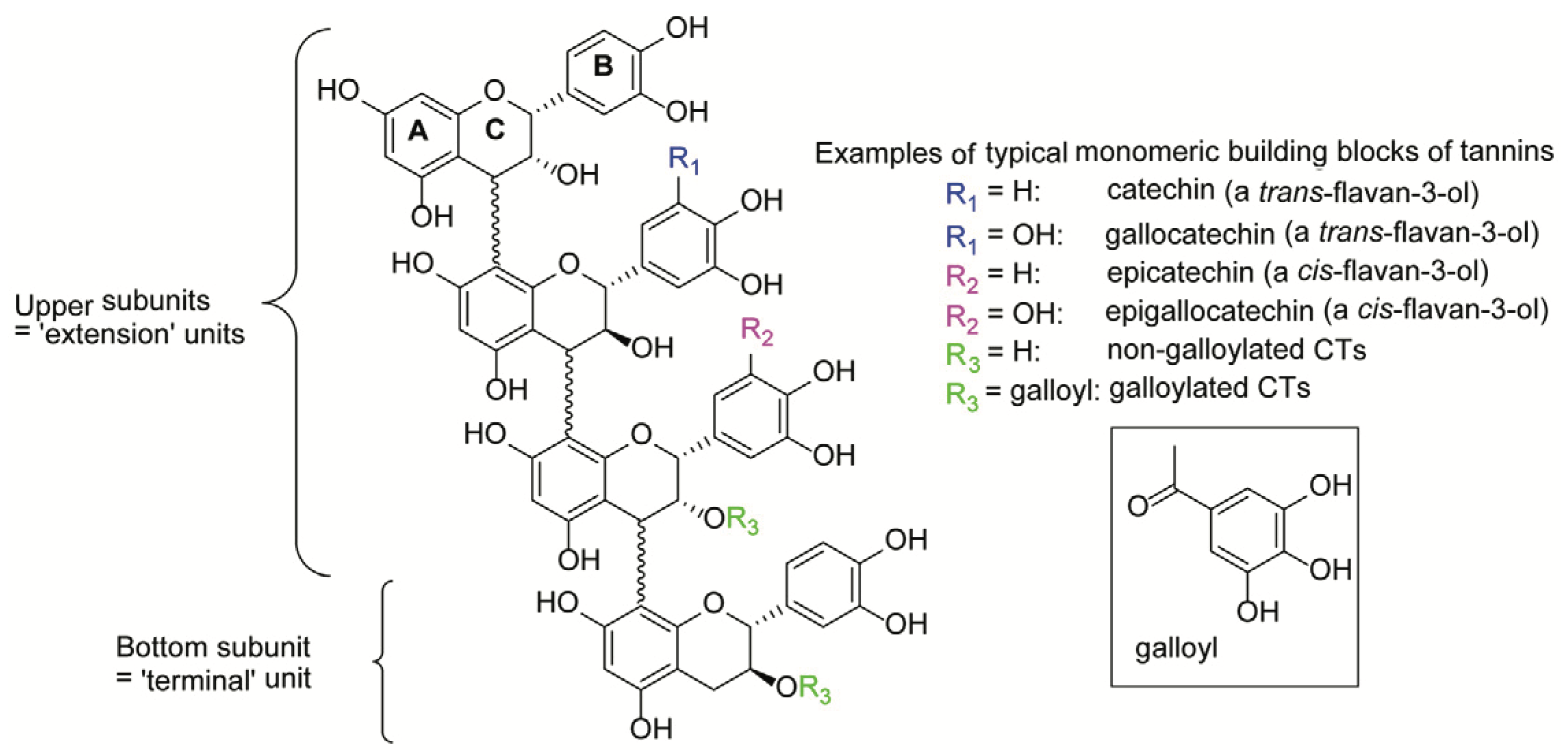
4.1. Condensed Tannin Structure
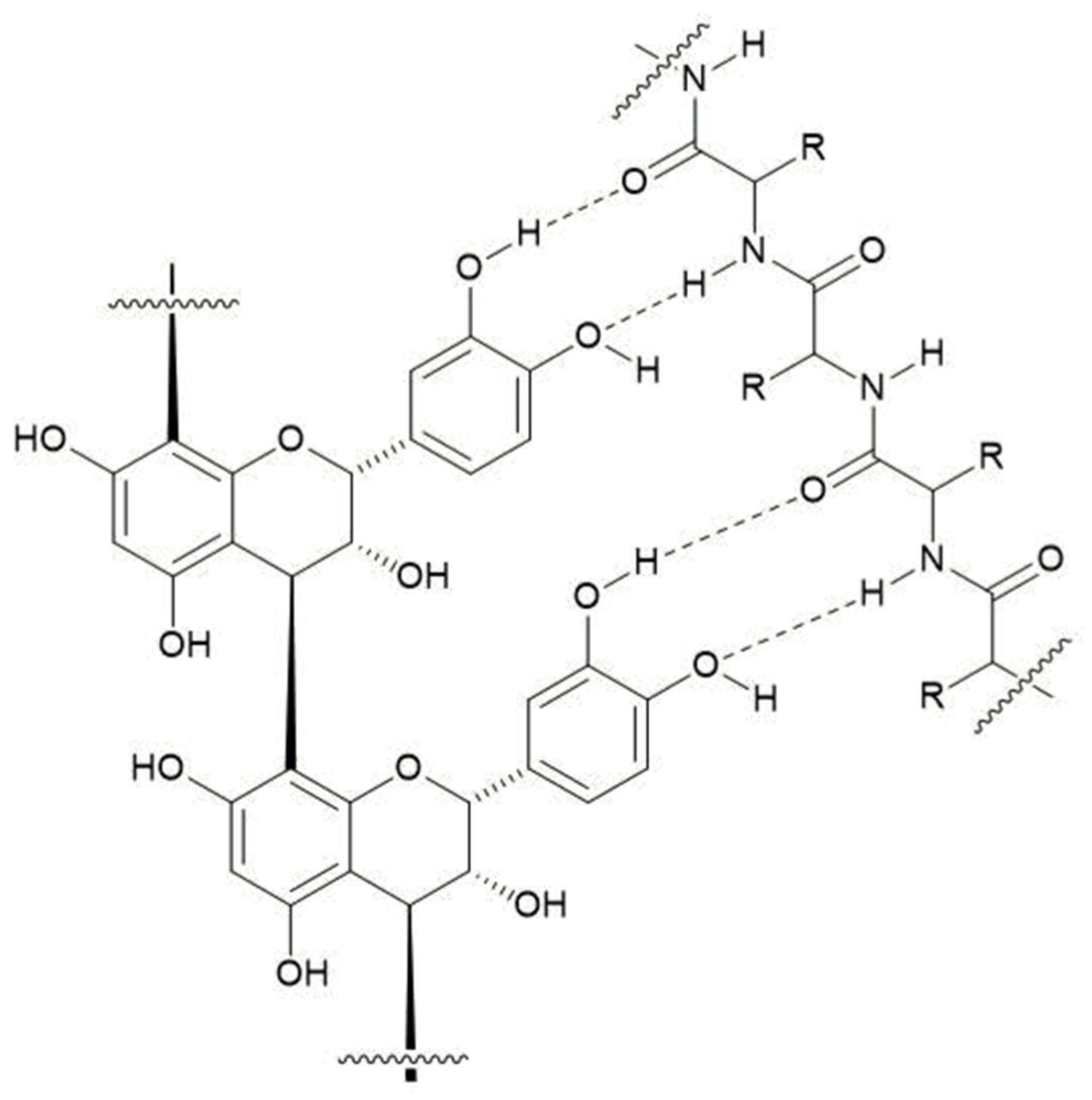
4.2. Condensed Tannin–Protein Complexes and Reductions in Urinary N Excretions
4.3. Effect of Condensed Tannins on Enteric Methane Emissions
5. Other Beneficial Effects of Tanniferous Legumes in Grazing Beef Production Systems
6. Forage Diversity in Beef Cattle Production Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rotz, C.A.; Asem-Hiablie, S.; Place, S.; Thoma, G. Environmental Footprints of Beef Cattle Production in the United States. Agric. Syst. 2019, 169, 1–13. [Google Scholar] [CrossRef]
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2017. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2017 (accessed on 24 January 2020).
- Beauchemin, K.A.; Henry Janzen, H.; Little, S.M.; McAllister, T.A.; McGinn, S.M. Life Cycle Assessment of Greenhouse Gas Emissions from Beef Production in Western Canada: A Case Study. Agric. Syst. 2010, 103, 371–379. [Google Scholar] [CrossRef]
- Gerber, P.J. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations, Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting Rumen Fermentation to Reduce Methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of Hydrogen on Rumen Methane Formation and Fermentation Balances through Microbial Growth Kinetics and Fermentation Thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Broucek, J. Options to Methane Production Abatement in Ruminants: A review. J. Anim. Plant. Sci. 2018, 28, 348–364. [Google Scholar]
- Haque, M.N. Dietary Manipulation: A Sustainable Way to Mitigate Methane Emissions from Ruminants. J. Anim. Sci. Technol. 2018, 60, 15. [Google Scholar] [CrossRef] [Green Version]
- Alemneh, T.; Getabalew, M. Strategies to Reduce Methane Emission in Ruminants. Int. J. Ecol. Ecosolution 2019, 6, 16–22. [Google Scholar]
- Pámanes-Carrasco, G.; Herrera-Torres, E.; Murillo-Ortiz, M.; Reyes-Jáques, D. Climate Change Mitigation in Livestock Production: Nonconventional Feedstuffs and Alternative Additives. In Livestock Health and Farming; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Lee, S.-S. Advanced Estimation and Mitigation Strategies: A Cumulative Approach to Enteric Methane Abatement from Ruminants. J. Anim. Sci. Technol. 2019, 61, 122–137. [Google Scholar] [CrossRef] [Green Version]
- Hegarty, R.S. Reducing Rumen Methane Emissions through Elimination of Rumen Protozoa. Aust. J. Agric. Res. 1999, 50, 1321. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane Mitigation in Ruminants: From Microbe to the Farm Scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [Green Version]
- Wright, A. Reducing Methane Emissions in Sheep by Immunization against Rumen Methanogens. Vaccine 2004, 22, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Williams, Y.J.; Popovski, S.; Rea, S.M.; Skillman, L.C.; Toovey, A.F.; Northwood, K.S.; Wright, A.-D.G. A Vaccine against Rumen Methanogens Can Alter the Composition of Archaeal Populations. Appl. Environ. Microbiol. 2009, 75, 1860–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickering, N.K.; Oddy, V.H.; Basarab, J.; Cammack, K.; Hayes, B.; Hegarty, R.S.; Lassen, J.; McEwan, J.C.; Miller, S.; Pinares-Patiño, C.S. Animal Board Invited Review: Genetic Possibilities to Reduce Enteric Methane Emissions from Ruminants. Animal 2015, 9, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of Ionophores in Cattle Diets for Mitigation of Enteric Methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, G.; Makkar, H.P.S.; Becker, K. Inhibition of Methanogens by Bromochloromethane: Effects on Microbial Communities and Rumen Fermentation Using Batch and Continuous Fermentations. Br. J. Nutr. 2009, 101, 1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Beauchemin, K.A. A Review of Feeding Supplementary Nitrate to Ruminant Animals: Nitrate Toxicity, Methane Emissions, and Production Performance. Can. J. Anim. Sci. 2014, 94, 557–570. [Google Scholar] [CrossRef]
- Pacheco, D.; Waghorn, G.; Janssen, P.H. Decreasing Methane Emissions from Ruminants Grazing Forages: A Fit with Productive and Financial Realities? Anim. Prod. Sci. 2014, 54, 1141–1154. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M. Methane Emissions from Feedlot Cattle Fed Barley or Corn Diets. J. Anim. Sci. 2005, 83, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the Addition of Fumarate on Methane Production by Ruminal Microorganisms In Vitro. J. Dairy Sci. 1999, 82, 780–787. [Google Scholar] [CrossRef]
- Tejido, M.; Ranilla, M.; García-Martínez, R.; Carro, M. In vitro microbial growth and rumen fermentation of different substrates as affected by the addition of disodium malate. Anim. Sci. 2005, 81, 31–38. [Google Scholar] [CrossRef]
- Grainger, C.; Beauchemin, K.A. Can Enteric Methane Emissions from Ruminants Be Lowered without Lowering Their Production? Anim. Feed Sci. Technol. 2011, 166–167, 308–320. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia Mearnsii Tannins Decreases Methanogenesis and Urinary Nitrogen in Forage-Fed Sheep. Aust. J. Agric. Res. 2005, 56, 961. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential Use of Acacia Mearnsii Condensed Tannins to Reduce Methane Emissions and Nitrogen Excretion from Grazing Dairy Cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Benchaar, C.; Greathead, H. Essential Oils and Opportunities to Mitigate Enteric Methane Emissions from Ruminants. Anim. Feed Sci. Technol. 2011, 166–167, 338–355. [Google Scholar] [CrossRef]
- McGinn, S.M.; Beauchemin, K.A.; Coates, T.; Colombatto, D. Methane Emissions from Beef Cattle: Effects of Monensin, Sunflower Oil, Enzymes, Yeast, and Fumaric Acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, W.P.; Wittenberg, K.; Corrigan, D. Impact of Pasture Type on Methane Production by Lactating Beef Cows. Can. J. Anim. Sci. 1999, 79, 221–226. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methanogenesis from Forages Fed to Sheep. In Proceedings of the Conference New Zeeland Grassland Association, Gore, NZ, USA, 12–16 November 2012; pp. 167–172. [Google Scholar]
- Allen, M.S. Physical Constraints on Voluntary Intake of Forages by Ruminants. J. Anim. Sci. 1996, 74, 3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.; Hummel, J.; Clauss, M. The Relationship between Forage Cell Wall Content and Voluntary Food Intake in Mammalian Herbivores. Mammal Rev. 2010, 40, 221–245. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane Production by Ruminants: Its Contribution to Global Warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef] [Green Version]
- Ominski, K.H.; Wittenberg, K.M. Strategies for Reducing Enteric Methane Emissions in Forage-Based Beef Production Systems. In Climate Change and Managed Ecosystems; CRC Press: Boca Raton, FL, USA, 2005; pp. 261–272. [Google Scholar]
- Pelletier, S.; Tremblay, G.F.; Bélanger, G.; Bertrand, A.; Castonguay, Y.; Pageau, D.; Drapeau, R. Forage Nonstructural Carbohydrates and Nutritive Value as Affected by Time of Cutting and Species. Agron. J. 2010, 102, 1388. [Google Scholar] [CrossRef]
- Phelan, P.; Moloney, A.P.; McGeough, E.J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P. Forage Legumes for Grazing and Conserving in Ruminant Production Systems. Crit. Rev. Plant Sci. 2015, 34, 281–326. [Google Scholar] [CrossRef]
- Fulkerson, W.J.; Neal, J.S.; Clark, C.F.; Horadagoda, A.; Nandra, K.S.; Barchia, I. Nutritive Value of Forage Species Grown in the Warm Temperate Climate of Australia for Dairy Cows: Grasses and Legumes. Livest. Sci. 2007, 107, 253–264. [Google Scholar] [CrossRef]
- Villalba, J.J.; Ates, S.; MacAdam, J.W. Non-Fiber Carbohydrates in Forages and Their Influence on Beef Production Systems. Front. Sustain. Food Syst. 2021, 5, 566338. [Google Scholar] [CrossRef]
- Van Soest, P.J.V. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 2018; ISBN 978-1-5017-3235-5. [Google Scholar]
- Waghorn, G.C.; Shelton, I.D.; Thomas, V.J. Particle Breakdown and Rumen Digestion of Fresh Ryegrass (Lolium perenne L.) and Lucerne (Medicago sativa L.) Fed to Cows during a Restricted Feeding Period. Br. J. Nutr. 1989, 61, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Henderson, G.; Cox, F.; Molano, G.; Harrison, S.J.; Luo, D.; Janssen, P.H.; Pacheco, D. Lambs Fed Fresh Winter Forage Rape (Brassica napus L.) Emit Less Methane than Those Fed Perennial Ryegrass (Lolium perenne L.), and Possible Mechanisms behind the Difference. PLoS ONE 2015, 10, e0119697. [Google Scholar] [CrossRef] [PubMed]
- Archimède, H.; Eugène, M.; Marie Magdeleine, C.; Boval, M.; Martin, C.; Morgavi, D.P.; Lecomte, P.; Doreau, M. Comparison of Methane Production between C3 and C4 Grasses and Legumes. Anim. Feed Sci. Technol. 2011, 166–167, 59–64. [Google Scholar] [CrossRef]
- Pitcher, L.R. Beef Average Daily Gain and Enteric Methane Emissions on Birdsfoot Trefoil, Cicer Milkvetch and Meadow Brome Pastures; All Graduate Theses and Dissertations 4015. Master’s Thesis, Utah State University, Logan, UT, USA, 2015. Available online: https://digitalcommons.usu.edu/etd/401590 (accessed on 12 August 2021).
- Berthiaume, R.; Benchaar, C.; Chaves, A.V.; Tremblay, G.F.; Castonguay, Y.; Bertrand, A.; Bélanger, G.; Michaud, R.; Lafrenière, C.; McAllister, T.A. Effects of Nonstructural Carbohydrate Concentration in Alfalfa on Fermentation and Microbial Protein Synthesis in Continuous Culture. J. Dairy Sci. 2010, 93, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Popp, J.D.; McCaughey, W.P.; Cohen, R.D.H.; McAllister, T.A.; Majak, W. Enhancing Pasture Productivity with Alfalfa: A Review. Can. J. Plant Sci. 2000, 80, 513–519. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Ward, R.E.; Griggs, T.C.; Min, B.R.; Aiken, G.E. Average Daily Gain and Blood Fatty Acid Composition of Cattle Grazing the Nonbloating Legumes Birdsfoot Trefoil and Cicer Milkvetch in the Mountain West. Prof. Anim. Sci. 2011, 27, 574–583. [Google Scholar] [CrossRef]
- MacAdam, J.; Villalba, J. Beneficial Effects of Temperate Forage Legumes That Contain Condensed Tannins. Agriculture 2015, 5, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Yost, M.; Allen, N.; Creech, E.; Putnam, D.; Gale, J.; Shewmaker, G. Ten Reasons Why Alfalfa Is Highly Suitable for the West; Utah State University Agriculture Extension; AG/Crops/2020-01pr.; Utah State University College of Agriculture and Applied Sciences: Logan, UT, USA, 2020; Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=3100&context=extension_curall (accessed on 12 August 2021).
- Wang, Y.; Majak, W.; McAllister, T.A. Frothy Bloat in Ruminants: Cause, Occurrence, and Mitigation Strategies. Anim. Feed Sci. Technol. 2012, 172, 103–114. [Google Scholar] [CrossRef]
- Majak, W.; McAllister, T.A.; McCartney, D.; Stanford, K.; Cheng, K.J. Bloat in Cattle; Alberta Agriculture Food and Rural Development, Information Packaging Center: Edmonton, AB, Canada, 2003; pp. 1–24. [Google Scholar]
- Cameron, A.R.; Malmo, J. A Survey of the Efficacy of Sustained-release Monensin Capsules in the Control of Bloat in Dairy. Cattle. Aust. Vet. J. 1993, 70, 1–4. [Google Scholar] [CrossRef]
- Thompson, D.J.; Brooke, B.M.; Garland, G.J.; Hall, J.W.; Majak, W. Effect of Stage of Growth of Alfalfa on the Incidence of Bloat in Cattle. Can. J. Anim. Sci. 2000, 80, 725–727. [Google Scholar] [CrossRef]
- Julier, B.; Guines, F.; Emile, J.-C.; Huyghe, C. Variation in Protein Degradability in Dried Forage Legumes. Anim. Res. 2003, 52, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Abdoun, K.; Stumpff, F.; Martens, H. Ammonia and Urea Transport across the Rumen Epithelium: A Review. Anim. Health Res. Rev. 2006, 7, 43–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobley, G.E.; Milano, G.D. Regulation of Hepatic Nitrogen Metabolism in Ruminants. Proc. Nutr. Soc. 1997, 56, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; van Vuuren, A.M. Strategies for Optimizing Nitrogen Use by Ruminants. Animal 2010, 4, 1184–1196. [Google Scholar] [CrossRef]
- Provenza, F.D. Postingestive Feedback as an Elementary Determinant of Food Preference and Intake in Ruminants. J. Range Manag. 1995, 48, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using Blood Urea Nitrogen to Predict Nitrogen Excretion and Efficiency of Nitrogen Utilization in Cattle, Sheep, Goats, Horses, Pigs, and Rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [Green Version]
- Getachew, G.; Depeters, E.J.; Pittroff, W.; Putnam, D.H.; Dandekar, A.M. Review: Does Protein in Alfalfa Need Protection from Rumen Microbes? Prof. Anim. Sci. 2006, 22, 364–373. [Google Scholar] [CrossRef]
- Dijkstra, J.; Oenema, O.; van Groenigen, J.W.; Spek, J.W.; van Vuuren, A.M.; Bannink, A. Diet Effects on Urine Composition of Cattle and N2O Emissions. Animal 2013, 7, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonderland-Thomassen, M.A.; Lieffering, M.; Ledgard, S.F. Water Footprint of Beef Cattle and Sheep Produced in New Zealand: Water Scarcity and Eutrophication Impacts. J. Clean. Prod. 2014, 73, 253–262. [Google Scholar] [CrossRef]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; de Vries, W.; Weiss, F. Impacts of European Livestock Production: Nitrogen, Sulphur, Phosphorus and Greenhouse Gas Emissions, Land-Use, Water Eutrophication and Biodiversity. Environ. Res. Lett. 2015, 10, 115004. [Google Scholar] [CrossRef]
- Oenema, O.; Wrage, N.; Velthof, G.L.; van Groenigen, J.W.; Dolfing, J.; Kuikman, P.J. Trends in Global Nitrous Oxide Emissions from Animal Production Systems. Nutr. Cycl. Agroecosystems 2005, 72, 51–65. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-Oxidation as an Engine to Generate Nitrous Oxide in an Intensively Managed Calcareous Fluvo-Aquic Soil. Sci. Rep. 2015, 4, 3950. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.; Mayer, L. Climate Change 2014: Synthesis Report; Intergovernmental Panel on Climate Change, Ed.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2015; ISBN 978-92-9169-143-2. [Google Scholar]
- Bao, Y.; Zhou, K.; Zhao, G. Nitrous Oxide Emissions from the Urine of Beef Cattle as Regulated by Dietary Crude Protein and Gallic Acid. J. Anim. Sci. 2018, 96, 3699–3711. [Google Scholar] [CrossRef]
- Cai, Y.; Chang, S.X.; Cheng, Y. Greenhouse Gas Emissions from Excreta Patches of Grazing Animals and Their Mitigation Strategies. Earth-Sci. Rev. 2017, 171, 44–57. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Acharya, S. Condensed Tannins in Sainfoin: Composition, Concentration, and Effects on Nutritive and Feeding Value of Sainfoin Forage. Crop Sci. 2015, 55, 13. [Google Scholar] [CrossRef]
- Lees, G.L.; Suttill, N.H.; Gruber, M.Y. Condensed Tannins in Sainfoin. 1. A Histological and Cytological Survey of Plant Tissues. Can. J. Bot. 1993, 71, 1147–1152. [Google Scholar] [CrossRef]
- Sengul, S. Performance of Some Forage Grasses or Legumes and Their Mixtures under Dry Land Conditions. Eur. J. Agron. 2003, 19, 401–409. [Google Scholar] [CrossRef]
- Karnezos, T.P.; Matches, A.G.; Brown, C.P. Spring Lamb Production on Alfalfa, Sainfoin, and Wheatgrass Pastures. Agron. J. 1994, 86, 497–502. [Google Scholar] [CrossRef]
- Maughan, B.; Provenza, F.D.; Tansawat, R.; Maughan, C.; Martini, S.; Ward, R.; Clemensen, A.; Song, X.; Cornforth, D.; Villalba, J.J. Importance of Grass-Legume Choices on Cattle Grazing Behavior, Performance, and Meat Characteristics. J. Anim. Sci. 2014, 92, 2309–2324. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, S.; Beauchemin, K.A.; MacAdam, J.; Villalba, J.J. Grazing Diverse Combinations of Tanniferous and Non-Tanniferous Legumes: Implications for Beef Cattle Performance and Environmental Impact. Sci. Total Environ. 2020, 746, 140788. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, S.P. Influence of Forage Diversity and Condensed Tannins on Livestock Foraging Behavior, Production and Environmental Impact; All Graduate Theses and Dissertations 7813. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2020. Available online: https://digitalcommons.usu.edu/etd/7813/ (accessed on 12 August 2021).
- Grabber, J.H.; Riday, H.; Cassida, K.A.; Griggs, T.C.; Min, D.H.; MacAdam, J.W. Yield, Morphological Characteristics, and Chemical Composition of European- and Mediterranean-Derived Birdsfoot Trefoil Cultivars Grown in the Colder Continental United States. Crop Sci. 2014, 54, 1893. [Google Scholar] [CrossRef]
- Gibb, M.; Orr, R. Grazing Behaviour of Ruminants. IGER Innov. 1997, 1, 54–57. [Google Scholar]
- Grabber, J.H.; Coblentz, W.K.; Riday, H.; Griggs, T.C.; Min, D.H.; MacAdam, J.W.; Cassida, K.A. Protein and Dry-Matter Degradability of European- and Mediterranean-Derived Birdsfoot Trefoil Cultivars Grown in the Colder Continental USA. Crop Sci. 2015, 55, 1356. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Griggs, T.C. Irrigated Birdsfoot Trefoil Variety Trial: Forage Yield; All Current Publications Paper 1337; Utah State University: Logan, UT, USA, 2013; Available online: https://digitalcommons.usu.edu/extension_curall/1337 (accessed on 12 August 2021).
- Barry, T.N.; McNabb, W.C. The Implications of Condensed Tannins on the Nutritive Value of Temperate Forages Fed to Ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Waghorn, G. Beneficial and Detrimental Effects of Dietary Condensed Tannins for Sustainable Sheep and Goat Production—Progress and Challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Zeller, W.E. Activity, Purification, and Analysis of Condensed Tannins: Current State of Affairs and Future Endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861. [Google Scholar] [CrossRef] [Green Version]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The Role of Condensed Tannins in Ruminant Animal Production: Advances, Limitations and Future Directions. Rev. Bras. Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Lees, G.L.; Gruber, M.Y.; Suttill, N.H. Condensed Tannins in Sainfoin. II. Occurrence and Changes during Leaf Development. Can. J. Bot. 1995, 73, 1540–1547. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrère, J.; Andueza, D.; Pourrat, J.; Le Morvan, A.; Stringano, E.; Mueller-Harvey, I.; Baumont, R. Effects of Condensed Tannins in Fresh Sainfoin (Onobrychis viciifolia) on in Vivo and in Situ Digestion in Sheep. Anim. Feed Sci. Technol. 2010, 160, 23–38. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed Tannin Concentrations Found in Vegetative and Mature Forage Legumes Grown in Western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- Aerts, R.J.; Barry, T.N.; McNabb, W.C. Polyphenols and Agriculture: Beneficial Effects of Proanthocyanidins in Forages. Agric. Ecosyst. Environ. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- Jonker, A.; Yu, P. The Occurrence, Biosynthesis, and Molecular Structure of Proanthocyanidins and Their Effects on Legume Forage Protein Precipitation, Digestion and Absorption in the Ruminant Digestive Tract. Int. J. Mol. Sci. 2017, 18, 1105. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Mcnabb, W.C.; Barry, T.N.; Peters, J.S. Solubilization and Degradation of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (EC 4.1.1.39; Rubisco) Protein from White Clover (Trifolium repens) and Lotus Corniculatus by Rumen Microorganisms and the Effect of Condensed Tannins on These Processes. J. Agric. Sci. 2000, 134, 305–317. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A. Effects of Sainfoin (Onobrychis viciifolia Scop.) Condensed Tannins on Growth and Proteolysis by Four Strains of Ruminal Bacteria. Appl. Environ. Microbiol. 1994, 60, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNabb, W.C.; Waghorn, G.C.; Peters, J.S.; Barry, T.N. The Effect of Condensed Tannins in Lotus Pedunculatus on the Solubilization and Degradation of Ribulose-1,5-Bisphosphate Carboxylase (EC 4.1.1.39; Rubisco) Protein in the Rumen and the Sites of Rubisco Digestion. Br. J. Nutr. 1996, 76, 535–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufrère, J.; Dudilieu, M.; Andueza, D.; Poncet, C.; Baumont, R. Mixing Sainfoin and Lucerne to Improve the Feed Value of Legumes Fed to Sheep by the Effect of Condensed Tannins. Animal 2013, 7, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Avila, S.C.; Kozloski, G.V.; Orlandi, T.; Mezzomo, M.P.; Stefanello, S. Impact of a Tannin Extract on Digestibility, Ruminal Fermentation and Duodenal Flow of Amino Acids in Steers Fed Maize Silage and Concentrate Containing Soybean Meal or Canola Meal as Protein Source. J. Agric. Sci. 2015, 153, 943–953. [Google Scholar] [CrossRef]
- Perez-Maldonado, R.A.; Norton, B.W.; Kerven, G.L. Factors Affecting in Vitro Formation of Tannin-protein Complexes. J. Sci. Food Agric. 1995, 69, 291–298. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Hutchinson, K.J.; Revell, D.K.; Brookes, I.M.; Mcnabb, W.C. The Effect of Condensed Tannins in Sainfoin (Onobrychis viciifolia) and Sulla (Hedysarum coronarium) on the Digestion of Amino Acids in Sheep. Proc. N. Z. Soc. Anim. Prod. 2001, 61, 5. [Google Scholar]
- Naumann, H.D.; Hagerman, A.E.; Lambert, B.D.; Muir, J.P.; Tedeschi, L.O.; Kothmann, M.M. Molecular Weight and Protein-Precipitating Ability of Condensed Tannins from Warm-Season Perennial Legumes. J. Plant Interact. 2014, 9, 212–219. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of Structural Features of Condensed Tannins That Affect Protein Aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef]
- AufrèRe, J.; Theodoridou, K.; Mueller-Harvey, I.; Yu, P.; Andueza, D. Ruminal Dry Matter and Nitrogen Degradation in Relation to Condensed Tannin and Protein Molecular Structures in Sainfoin (Onobrychis viciifolia) and Lucerne (Medicago sativa). J. Agric. Sci. 2014, 152, 333–345. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- McAllister, T.A.; Martinez, T.; Bae, H.D.; Muir, A.D.; Yanke, L.J.; Jones, G.A. Characterization of Condensed Tannins Purified from Legume Forages: Chromophore Production, Protein Precipitation, and Inhibitory Effects on Cellulose Digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef]
- Aufrère, J.; Dudilieu, M.; Poncet, C. In Vivo and in Situ Measurements of the Digestive Characteristics of Sainfoin in Comparison with Lucerne Fed to Sheep as Fresh Forages at Two Growth Stages and as Hay. Animal 2008, 2, 1331–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufino-Moya, P.J.; Blanco, M.; Bertolín, J.R.; Joy, M. Methane Production of Fresh Sainfoin, with or without PEG, and Fresh Alfalfa at Different Stages of Maturity is Similar but the Fermentation End Products Vary. Animals 2019, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Wyss, U.; Hess, H.D.; Kreuzer, M.; Dohme, F. Effect of Feeding Dehydrated and Ensiled Tanniferous Sainfoin (Onobrychis viciifolia) on Nitrogen and Mineral Digestion and Metabolism of Lambs. Arch. Anim. Nutr. 2007, 61, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Theodoridou, K.; Aufrère, J.; Andueza, D.; Le Morvan, A.; Picard, F.; Pourrat, J.; Baumont, R. Effects of Condensed Tannins in Wrapped Silage Bales of Sainfoin (Onobrychis viciifolia) on in Vivo and in Situ Digestion in Sheep. Animal 2012, 6, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Williams, C.M.; Eun, J.-S.; MacAdam, J.W.; Young, A.J.; Fellner, V.; Min, B.R. Effects of Forage Legumes Containing Condensed Tannins on Methane and Ammonia Production in Continuous Cultures of Mixed Ruminal Microorganisms. Anim. Feed Sci. Technol. 2011, 166–167, 364–372. [Google Scholar] [CrossRef]
- Grosse Brinkhaus, A.; Bee, G.; Silacci, P.; Kreuzer, M.; Dohme-Meier, F. Effect of Exchanging Onobrychis Viciifolia and Lotus Corniculatus for Medicago Sativa on Ruminal Fermentation and Nitrogen Turnover in Dairy Cows. J. Dairy Sci. 2016, 99, 4384–4397. [Google Scholar] [CrossRef] [Green Version]
- Aufrère, J.; Dudilieu, M.; Poncet, C.; Baumont, R. Effect of Condensed Tannins in Sainfoin on in Vitro Protein Solubility of Lucerne. In Proceedings of the XX International Grassland Congress: Grasslands–A Global Resource, Dublin, Ireland, 31 August 2005; O’Mara, F.P., Wilkins, R.J., Mannetje, L., Lovett, D.K., Rogers, P.A.M., Boland, T.M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; p. 248. [Google Scholar]
- Stewart, E.K.; Beauchemin, K.A.; Dai, X.; MacAdam, J.W.; Christensen, R.G.; Villalba, J.J. Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. J. Anim. Sci. 2019, 97, 3286–3299. [Google Scholar] [CrossRef]
- McNabb, W.C.; Peters, J.S.; Foo, L.Y.; Waghorn, G.C.; Jackson, F.S. Effect of Condensed Tannins Prepared from Several Forages on the in Vitro Precipitation of Ribulose-1,5-Bisphosphate Carboxylase (Rubisco) Protein and its Digestion by Trypsin (EC 2.4.21.4) and Chymotrypsin (EC 2.4.21.1). J. Sci. Food Agric. 1998, 77, 201–212. [Google Scholar] [CrossRef]
- Lagrange, S.; Villalba, J.J. Tannin-Containing Legumes and Forage Diversity Influence Foraging Behavior, Diet Digestibility, and Nitrogen Excretion by Lambs. J. Anim. Sci. 2019, 97, 3994–4009. [Google Scholar] [CrossRef] [PubMed]
- Azuhnwi, B.N.; Hertzberg, H.; Arrigo, Y.; Gutzwiller, A.; Hess, H.D.; Mueller-Harvey, I.; Torgerson, P.R.; Kreuzer, M.; Dohme-Meier, F. Investigation of Sainfoin (Onobrychis viciifolia) Cultivar Differences on Nitrogen Balance and Fecal Egg Count in Artificially Infected Lambs. J. Anim. Sci. 2013, 91, 2343–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.K.; Saxena, J. A New Perspective on the Use of Plant Secondary Metabolites to Inhibit Methanogenesis in the Rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; McNABB, W.C.; Barry, T.N.; Kemp, P.D.; Waghorn, G.C.; McDonald, M.F. The Effect of Condensed Tannins in Lotus Corniculatus upon Reproductive Efficiency and Wool Production in Sheep during Late Summer and Autumn. J. Agric. Sci. 1999, 132, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.G.; Yang, S.Y.; Eun, J.-S.; Young, A.J.; Hall, J.O.; MacAdam, J.W. Effects of Feeding Birdsfoot Trefoil Hay on Neutral Detergent Fiber Digestion, Nitrogen Utilization Efficiency, and Lactational Performance by Dairy Cows. J. Dairy Sci. 2015, 98, 7982–7992. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.R.; MacAdam, J.W.; Griggs, T.C. Lignification and Tannin Localization during the Development of Birdsfoot Trefoil Stems. Crop Sci. Madison 2014, 54, 1876–1886. [Google Scholar] [CrossRef]
- Hunt, S.R.; Griggs, T.C.; MacAdam, J.W. Change in Birdsfoot Trefoil (Lotus corniculatus L.) Nutritive Value with Stem Elongation, Flowering and Pod Formation. In EGF at 50: The Future of European Grasslands, Proceedings of the 25th General Meeting of the European Grassland Federation, Aberystwyth, Wales, 7–11 September 2014; IBERS, Aberystwyth University: Aberystwyth, UK, 2014; pp. 884–886. [Google Scholar]
- Douglas, G.B.; Wang, Y.; Waghorn, G.C.; Barry, T.N.; Purchas, R.W.; Foote, A.G.; Wilson, G.F. Liveweight Gain and Wool Production of Sheep Grazing Lotus Corniculatus and Lucerne (Medicago sativa). N. Z. J. Agric. Res. 1995, 38, 95–104. [Google Scholar] [CrossRef]
- Harris, S.L.; Clark, D.A.; Laboyrie, P.J. Birdsfoot Trefoil—An Alternative Legume for New Zealand Dairy Pastures; New Zealand Grassland Association: Wellington, New Zealand, 1998; Volume 60, pp. 99–103. [Google Scholar]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed Tannins in Birdsfoot Trefoil (Lotus corniculatus) Reduce Methane Emissions from Dairy Cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 6. [Google Scholar]
- Moreira, G.D.; Lima, P.D.M.T.; Borges, B.O.; Primavesi, O.; Longo, C.; McManus, C.; Abdalla, A.; Louvandini, H. Tropical Tanniniferous Legumes Used as an Option to Mitigate Sheep Enteric Methane Emission. Trop. Anim. Health Prod. 2013, 45, 879–882. [Google Scholar] [CrossRef]
- Wang, S.; Terranova, M.; Kreuzer, M.; Marquardt, S.; Eggerschwiler, L.; Schwarm, A. Supplementation of Pelleted Hazel (Corylus avellana) Leaves Decreases Methane and Urinary Nitrogen Emissions by Sheep at Unchanged Forage Intake. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of Quebracho Tannin Extract on Intake, Digestibility, Rumen Fermentation, and Methane Production in Crossbred Heifers Fed Low-Quality Tropical Grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population in Vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Hatew, B.; Stringano, E.; Mueller-Harvey, I.; Hendriks, W.H.; Carbonero, C.H.; Smith, L.M.J.; Pellikaan, W.F. Impact of Variation in Structure of Condensed Tannins from Sainfoin (Onobrychis viciifolia) on in Vitro Ruminal Methane Production and Fermentation Characteristics. J. Anim. Physiol. Anim. Nutr. 2016, 100, 348–360. [Google Scholar] [CrossRef]
- McMahon, L.R.; Majak, W.; McAllister, T.A.; Hall, J.W.; Jones, G.A.; Popp, J.D.; Cheng, K.J. Effect of Sainfoin on in Vitro Digestion of Fresh Alfalfa and Bloat in Steers. Can. J. Anim. Sci. 1999, 79, 203–212. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrère, J.; Niderkorn, V.; Andueza, D.; Le Morvan, A.; Picard, F.; Baumont, R. In Vitro Study of the Effects of Condensed Tannins in Sainfoin on the Digestive Process in the Rumen at Two Vegetation Cycles. Anim. Feed Sci. Technol. 2011, 170, 147–159. [Google Scholar] [CrossRef]
- Niderkorn, V.; Barbier, E.; Macheboeuf, D.; Torrent, A.; Mueller-Harvey, I.; Hoste, H. In Vitro Rumen Fermentation of Diets with Different Types of Condensed Tannins Derived from Sainfoin (Onobrychis viciifolia Scop.) Pellets and Hazelnut (Corylus avellana L.) Pericarps. Anim. Feed Sci. Technol. 2020, 259, 114357. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane Production from in Vitro Rumen Incubations with Lotus Pedunculatus and Medicago Sativa, and Effects of Extractable Condensed Tannin Fractions on Methanogenesis. Anim. Feed Sci. Technol. 2005, 123–124, 403–419. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W. Effects of Condensed Tannin Fractions of Different Molecular Weights on Population and Diversity of Bovine Rumen Methanogenic Archaea in Vitro, as Determined by High-Throughput Sequencing. Anim. Feed Sci. Technol. 2016, 216, 146–160. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of Condensed Tannins from Leucaena on Methane Production, Rumen Fermentation and Populations of Methanogens and Protozoa in Vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of Rumen Fermentation and Methane Production with Plant Secondary Metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited Review: Plant Polyphenols and Rumen Microbiota Responsible for Fatty Acid Biohydrogenation, Fiber Digestion, and Methane Emission: Experimental Evidence and Methodological Approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Bae, H.D.; McAllister, T.A.; Yanke, J.; Cheng, K.J.; Muir, A.D. Effects of Condensed Tannins on Endoglucanase Activity and Filter Paper Digestion by Fibrobacter Succinogenes S85t. Appl. Environ. Microbiol. 1993, 59, 2132–2138. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, C.S.; Palmer, B.; Bunch, R.; Krause, D.O. Effect of the Tropical Forage Calliandra on Microbial Protein Synthesis and Ecology in the Rumen. J. Appl. Microbiol. 2001, 90, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Bento, M.H.L.; Acamovic, T.; Makkar, H.P.S. The Influence of Tannin, Pectin and Polyethylene Glycol on Attachment of 15N-Labelled Rumen Microorganisms to Cellulose. Anim. Feed Sci. Technol. 2005, 122, 41–57. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Mc Geough, E.J.; Acharya, S.; McAllister, T.A.; McGinn, S.M.; Harstad, O.M.; Beauchemin, K.A. Enteric Methane Emission, Diet Digestibility, and Nitrogen Excretion from Beef Heifers Fed Sainfoin or Alfalfa. J. Anim. Sci. 2013, 91, 4861–4874. [Google Scholar] [CrossRef] [Green Version]
- Vaithiyanathan, S.; Bhatta, R.; Mishra, A.S.; Prasad, R.; Verma, D.L.; Singh, N.P. Effect of Feeding Graded Levels of Prosopis Cineraria Leaves on Rumen Ciliate Protozoa, Nitrogen Balance and Microbial Protein Supply in Lambs and Kids. Anim. Feed Sci. Technol. 2007, 133, 177–191. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the Nature of Tannins on in Vitro Ruminal Methane and Volatile Fatty Acid Production and on Methanogenic Archaea and Protozoal Populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, B.R.; Parker, D.; Brauer, D.; Waldrip, H.; Lockard, C.; Hales, K.; Akbay, A.; Augyte, S. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim. Nutr. 2021, 7, 1371–1387. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Gómez-Bravo, C.A.; Aguilar-Pérez, C.F.; Solorio-Sánchez, F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 7, 584. [Google Scholar] [CrossRef] [PubMed]
- Mbiriri, D.T.; Cho, S.; Mamvura, C.I.; Choi, N.J. Assessment of rumen microbial adaptation to garlic oil, carvacrol and thymol using the consecutive batch culture system. J. Vet. Sci. Anim. Husb. 2015, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.J. A Review of the Effects of Forage Condensed Tannins on Ruminal Fermentation and Bloat in Grazing Cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-G.; Tanner, G.; Larkin, P. The DMACA–HCl Protocol and the Threshold Proanthocyanidin Content for Bloat Safety in Forage Legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Wang, Y.; Berg, B.P.; Barbieri, L.R.; Veira, D.M.; McAllister, T.A. Comparison of Alfalfa and Mixed Alfalfa-Sainfoin Pastures for Grazing Cattle: Effects on Incidence of Bloat, Ruminal Fermentation, and Feed Intake. Can. J. Anim. Sci. 2006, 86, 383–392. [Google Scholar] [CrossRef]
- Chail, A.; Legako, J.F.; Pitcher, L.R.; Griggs, T.C.; Ward, R.E.; Martini, S.; MacAdam, J.W. Legume Finishing Provides Beef with Positive Human Dietary Fatty Acid Ratios and Consumer Preference Comparable with Grain-Finished Beef. J. Anim. Sci. 2016, 94, 2184–2197. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty Acids from Fish: The Anti-Inflammatory Potential of Long-Chain Omega-3 Fatty Acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Gomez Candela, C.; López, L.B.; Kohen, V.L. Importance of a Balanced Omega 6/Omega 3 Ratio for the Maintenance of Health Nutritional Recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Makkar, H.P.S.; Mele, M.; Priolo, A. Ruminal Biohydrogenation as Affected by Tannins in Vitro. Br. J. Nutr. 2008, 102, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasta, V.; Mele, M.; Serra, A.; Scerra, M.; Luciano, G.; Lanza, M.; Priolo, A. Metabolic Fate of Fatty Acids Involved in Ruminal Biohydrogenation in Sheep Fed Concentrate or Herbage with or without Tannins. J. Anim. Sci. 2009, 87, 2674–2684. [Google Scholar] [CrossRef] [Green Version]
- Temperton, V.M.; Mwangi, P.N.; Scherer-Lorenzen, M.; Schmid, B.; Buchmann, N. Positive interactions between nitrogen-fixing legumes and four different neighboring species in a biodiversity experiment. Oecologia 2007, 151, 190–205. [Google Scholar] [CrossRef]
- Pirhofer-Walzl, K.; Rasmussen, J.; Høgh-Jensen, H.; Eriksen, J.; Søegaard, K.; Rasmussen, J. Nitrogen transfer from forage legumes to nine neighbouring plants in a multi-species grassland. Plant Soil 2012, 350, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Rochette, P.; Janzen, H.H. Towards a revised coefficient for estimating N2O emissions from legumes. Nutr. Cycl. Agroecosyst. 2005, 73, 171–179. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982; ISBN 0-691-08302-9. [Google Scholar]
- Provenza, F.D.; Villalba, J.J.; Haskell, J.; MacAdam, J.W.; Griggs, T.C.; Wiedmeier, R.D. The Value to Herbivores of Plant Physical and Chemical Diversity in Time and Space. Crop Sci. 2007, 47, 382. [Google Scholar] [CrossRef]
- Westoby, M. What Are the Biological Bases of Varied Diets? Am. Nat. 1978, 112, 627–631. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenza, F.D.; Catanese, F.; Distel, R.A. Understanding and Manipulating Diet Choice in Grazing Animals. Anim. Prod. Sci. 2015, 55, 261. [Google Scholar] [CrossRef]
- Waghorn, G.C.; McNabb, W.C. Consequences of Plant Phenolic Compounds for Productivity and Health of Ruminants. Proc. Nutr. Soc. 2003, 62, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant Bioactives for Ruminant Health and Productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niderkorn, V.; Mueller-Harvey, I.; Le Morvan, A.; Aufrère, J. Synergistic Effects of Mixing Cocksfoot and Sainfoin on In Vitro Rumen Fermentation. Role of Condensed Tannins. Anim. Feed Sci. Technol. 2012, 178, 48–56. [Google Scholar] [CrossRef]
- Sinz, S.; Marquardt, S.; Soliva, C.R.; Braun, U.; Liesegang, A.; Kreuzer, M. Phenolic Plant Extracts are Additive in their Effects Against in Vitro Ruminal Methane and Ammonia Formation. Asian-Australas. J. Anim. Sci. 2019, 32, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Aufrère, J.; Dudilieu, M.; Poncet, C.; Baumont, R.; Dumont, B. Effect of Condensed Tannins in Sainfoin on In Vitro Protein Solubility of Lucerne as Affected by the Proportion of Sainfoin in the Mixture and the Preserving Conditions; Serie A; Options Méditerranéennes: Paris, France, 2007; pp. 63–66. Available online: http://om.ciheam.org/article.php?IDPDF=800355 (accessed on 12 August 2021).
- Villalba, J.J.; Provenza, F.D.; Han, G. Experience Influences Diet Mixing by Herbivores: Implications for Plant Biochemical Diversity. Oikos 2004, 107, 100–109. [Google Scholar] [CrossRef]
- Rogosic, J.; Estell, R.E.; Skobic, D.; Stanic, S. Influence of Secondary Compound Complementarity and Species Diversity on Consumption of Mediterranean Shrubs by Sheep. Appl. Anim. Behav. Sci. 2007, 107, 58–65. [Google Scholar] [CrossRef]
- Meuret, M.; Bruchou, C. Modélisation de l’ingestion Selon La Diversité Des Choix Alimentaires Réalisés Par La Chèvre Au Pâturage Sur Parcours. Rencontres Rech. Rumin. 1994, 1, 225–228. [Google Scholar]
- Provenza, F.D. Acquired Aversions as the Basis for Varied Diets of Ruminants Foraging on Rangelands. J. Anim. Sci. 1996, 74, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.G. Improvement of Nutrient Utilization Efficiency, Ruminal Fermentation and Lactational Performance of Dairy Cows by Feeding Birdsfoot Trefoil. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2015. Available online: https://digitalcommons.usu.edu/etd/4286207 (accessed on 15 August 2021).
- Senft, R.L.; Coughenour, M.B.; Bailey, D.W.; Rittenhouse, L.R.; Sala, O.E.; Swift, D.M. Large Herbivore Foraging and Ecological Hierarchies. BioScience 1987, 37, 789–799. [Google Scholar] [CrossRef]
- Chapman, D.F.; Parsons, A.J.; Cosgrove, G.P.; Barker, D.J.; Marotti, D.M.; Venning, K.J.; Rutter, S.M.; Hill, J.; Thompson, A.N. Impacts of Spatial Patterns in Pasture on Animal Grazing Behavior, Intake, and Performance. Crop Sci. 2007, 47, 399. [Google Scholar] [CrossRef]
- Prache, S.J.; Gordon, I.; Rook, A.J. Foraging Behaviour and Diet Selection in Domestic Herbivores. Ann. Zootech. 1998, 47, 335–345. [Google Scholar] [CrossRef]
- Acharya, S.; Sottie, E.; Coulman, B.; Iwaasa, A.; McAllister, T.; Wang, Y.; Liu, J. New Sainfoin Populations for Bloat-Free Alfalfa Pasture Mixtures in Western Canada. Crop Sci. 2013, 53, 2283–2293. [Google Scholar] [CrossRef]
- Sottie, E.T.; Acharya, S.N.; McAllister, T.; Thomas, J.; Wang, Y.; Iwaasa, A. Alfalfa Pasture Bloat Can Be Eliminated by Intermixing with Newly-Developed Sainfoin Population. Agron. J. 2014, 106, 1470. [Google Scholar] [CrossRef] [Green Version]
- Villalba, J.J.; Manteca, X. A Case for Eustress in Grazing Animals. Front. Vet. Sci. 2019, 6, 303. [Google Scholar] [CrossRef] [PubMed]
- Catanese, F.; Obelar, M.; Villalba, J.J.; Distel, R.A. The Importance of Diet Choice on Stress-Related Responses by Lambs. Appl. Anim. Behav. Sci. 2013, 148, 37–45. [Google Scholar] [CrossRef]
- Lyons, D.M.; Parker, K.J. Stress Inoculation-Induced Indications of Resilience in Monkeys. J. Trauma. Stress. 2007, 20, 423–433. [Google Scholar] [CrossRef]
- Manteca, X.; Villalba, J.J.; Atwood, S.B.; Dziba, L.; Provenza, F.D. Is Dietary Choice Important to Animal Welfare? J. Vet. Behav. 2008, 3, 229–239. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Goslee, S.C.; Soder, K.J.; Skinner, R.H.; Tracy, B.F.; Deak, A. Plant Species Diversity, Ecosystem Function, and Pasture Management—A Perspective. Can. J. Plant Sci. 2007, 87, 479–487. [Google Scholar] [CrossRef]
- Villalba, J.J.; Beauchemin, K.A.; Gregorini, P.; MacAdam, J.W. Pasture Chemoscapes and Their Ecological Services. Transl. Anim. Sci. 2019, 3, txz003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caroprese, M.; Ciliberti, M.G.; Marino, R.; Napolitano, F.; Braghieri, A.; Sevi, A.; Albenzio, M. Effect of Information on Geographical Origin, Duration of Transport and Welfare Condition on Consumer’s Acceptance of Lamb Meat. Sci. Rep. 2020, 10, 9754. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L. Review: An Overview of Beef Production from Pasture and Feedlot Globally, as Demand for Beef and the Need for Sustainable Practices Increase. Animal 2021, 100295. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagrange, S.P.; MacAdam, J.W.; Villalba, J.J. The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production. Agronomy 2021, 11, 2264. https://doi.org/10.3390/agronomy11112264
Lagrange SP, MacAdam JW, Villalba JJ. The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production. Agronomy. 2021; 11(11):2264. https://doi.org/10.3390/agronomy11112264
Chicago/Turabian StyleLagrange, Sebastian P., Jennifer W. MacAdam, and Juan J. Villalba. 2021. "The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production" Agronomy 11, no. 11: 2264. https://doi.org/10.3390/agronomy11112264
APA StyleLagrange, S. P., MacAdam, J. W., & Villalba, J. J. (2021). The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production. Agronomy, 11(11), 2264. https://doi.org/10.3390/agronomy11112264






