Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Solid Organic Fertilisers
2.2. Analytical Methods
2.2.1. Solid Organic Fertiliser Characterisation
2.2.2. P Extraction Procedures
2.3. Data Analysis
3. Results and Discussion
3.1. General Composition
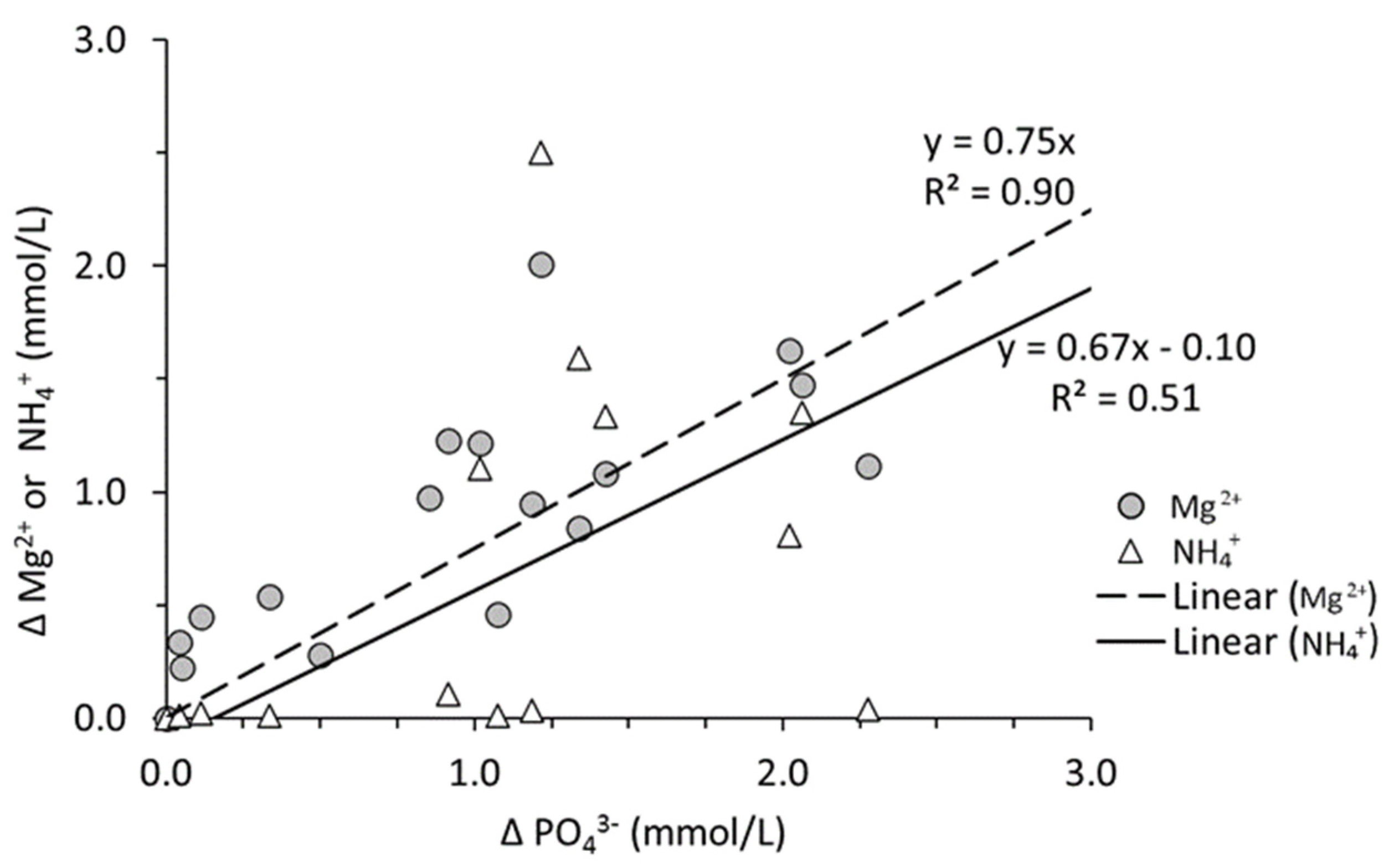
3.2. Total and Reactive Fe
3.3. Inorganic and Organic P
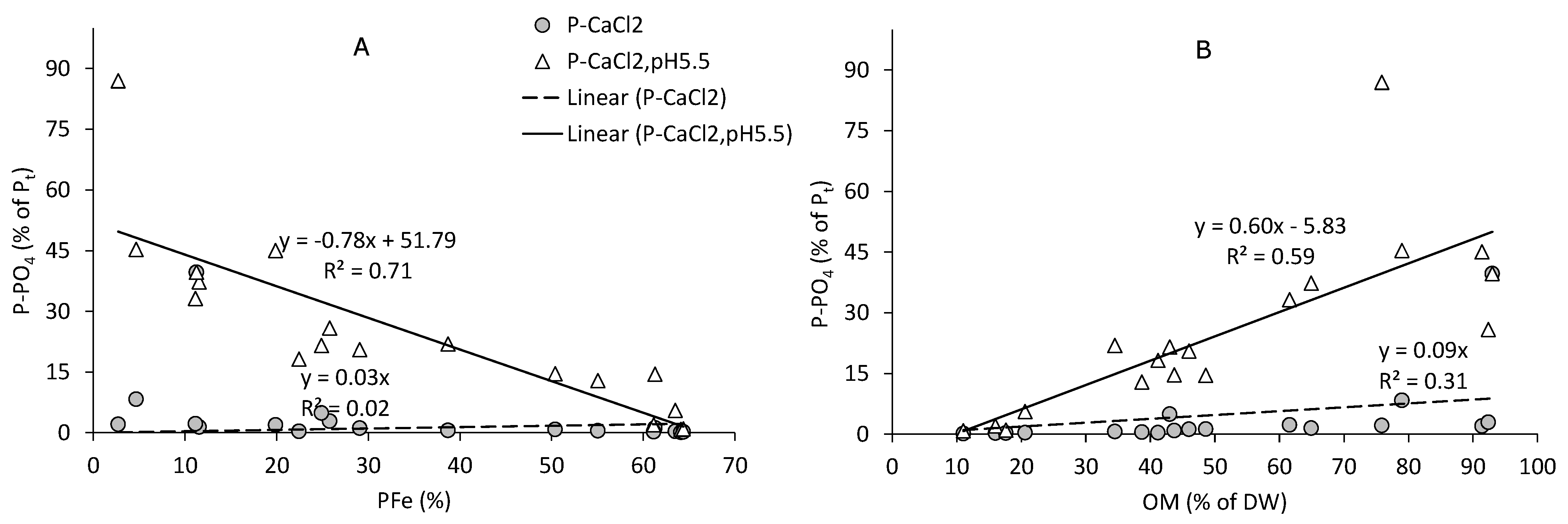
3.4. P Associated with Fe/Al-Oxides
3.5. Easily Available P
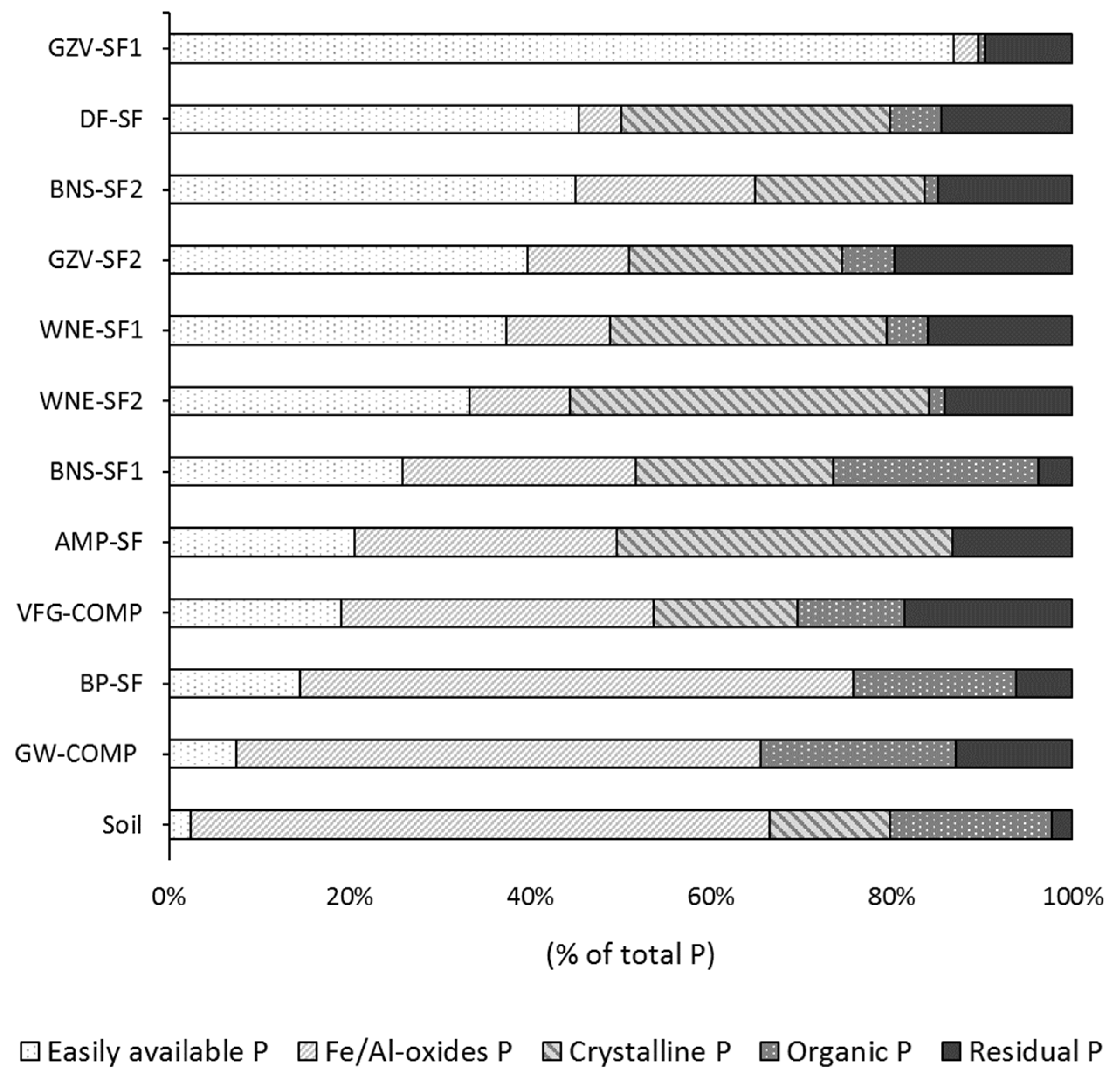
3.6. Speciation of P in SOFs and Implications for Their Use in Agriculture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, e00419. [Google Scholar] [CrossRef]
- Wellmer, F.-W.; Scholz, R.W. The Right to Know the Geopotential of Minerals for Ensuring Food Supply Security: The Case of Phosphorus. J. Ind. Ecol. 2015, 19, 3–6. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total. Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef]
- Christel, W.; Bruun, S.; Magid, J.; Jensen, L.S. Phosphorus availability from the solid fraction of pig slurry is altered by composting or thermal treatment. Bioresour. Technol. 2014, 169, 543–551. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Meers, E.; Tack, F.M.G. Efficiency of soil and fertilizer phosphorus use in time: A comparison between recovered struvite, FePO4-sludge, digestate, animal manure, and synthetic fertilizer. In Nutrient Use Efficiency: From Basics to Advance; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–417. ISBN 9788132221692. [Google Scholar]
- Grigatti, M.; Boanini, E.; Cavani, L.; Ciavatta, C.; Marzadori, C. Phosphorus in Digestate-Based Compost: Chemical Speciation and Plant-Availability. Waste Biomass Valorization 2015, 6, 481–493. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.; Meers, E. Phosphorus Use Efficiency of Bio-Based Fertilizers: Bioavailability and Fractionation. Pedosphere 2016, 26, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, B.; Demers, I.; Ziadi, N.; Chantigny, M.H.; Parent, L.-É.; Forge, T.A.; Larney, F.J.; Buckley, K.E. Forms of phosphorus in composts and in compost-amended soils following incubation. Can. J. Soil Sci. 2012, 92, 711–721. [Google Scholar] [CrossRef] [Green Version]
- Grigatti, M.; Boanini, E.; Mancarella, S.; Simoni, A.; Centemero, M.; Veeken, A.H. Phosphorous extractability and ryegrass availability from bio-waste composts in a calcareous soil. Chemosphere 2017, 174, 722–731. [Google Scholar] [CrossRef]
- Elliott, H.A.; O’Connor, G.A.; Brinton, S. Phosphorus Leaching from Biosolids-Amended Sandy Soils. J. Environ. Qual. 2002, 31, 681–689. [Google Scholar] [CrossRef]
- Meyer, G.; Frossard, E.; Mäder, P.; Nanzer, S.; Randall, D.; Udert, K.M.; Oberson, A. Water soluble phosphate fertilizers for crops grown in calcareous soils—An outdated paradigm for recycled phosphorus fertilizers? Plant Soil 2018, 424, 367–388. [Google Scholar] [CrossRef]
- Vogel, T.; Kruse, J.; Siebers, N.; Nelles, M.; Eichler-Löbermann, B. Recycled Products from Municipal Wastewater: Composition and Effects on Phosphorus Mobility in a Sandy Soil. J. Environ. Qual. 2017, 46, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Nest, T.V.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total. Environ. 2021, 750, 141699. [Google Scholar] [CrossRef]
- Talboys, P.J.; Heppell, J.; Roose, T.; Healey, J.; Jones, D.; A Withers, P.J. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Zhou, Q.; Cusick, R.D.; Margenot, A.J. Evaluating agronomic soil phosphorus tests for soils amended with struvite. Geoderma 2021, 399, 115093. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.-J.; van Loosdrecht, M. The Relevance of Phosphorus and Iron Chemistry to the Recovery of Phosphorus from Wastewater: A Review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Holford, I.C.R. Soil phosphorus: Its measurement, and its uptake by plants. Soil Res. 1997, 35, 227. [Google Scholar] [CrossRef]
- Sánchez-Alcalá, I.; del Campillo, M.C.; Torrent, J. Critical Olsen P and CaCl2-P levels as related to soil properties: Results from micropot experiments. Soil Use Manag. 2015, 31, 233–240. [Google Scholar] [CrossRef]
- Chinault, S.L.; O’Connor, G.A. Phosphorus Release from a Biosolids-Amended Sandy Spodosol. J. Environ. Qual. 2008, 37, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Cherosky, P.; Li, Y. Hydrogen sulfide removal from biogas by bio-based iron sponge. Biosyst. Eng. 2013, 114, 55–59. [Google Scholar] [CrossRef]
- Øgaard, A.F.; Brod, E. Efficient Phosphorus Cycling in Food Production: Predicting the Phosphorus Fertilization Effect of Sludge from Chemical Wastewater Treatment. J. Agric. Food Chem. 2016, 64, 4821–4829. [Google Scholar] [CrossRef]
- Schwertmann, U. Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z. Für Pflanz. Düngung Bodenkd. 1964, 105, 194–202. [Google Scholar] [CrossRef]
- Ayenew, B.; Taddesse, A.M.; Kibret, K.; Melese, A. Chemical forms of phosphorous and physicochemical properties of acid soils of Cheha and Dinsho districts, southern highlands of Ethiopia. Environ. Syst. Res. 2018, 7, 15. [Google Scholar] [CrossRef]
- Guo, F.; Yost, R.S. Quantifying the Available Soil Phosphorus Pool with the Acid Ammonium Oxalate Method. Soil Sci. Soc. Am. J. 1999, 63, 651–656. [Google Scholar] [CrossRef]
- Maguire, R.O.; Sims, J.T.; Coale, F.J. Phosphorus Solubility in Biosolids-Amended Farm Soils in the Mid-Atlantic Region of the USA. J. Environ. Qual. 2000, 29, 1225–1233. [Google Scholar] [CrossRef]
- Warrinnier, R.; Goossens, T.; Amery, F.; Nest, T.V.; Verbeeck, M.; Smolders, E. Investigation on the control of phosphate leaching by sorption and colloidal transport: Column studies and multi-surface complexation modelling. Appl. Geochem. 2019, 100, 371–379. [Google Scholar] [CrossRef]
- Elliot, H.A.; Potter, J.M.; Kang, J.; Brandt, R.C.; O’Connor, G.A. Neutral Ammonium Citrate Extraction of Biosolids Phosphorus. Commun. Soil Sci. Plant Anal. 2005, 36, 2447–2459. [Google Scholar] [CrossRef]
- Janßen, E. Extraction of Soluble Phosphorus in Soil, Sludge, Biowaste and Treated Biowaste; Horizontal-25; Giessen: Frankfurt, German, 2004; pp. 1–18. Available online: https://horizontal.ecn.nl/docs/society/horizontal/hor_desk_25_solubleP_revised.pdf (accessed on 25 April 2021).
- Houba, V.J.G.; Novozamsky, I.; Lexmond, T.M.; Van Der Lee, J.J. Applicability of 0.01 M CaCl2 as a single extraction solution for the assessment of the nutrient status of soils and other diagnostic purposes. Commun. Soil Sci. Plant Anal. 1990, 21, 2281–2290. [Google Scholar] [CrossRef]
- Kratz, S.; Schick, J.; Øgaard, A.F. P Solubility of Inorganic and Organic P Sources. In Phosphorus in Agriculture: 100% Zero; Schnug, E., De Kok, L.J., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–926. ISBN 9789401776127. [Google Scholar]
- Idowu, M.K.; Ige, D.V.; O Akinremi, O. Elution of inorganic and organic phosphorus from surface applied organic amendments. Can. J. Soil Sci. 2008, 88, 709–718. [Google Scholar] [CrossRef]
- Iyamuremye, F.; Dick, R.P.; Baham, J. Organic Amendments and Phosphorus Dynamics: I. Phosphorus Chemistry and Sorption. Soil Sci. 1996, 161, 426–435. [Google Scholar] [CrossRef]
- Siddique, M.T.; Robinson, J.S. Phosphorus Sorption and Availability in Soils Amended with Animal Manures and Sewage Sludge. J. Environ. Qual. 2003, 32, 1114–1121. [Google Scholar] [CrossRef]
- Nest, T.V.; Ruysschaert, G.; Vandecasteele, B.; Houot, S.; Baken, S.; Smolders, E.; Cougnon, M.; Reheul, D.; Merckx, R. The long term use of farmyard manure and compost: Effects on P availability, orthophosphate sorption strength and P leaching. Agric. Ecosyst. Environ. 2016, 216, 23–33. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F.; Hansen, E.; Wragg, D.; Haraldsen, T.K.; Krogstad, T. Waste products as alternative phosphorus fertilisers part I: Inorganic P species affect fertilisation effects depending on soil pH. Nutr. Cycl. Agroecosystems 2015, 103, 167–185. [Google Scholar] [CrossRef]
- Biowaste Solution—SYSTEMIC European Union Project. Available online: www.systemicproject.eu (accessed on 5 May 2021).
- Brienza, C.; Sigurnjak, I.; Meier, T.; Michels, E.; Adani, F.; Schoumans, O.; Vaneeckhaute, C.; Meers, E. Techno-economic assessment at full scale of a biogas refinery plant receiving nitrogen rich feedstock and producing renewable energy and biobased fertilisers. J. Clean. Prod. 2021, 308, 127408. [Google Scholar] [CrossRef] [PubMed]
- Fertimanure. Available online: www.fertimanure.eu (accessed on 5 May 2021).
- EN13650. Soil Improvers and Growing Media Extraction of Aqua Regia Soluble Elements; BSI: London, UK, 2001. [Google Scholar]
- EN13654-1. Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method; CEN: Brussels, Belgium, 2002. [Google Scholar]
- Houba, V.; Lexmond, T.; Novozamsky, I.; van der Lee, J. State of the art and future developments in soil analysis for bioavailability assessment. Sci. Total. Environ. 1996, 178, 21–28. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis; Sparks, D.L., Ed.; Agronomy 9; ASA-SSSA: Madison, WI, USA, 1996; pp. 1035–1049. ISBN 9780891182047. [Google Scholar]
- van Reeuwijk, L. Procedures for Soil Analysis, 6th ed.; ISRIC, FAO: Wageningen, The Netherlands, 2002. [Google Scholar]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid fraction of separated digestate as soil improver: Implications for soil fertility and carbon sequestration. J. Soils Sediments 2021, 21, 678–688. [Google Scholar] [CrossRef]
- Prasad, M. A Literature Review on the Availability of Phosphorus from Compost in Relation to the Nitrate Regulations SI 378 of 2006; Environmental Protection Agency: Wexford, Ireland, 2013. [Google Scholar]
- Kern, J.; Heinzmann, B.; Markus, B.; Kaufmann, A.C.; Soethe, N.; Engels, C. Recycling and Assessment of Struvite Phosphorus from Sewage Sludge. Agric. Eng. Int. CIGR J. 2008, 10, 13. [Google Scholar]
- Ehlert, P.; van Middelkoop, J.; van Geel, W.; de Haan, J.; Regelink, I.C. Veeljarige Fosfaatveldproeven op Gras- en Bouwland; Wageningen, The Netherland, 2018; p. 114. Available online: https://edepot.wur.nl/460816 (accessed on 5 June 2021).
- Güsewell, S. N : P ratios in terrestrial plants: Variation and functional significance. N. Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- García-Albacete, M.; Martín, A.; Cartagena, M.C. Fractionation of phosphorus biowastes: Characterisation and environmental risk. Waste Manag. 2012, 32, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Dormaar, J.F.; Webster, G.R. Losses Inherent in Ignition Procedures for Determining Total Organic Phosphorus. Can. J. Soil Sci. 1964, 44, 1–6. [Google Scholar] [CrossRef]
- Williams, J.D.H.; Syers, J.K.; Walker, T.W.; Rex, R.W. A comparison of methods for the determination of soil organic phosphorus. Soil Sci. 1970, 110, 13–18. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Chardon, W.; Ehlert, P.A.I.; Dolfing, J.; Suurs, R.A.A.; Oenema, O.; Van Riemsdijk, W.H. Phosphorus Availability for Plant Uptake in a Phosphorus-Enriched Noncalcareous Sandy Soil. J. Environ. Qual. 2004, 33, 965–975. [Google Scholar] [CrossRef]
- Gasparatos, D.; Haidouti, C.; Haroulis, A.; Tsaousidou, P. Estimation of Phosphorus Status of Soil Fe-Enriched Concretions with the Acid Ammonium Oxalate Method. Commun. Soil Sci. Plant Anal. 2006, 37, 2375–2387. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Modelling competitive anion adsorption on oxide minerals and an allophane-containing soil. Eur. J. Soil Sci. 2001, 52, 639–653. [Google Scholar] [CrossRef]
- Van Der Zee, S.E.A.T.M.; Fokkink, L.G.J.; Van Riemsdijk, W.H. A New Technique for Assessment of Reversibly Adsorbed Phosphate. Soil Sci. Soc. Am. J. 1987, 51, 599–604. [Google Scholar] [CrossRef]
- Van Der Zee, S.; Van Riemsdijk, W. Sorption kinetics and transport of phosphate in sandy soil. Geoderma 1986, 38, 293–309. [Google Scholar] [CrossRef]
- Hylander, L.D.; Svensson, H.; Siman, G. Different methods for determination of plant available soil phosphorus. Commun. Soil Sci. Plant Anal. 1996, 27, 1501–1512. [Google Scholar] [CrossRef]
- Dail, H.W.; He, Z.; Erich, M.S.; Honeycutt, C.W. Effect of Drying on Phosphorus Distribution in Poultry Manure. Commun. Soil Sci. Plant Anal. 2007, 38, 1879–1895. [Google Scholar] [CrossRef] [Green Version]
- Achat, D.L.; Augusto, L.; Gallet-Budynek, A.; Bakker, M.R. Drying-induced changes in phosphorus status of soils with contrasting soil organic matter contents—Implications for laboratory approaches. Geoderma 2012, 187–188, 41–48. [Google Scholar] [CrossRef]
- Weng, L.; Vega, F.A.; Van Riemsdijk, W.H. Competitive and Synergistic Effects in pH Dependent Phosphate Adsorption in Soils: LCD Modeling. Environ. Sci. Technol. 2011, 45, 8420–8428. [Google Scholar] [CrossRef] [PubMed]
- Yaron, B.; Calvet, R.; Prost, R. The Soil Pollutants. In Soil Pollution: Processes and Dynamics; Springer: Berlin/Heidelberg, Germany, 1996; pp. 25–53. [Google Scholar]
- Roboredo, M.; Fangueiro, D.; Lage, S.; Coutinho, J. Phosphorus dynamics in soils amended with acidified pig slurry and derived solid fraction. Geoderma 2012, 189–190, 328–333. [Google Scholar] [CrossRef]
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A. Hydrogen Sulfide Adsorption by Iron Oxides and Their Polymer Composites: A Case-Study Application to Biogas Purification. Materials 2020, 13, 4725. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, T.; Antelo, J.; van Rotterdam, A.; van Riemsdijk, W.H. Nanoparticles in natural systems II: The natural oxide fraction at interaction with natural organic matter and phosphate. Geochim. Cosmochim. Acta 2010, 74, 59–69. [Google Scholar] [CrossRef]
| Type | Processing Plant | Code | Feedstock and Processing | Fe-Salts d |
|---|---|---|---|---|
| SF of digestate | AM-Power b | AMP-SF | Dried SF of digestate of biowaste including incl. sludge from agro-food industry | N |
| Groot Zevert Vergisting b | GZV-SF1 | SF of digestate of pig manure and biowaste | N | |
| GZV-SF2 | Low-P SF obtained after leaching P with water and sulphuric acid | N | ||
| Benas b | BNS-SF1 | SF of digestate of energy crops and poultry litter | N | |
| BNS-SF2 | Low N SF obtained by removing N through stripping | N | ||
| Waterleau New Energy b | WNE-SF1 | Dried SF of digestate of manure and biowaste incl. sludge from agro-food industry | Y | |
| WNE-SF2 | Dried SF mixed with concentrated liquid fraction of digestate | Y | ||
| Dairy farm c | DF-SF | SF of digestate of dairy manure | N | |
| Sugar-beet processing company | BP-SF | SF of digestate of residues from sugar beets | Y | |
| Compost | Biowaste treatment facilities | VFG-COMP1 | Compost of source separated VFG waste | N |
| VFG-COMP2 | N | |||
| VFG-COMP3 | Compost of digestate of source separated VFG waste. | Y | ||
| VFG-COMP4 | Y | |||
| GW-COMP1 | Compost of garden waste | N | ||
| GW-COMP2 | N | |||
| Sieved soil | Biowaste treatment facilities | SOIL1 | Fraction < 2 mm obtained by sieving garden waste prior to composting | N |
| SOIL2 | N | |||
| SOIL3 | N |
| Name | P-Pool | Analytical Method |
|---|---|---|
| Pt | Total P | P soluble after destruction with aqua regia |
| Pt,kuo | Total P | P-PO4 extracted with 0.5 M H2SO4 after oxidation at 550 °C |
| Pio | Total inorganic P | P-PO4 soluble in 0.5 M H2SO4 |
| Po | Total organic P | Difference Pt,kuo and Pio |
| Pox | P associated with Fe- and Al-oxides, acid-soluble P salts and a part of the organic P | P extracted in acid ammonium-oxalate, measured as total P (ICP-OES) |
| P-CaCl2 | Directly available P without pH adjustment | P extractable in 10 mM CaCl2 using fresh material and at a predetermined total P concentration of 150 mg/L in the suspension a |
| P-CaCl2 (pH5.5) | Easily soluble P at pH 5.5 | As P-CaCl2 but after lowering the pH of the suspension to 5.0 (at equilibrium, average pH was 5.5) |
| Sample | DW | OM | pH-CaCl2 | Pt | Nt | Kt | Mgt | Cat | St | Fet | Feox | Alox | Feox/Fet | Pt/OM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % of DW | - | g/kg DW | - | g/kg | |||||||||
| AMP-SF | 80 | 46 | 8.6 | 28 | 18 | 16 | 11 | 32 | 17 | 35 | 25.9 | 1.4 | 0.74 | 60 |
| WNE-SF1 | 95 | 65 | 8.1 | 26 | 28 | 22 | 11 | 49 | 13 | 13 | 9.6 | 0.5 | 0.73 | 39 |
| WNE-SF2 | 56 | 62 | 7.9 | 23 | 31 | 51 | 9.7 | 41 | 19 | 11 | 8.4 | 0.4 | 0.74 | 38 |
| GZV-SF1 | 31 | 76 | 8.7 | 28 | 38 | 16 | 19 | 30 | 6.5 | 2.8 | 2.4 | 0.2 | 0.86 | 37 |
| GZV-SF2 | 23 | 93 | 5.0 | 3.8 | 14 | <1.0 | 2.1 | 9.6 | 10 | 2.3 | 1.6 | <0.05 | 0.69 | 4.1 |
| BNS-SF1 | 25 | 91 | 8.7 | 6.2 | 34 | 23 | 3.4 | 7.0 | 3.9 | 5.5 | 4.5 | <0.05 | 0.81 | 6.8 |
| BNS-SF2 | 66 | 92 | 7.1 | 4.7 | 12 | 16 | 2.9 | 8.3 | 4.2 | 5.5 | 4.4 | <0.05 | 0.80 | 5.1 |
| BP-SF | 33 | 49 | 8.4 | 5.1 | 26 | 14 | 4.8 | 64 | 9.8 | 20 | 15.2 | 0.3 | 0.76 | 10 |
| DF-SF | 26 | 79 | 7.1 | 12 | 25 | 29 | 12 | 26 | 7.5 | 2.6 | 1.8 | 0.1 | 0.69 | 15 |
| VFG-COMP1 | 69 | 41 | 7.8 | 3.4 | 16 | 13 | 4.8 | 35 | 2.9 | 11 | 2.0 | 0.3 | 0.18 | 8.2 |
| VFG-COMP2 | 69 | 43 | 7.6 | 3.5 | 15 | 9.6 | 3.7 | 32 | 6.6 | 5.0 | 1.7 | 0.7 | 0.35 | 8.1 |
| VFG-COMP3 | 60 | 35 | 8.0 | 2.6 | 14 | 9.1 | 3.6 | 23 | 2.1 | 7.3 | 2.4 | 0.6 | 0.33 | 7.5 |
| VFG-COMP4 | 67 | 44 | 7.5 | 3.1 | 18 | 13 | 4.5 | 30 | 2.6 | 11 | 4.6 | 0.5 | 0.41 | 7.1 |
| GW-COMP1 | 42 | 39 | 7.9 | 1.5 | 7.9 | 7.0 | 2.9 | 24 | 1.1 | 10 | 2.7 | 0.3 | 0.27 | 4.0 |
| GW-COMP2 | 67 | 16 | 7.0 | 1.1 | 6.0 | 3.8 | 1.9 | 8.8 | 1.5 | 8.6 | 3.0 | 0.6 | 0.35 | 6.7 |
| Soil1 | 70 | 21 | 6.9 | 1.3 | 7.0 | 4.6 | 1.7 | 12 | 1.1 | 6.3 | 2.5 | 0.7 | 0.39 | 6.2 |
| Soil2 | 75 | 11 | 6.8 | 0.8 | 3.6 | 1.8 | 1.4 | 8.6 | 0.6 | 5.1 | 2.6 | 0.8 | 0.50 | 7.4 |
| Soil3 | 66 | 18 | 6.7 | 0.8 | 3.9 | 1.7 | 1.1 | 5.8 | 0.5 | 4.5 | 2.3 | 0.9 | 0.52 | 4.3 |
| Sample b | Inorganic P | Organic P | Pox | P-CaCl2 | P-CaCl2,pH5.5 | DPS | PFe |
|---|---|---|---|---|---|---|---|
| % of Pt | (-) | % of Pt | |||||
| AMP-SF | 97 | 0.0 | 87 | 1.2 | 21 | 3.0 | 29 |
| WNE-SF1 | 90 | 4.6 | 79 | 1.5 | 37 | 6.9 | 12 |
| WNE-SF2 | 86 | 1.7 | 84 | 2.3 | 33 | 7.6 | 11 |
| GZV-SF1 | 75 | 0.6 | 74 | 2.1 | 87 | 27 | 3 |
| GZV-SF2 | 75 | 5.9 | 74 | 40 | 40 | 6.6 | 11 |
| BNS-SF1 | 89 | 1.5 | 84 | 2.0 | 45 | 4.2 | 20 |
| BNS-SF2 | 65 | 23 | 74 | 2.9 | 26 | 2.9 | 26 |
| BP-SF | 65 | 18 | 61 | 1.2 | 14 | 0.7 | 87 |
| DF-SF | 75 | 5.6 | 80 | 8.3 | 45 | 17 | 5 |
| VFG-COMP1 | 76 | 8.5 | 62 | 0.4 | 18 | 2.8 | 22 |
| VFG-COMP2 | 86 | 9.5 | 74 | 4.9 | 22 | 3.0 | 25 |
| VFG-COMP3 | 89 | 8.1 | 71 | 0.6 | 22 | 1.8 | 39 |
| VFG-COMP4 | 83 | 20 | 71 | 0.9 | 15 | 1.4 | 50 |
| GW-COMP1 | 69 | 21 | 55 | 0.5 | 13 | 0.9 | 55 |
| GW-COMP2 | 70 | 22 | 61 | 0.3 | 2 | 0.6 | 61 |
| Soil1 | 76 | 13 | 64 | 0.4 | 6 | 0.7 | 64 |
| Soil2 | 63 | 18 | 64 | 0.1 | 1 | 0.4 | 64 |
| Soil3 | 65 | 22 | 64 | 0.2 | 1 | 0.4 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regelink, I.C.; Egene, C.E.; Tack, F.M.G.; Meers, E. Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste. Agronomy 2021, 11, 2233. https://doi.org/10.3390/agronomy11112233
Regelink IC, Egene CE, Tack FMG, Meers E. Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste. Agronomy. 2021; 11(11):2233. https://doi.org/10.3390/agronomy11112233
Chicago/Turabian StyleRegelink, Inge C., Caleb E. Egene, Filip M. G. Tack, and Erik Meers. 2021. "Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste" Agronomy 11, no. 11: 2233. https://doi.org/10.3390/agronomy11112233
APA StyleRegelink, I. C., Egene, C. E., Tack, F. M. G., & Meers, E. (2021). Speciation of P in Solid Organic Fertilisers from Digestate and Biowaste. Agronomy, 11(11), 2233. https://doi.org/10.3390/agronomy11112233







