Nitrogen Fractions in Soil Fertilized with Waste Organic Materials
Abstract
:1. Introduction
2. Material and Methods
- N-NH4—ammonium form of mineral nitrogen, after extraction with 1% K2SO4 determined with the direct Nesslerization method [33];
- N-NO3—nitrate forms after extraction with 1% K2SO4 determined with the phenol-di-sulfate method [29];
- N-min.—nitrogen in mineral compounds: N-min. = N-NH4 + N-NO3;
- N-eh—easily hydrolysable organic nitrogen, after hot hydrolysis in 0.25 mol H2SO4⋅dm−3;
- N-hh—hardly hydrolysable nitrogen, after hot hydrolysis in 2.5 mol H2SO4·dm−3 and mineralization of the solution;
- N-nh—non-hydrolysable organic nitrogen, remaining in soil material after extraction with 1% K2SO4 and two-step acid hydrolysis.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
- Wierzbowska, J., Kovačik, P., Sienkiewicz, S. Krzebietke S. Bowszys T. 2018. Determination of heavy metals and their availability to plants in soil fertilized with different waste substances. Environ Monit Assess 190, 567. https://doi.org/10.1007/s10661-018-6941-7.
- Wierzbowska, J., Sienkiewicz, S., Krzebietke, S. Bowszys T. 2016. Heavy Metals in Water Percolating Through Soil Fertilized with Biodegradable Waste Materials. Water Air Soil Pollut 227, 456 (2016). https://doi.org/10.1007/s11270-016-3147-x.
- Wierzbowska, J., Sienkiewicz, S., Sternik, P., & Bowszys, T. 2016. Content of macroelements in leachate from soils fertilized with organic materials. Fresenius Environmental Bulletin, 25, 6132–6138.
- Bowszys, T., Wierzbowska, J., Sternik, P., Busse, M. K. 2015. Effect of the application of sewage sludge compost on the content and leaching of zinc and copper from soils under agricultural use. Journal of Ecological Engineering, 16(1), 1-7. https://doi.org/10.12911/22998993/580.
Conflicts of Interest
References
- Leip, A.; Marchi, G.; Koeble, R.; Kempen, M.; Britz, W.; Li, C. Linking an economic model for European agriculture with a mechanistic model to estimate nitrogen and carbon losses from arable soils in Europe. Biogeosciences 2008, 5, 73–94. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.T.; Yoh, M.; Mo, J.M.; Gundersen, P.; Zhou, G.Y. Response of nitrogen leaching to nitrogen deposition in disturbed and mature forests of Southern China. Pedosphere 2009, 19, 111–120. [Google Scholar] [CrossRef]
- Murphy, O.V.; Macdonald, A.J.; Stockdale, K.W.T.; Goulding, K.W.T.; Fortune, S.; Gaunt, J.L.; Poulton, P.R.; Wakefield, J.A.; Webster, C.P.; Wilmf, R.W.S. Soluble organic nitrogen in agricultural soils. Biol. Fertil. Soils 2000, 30, 374–387. [Google Scholar] [CrossRef]
- Kelley, K.R.; Stevenson, F.J. Forms and nature of organic N in soil. Fertil. Res. 1995, 42, 1–11. [Google Scholar] [CrossRef]
- Deng, S.P.; Moore, J.M.; Tabatabai, M.A. Characterization of active nitrogen pools in soils under different cropping systems. Biol. Fertil. Soils 2000, 32, 302–309. [Google Scholar] [CrossRef]
- Fang, Y.T.; Gundersen, P.; Mo, J.M.; Zhu, W.X. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 2008, 5, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Blackmer, A.M.; Blackmer, T.M. Differences in physiological age affect diagnosis of nitrogen deficiencies in cornfields. Pedosphere 2008, 18, 45–553. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Wang, Y.; Zhang, X.; Huang, C. Spatial variability of soil total nitrogen and soil total phosphorus under different land uses in a small watershed on the Loess Plateau, China. Geoderma 2009, 150, 141–149. [Google Scholar] [CrossRef]
- Xu, G.; Li, Z.; Li, P. Fractal features of soil particle-size distribution and total soil nitrogen distribution in a typical watershed in the source area of the middle Dan River, China. Catena 2013, 101, 17–23. [Google Scholar] [CrossRef]
- Aula, L.; Macnack, N.; Omara, P.; Mullock, J.; Raun, W. Effect of fertilizer nitrogen (N) on soil organic carbon, total N, and soil pH in long-term continuous winter wheat (Triticum aestivum L.). Commun. Soil Sci. Plant Anal. 2016, 47, 863–874. [Google Scholar] [CrossRef]
- Gutser, R.; Ebertseder, T.H.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Pan, W.L.; Port, L.E.; Xiao, Y.; Bary, A.I.; Cogger, C.G. Soil Carbon and Nitrogen Fraction Accumulation with Long-Term Biosolids Applications. Soil Sci. Soc. Am. J. 2017, 81, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Omar, L.; Ahmed, O.H.; Jalloh, M.B.; Nik Muhamad, A.M. Soil Nitrogen Fractions, Nitrogen Use Efficiency and Yield of Zea mays L. Grown on a Tropical Acid Soil Treated with Composts and Clinoptilolite Zeolite. Appl. Sci. 2020, 10, 4139. [Google Scholar] [CrossRef]
- Yang, L.; Bai, J.; Liu, J.; Zeng, N.; Cao, W. Green Manuring Effect on Changes of Soil Nitrogen Fractions, Maize Growth, and Nutrient Uptake. Agronomy 2018, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Elsalam, H.E.A.; El-Sharnouby, M.E.; Mohamed, A.E.; Raafat, B.M.; El-Gamal, E.H. Effect of Sewage Sludge Compost Usage on Corn and Faba Bean Growth, Carbon and Nitrogen Forms in Plants and Soil. Agronomy 2021, 11, 628. [Google Scholar] [CrossRef]
- Rodrigues Prates, A.; Renée Coscione, A.; Carvalho Minhoto Teixeira Filho, M.; Gasparoti Miranda, B.; Arf, O.; Hamilton Abreu-Junior, C.; Carvalho Oliveira, F.; Moreira, A.; Shintate Galindo, F.; Márcia Pereira Sartori, M. Composted Sewage Sludge Enhances Soybean Production and Agronomic Performance in Naturally Infertile Soils (Cerrado Region, Brazil). Agronomy 2020, 10, 1677. [Google Scholar] [CrossRef]
- Statistics Poland. Environment 2019. Available online: https://stat.gov.pl/en/topics/environment-energy/environment/environment-2019,1,11.html# (accessed on 1 May 2021). (In Polish)
- Roiga, N.; Sierra, J.; Martíc, E.; Nadalb, M.; Schuhmachera, M.; Domingob, J.L. Long-term amendment of Spanish soils with sewage sludge: Effects on soil functioning. Agric. Ecosyst. Environ. 2012, 158, 41–48. [Google Scholar] [CrossRef]
- Poulsen, P.H.B.; Magid, J.; Luxhøi, J.; de Neergaard, A. Effects of fertilization with urban and agricultural organic wastes in a field trial–Waste imprint on soil microbial activity. Soil Biol. Biochem. 2013, 57, 794–802. [Google Scholar] [CrossRef]
- Börjesson, G.; Kätterer, T. Soil fertility effects of repeated application of sewage sludge in two 30-year-old field experiments. Nutr. Cycl. Agroecosyst. 2018, 112, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Kurzemann, F.R.; Plieger, U.; Probst, M.; Spiegel, H.; Sandén, T.; Ros, M.; Insam, H. Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil. Agronomy 2020, 10, 1664. [Google Scholar] [CrossRef]
- Schröder, C.; Häfner, F.; Larsen, O.C.; Krause, A. Urban Organic Waste for Urban Farming: Growing Lettuce Using Vermicompost and Thermophilic Compost. Agronomy 2021, 11, 1175. [Google Scholar] [CrossRef]
- Sądej, W.; Bowszys, T.; Namiotko, A. Leaching of nitrogen forms from soil fertilized with sewage sludge. Ecol. Chem Eng. A 2009, 16, 1001–1008. [Google Scholar]
- Wierzbowska, J.; Sienkiewicz, S.; Sternik, P.; Bowszys, T. Content of macroelements in leachate from soils fertilised with organic materials. Fresen. Environ. Bull. 2016, 25, 6132–6138. [Google Scholar]
- Krzywy-Gawrońska, E. Changes in the content of total, nitrate and ammonium nitrogen in mass of composted municipal sewage sludge and potato pulp during decomposition process. Zesz. Probl. Post Nauk. Rol. 2006, 513, 243–249. (In Polish) [Google Scholar]
- Iuss Working Group Wrb. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Iuss Working Group Wrb: Rome, Italy, 2015; p. 188. [Google Scholar]
- PN-EN 15934:2013-02E. Sewage Sludge, Treated Bio-Waste, Soil and Waste–Determination of Dry Matter by Determining the Dry Residue Content or Water Content; The Polish Committee for Standardization: Warsaw, Poland, 2013; p. 22. (In Polish) [Google Scholar]
- PN-EN 15936:2013-02E. Sewage Sludge, Treated Bio-Waste, Soil and Waste–Determination of Total Organic Carbon (TOC) after Dry Combustion; The Polish Committee for Standardization: Warsaw, Poland, 2013; p. 28. (In Polish) [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Evaluation of the Properties of Soils and Plants; The Institute of Environmental Protection: Warsaw, Poland, 1991; p. 334. (In Polish) [Google Scholar]
- PN-ISO 10390:1997. Soil Quality–Determination of pH; The Polish Committee for Standardization: Warsaw, Poland, 1997; p. 15. (In Polish) [Google Scholar]
- Kalembasa, S. Use Of 15 N and 13 N Isotopes in Soil Science and Agricultural Chemistry Researchs; WNT: Warsaw, Poland, 1995; p. 251. (In Polish) [Google Scholar]
- PN-R-04028:1997. Chemical and Agricultural Analysis of Soil. Method of Sampling and Determination of The Content of Nitrate and Ammonium Ions in Mineral Soils; The Polish Committee For Standardization: Warsaw, Poland, 1997; p. 5. (In Polish) [Google Scholar]
- Emmerling, C.; Udelhoven, T.; Schneider, R. Long-lasting impact of biowaste-compost application in agriculture on soil-quality parameters in three different crop-rotation systems. J. Plant Nutr. Soil Sci. 2010, 173, 391–398. [Google Scholar] [CrossRef]
- Nguyen, T.; Shindo, H. Effects of different levels of compost application on amounts and distribution of organic nitrogen forms in soil particle size fractions subjected mainly to double cropping. Agric. Sci. 2011, 2, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Omara, P.; Aula, L.; Raun, W.R. Nitrogen Uptake Efficiency and Total Soil Nitrogen Accumulation in Long-Term Beef Manure and Inorganic Fertilizer Application. Int. J. Agron. 2019, 2019, 9594369. [Google Scholar] [CrossRef]
- Mazur, Z.; Mazur, T. Effects of Long-Term Organic and Mineral Fertilizer Applications on Soil Nitrogen Content. Pol. J. Environ. Stud. 2015, 24, 2073–2078. [Google Scholar] [CrossRef]
- Hernández, T.; Moralb, R.; Perez-Espinosab, A.; Moreno-Casellesb, J.; Perez-Murciab, M.D.; Garcíaa, C. Nitrogen mineralisation potential in calcareous soils amended with sewage sludge. Bioresour. Technol. 2002, 83, 213–219. [Google Scholar] [CrossRef]
- Sienkiewicz, S.; Wierzbowska, J.; Kováčik, P.; Krzebietke, S.J.; Żarczyński, P. Digestate as a source of nutrients in the cultivation of Virginia fanpetals. Fresen. Environ. Bull. 2018, 27, 3970–3976. [Google Scholar]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef] [Green Version]
- Wierzbowska, J.; Sienkiewicz, S.; Zalewska, M.; Żarczyński, P.; Krzebietke, S. Phosphorus fractions in soil fertilised with organic waste. Environ. Monit. Assess. 2020, 192, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Olk, D.C. Organic Forms of Soil Nitrogen. In Nitrogen in Agricultural Systems; Book Series: Agronomy Monographs, 49; Schepers, J.S., Raun, W.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 57–100. [Google Scholar]
- Khan, S.A.; Mulvaney, R.L.; Hoeft, R.G. A simple soil test for detecting sites that are nonresponsive to nitrogen fertilization. Soil Sci. Soc. Am. J. 2001, 65, 1751–1760. [Google Scholar] [CrossRef]
- Sharma, R.P.; Verma, T.S. Dynamics of nitrogen fractions with long-term addition of Lantana camara biomass in rice-wheat cropping sequence. J. Ind. Soc. Soil Sci. 2001, 49, 407–412. [Google Scholar]
- Chadwick, D.R.; John, F.; Pain, B.; Chambers, B.J.; Williams, J. Plant uptake of nitrogen from the organic nitrogen fraction of animal manures: A laboratory experiment. J. Agric. Sci. 2000, 134, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Sądej, W.; Przekwas, K. Fluctuations of nitrogen levels in soil profile under conditions of a long-term fertilization experiment. Plant Soil Environ. 2008, 54, 97–203. [Google Scholar] [CrossRef] [Green Version]
- Gonet, S.S.; Debska, B. Dissolved organic carbon and dissolved nitrogen in soil under different fertilization treatments. Plant Soil Environ. 2006, 52, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Khandagle, A.; Dwivedi, B.S.; Aher, S.B.; Dwivedi, A.K.; Yashona, D.S.; Mohbe, S.; Panwar, S. Distribution of nitrogen fractions under long term fertilizer and manure application in a vertisol. Biosc. Biotech. Res. Commun. 2019, 12, 186–193. [Google Scholar]
- Skowrońska, M. The easily hydrolyzable nitrogen content in the soil under the conditions of organic wastes application. Zesz. Probl. Post Nauk. Rol. 2004, 499, 307–313. (In Polish) [Google Scholar]
- Rathi, D.; Devraj; Sharma, M.K. Effect of FYM and Fertilizer Application on Soil Organic Nitrogen Fractions: A Review. Int. Adv. Res. J. Sci. Eng. Technol. 2019, 9, 38–42. [Google Scholar]
- Souza, W.J.O.; Melo, W.J. Nitrogen in soil and soil organic matter fractions affected by different corn production systems. Rev. Bras. Ciência Solo 2000, 24, 885–896. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Sharifi, M.; Zebarth, B.J.; Burton, D.L.; Grant, C.A.; Bittman, S.; Drury, C.F.; McConkey, B.G.; Ziadi, N. Response of Potentially Mineralizable Soil Nitrogen and Indices of Nitrogen Availability to Tillage System. Soil Sci. Soc. Am. J. 2008, 72, 1124–1131. [Google Scholar] [CrossRef]
- Sano, S.; Yanai, J.; Kosaki, T. Evaluation of soil nitrogen status in japanese agricultural lands with reference to land use and soil types. Soil Sci. Plant. Nutr. 2004, 50, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Leinweber, P.; Schulten, H.-R. Nonhydrolyzable forms of soil organic nitrogen: Extractability and composition. J. Plant Nutr. Soil Sci. 2000, 163, 433–439. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, F.; Zhang, Z.; Zhu, T.; Xiao, H. Biotic and abiotic nitrogen immobilization in soil incorporated with crop residue. Soil Tillage Res. 2020, 202, 104664. [Google Scholar]
- Sapek, B. Nitrogen and phosphorus release from soil organic matter. Water Environ. Rural Areas 2010, 10, 229–256. (In Polish) [Google Scholar]
- Bregliani, M.M.; Temminghoff, E.J.M.; van Riemsdijk, W.; Haggi, E.S. Nitrogen Fractions in Arable Soils in Relation to Nitrogen Mineralization and Plant Uptake. Commun. Soil Sci. Plant. Anal. 2006, 37, 1571–1586. [Google Scholar] [CrossRef]


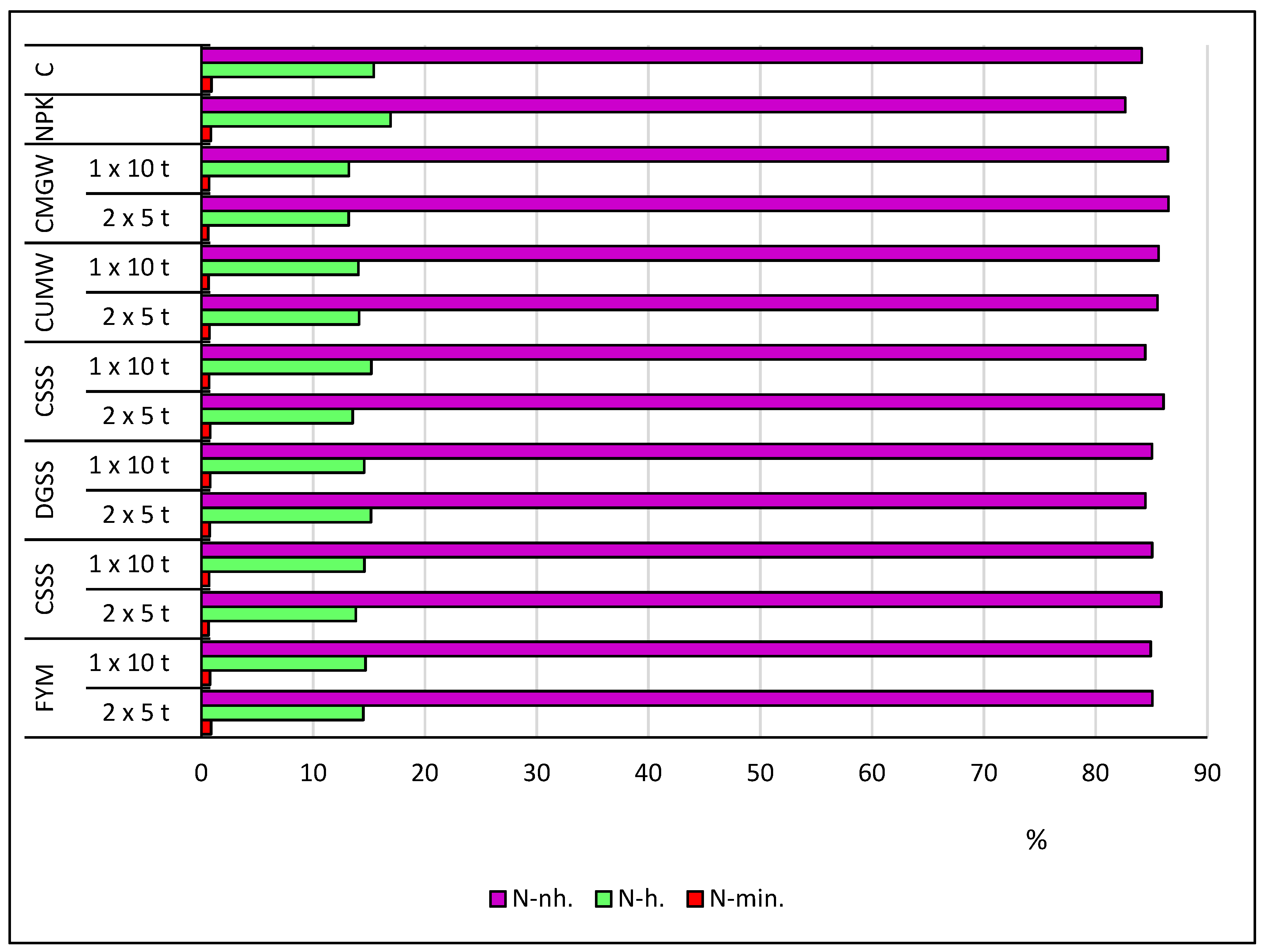
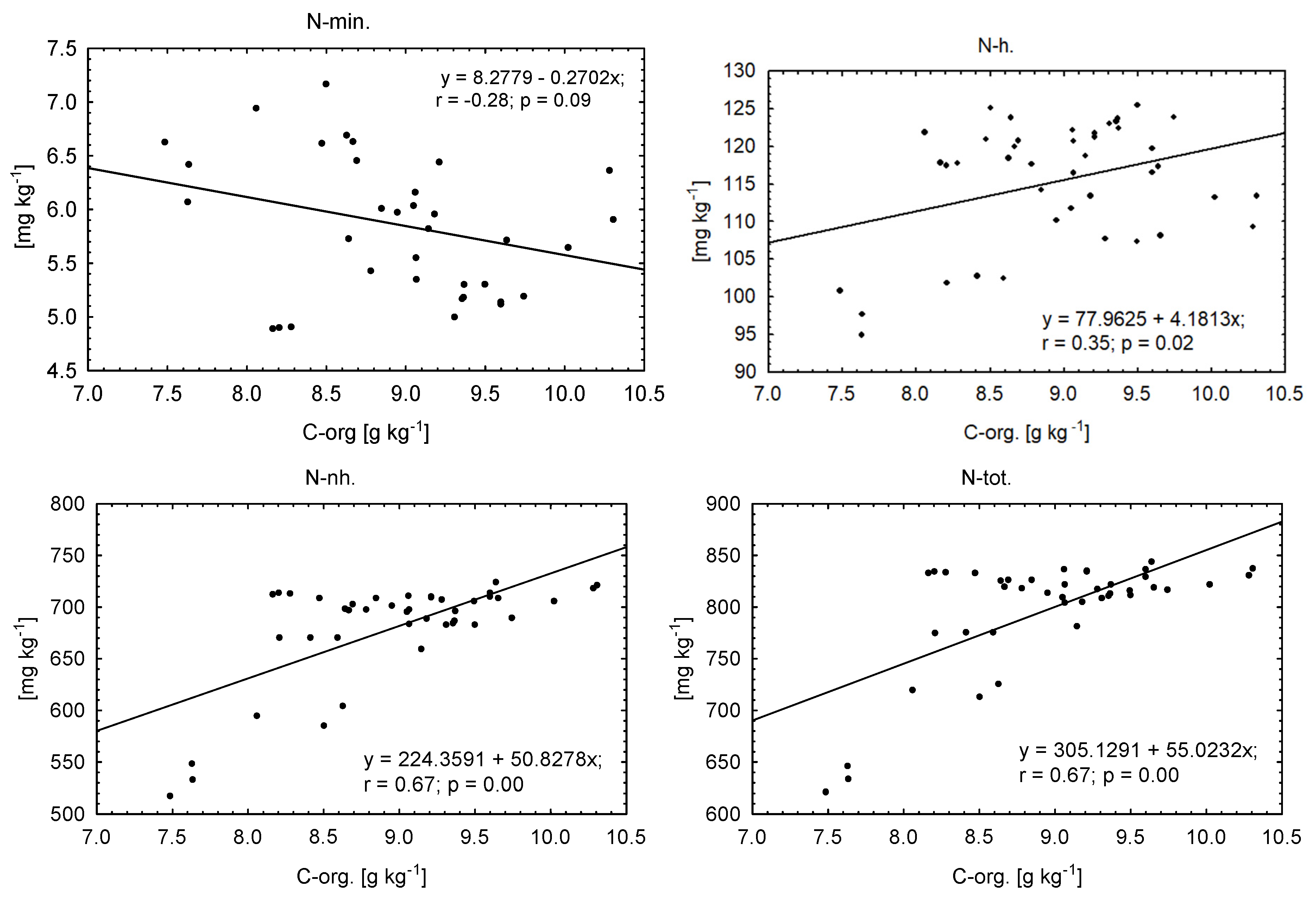
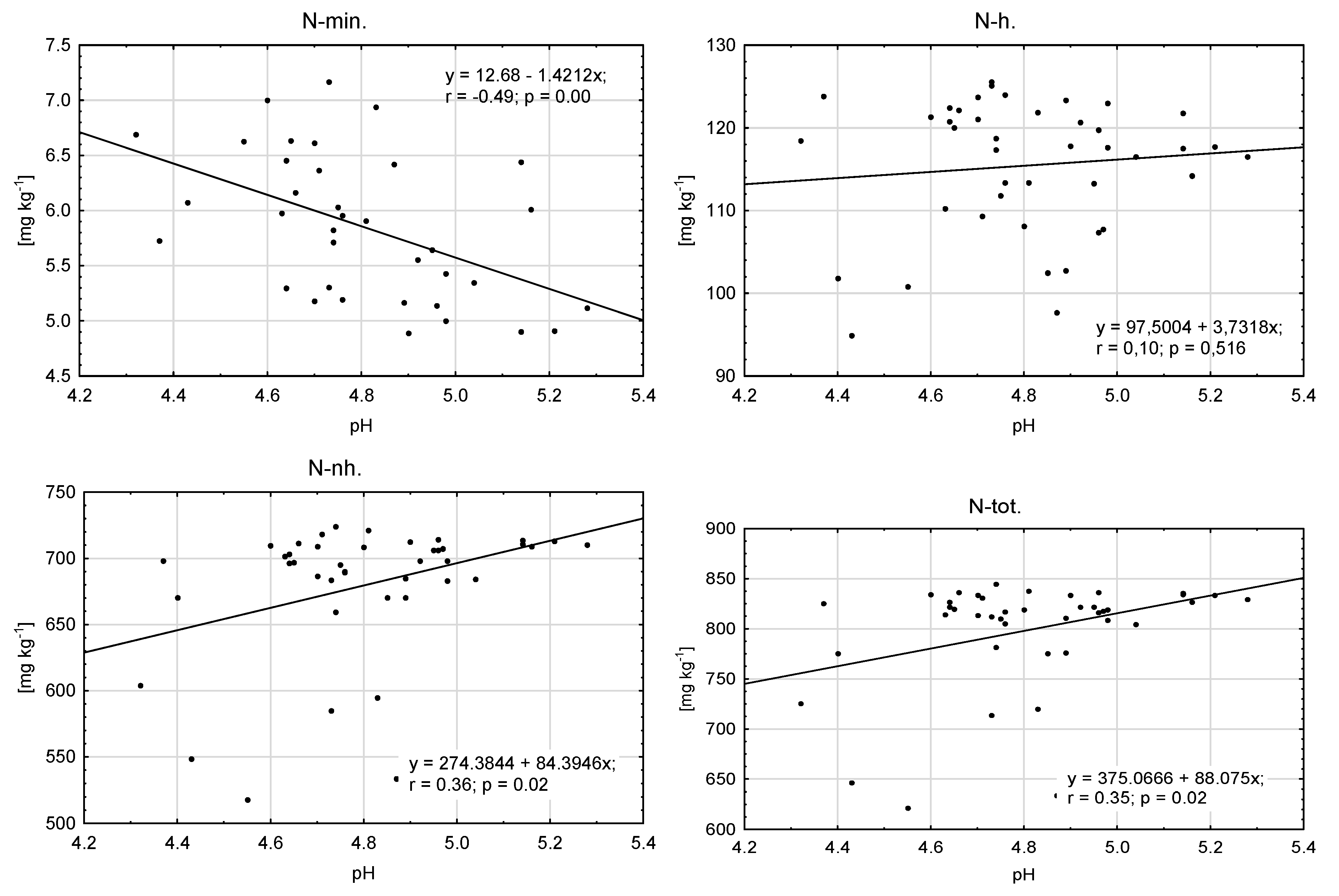
| Element | Manure (FYM) | Composted Municipal Sewage Sludge and Straw (CSSS) | Composted Sewage Sludge (CSS) | Dried and Granulated Sewage Sludge (DGSS) | “Dano” Compost Made from Unsorted Municipal Waste (CUMW) | Composted Municipal Green Waste (CMGW) |
|---|---|---|---|---|---|---|
| Content (g kg−1) | ||||||
| d.m. | 222.4 | 586.8 | 403.1 | 851.4 | 746.4 | 788.8 |
| Content (g kg−1 d.m.) | ||||||
| C-org. | 76.2 | 108.4 | 86.2 | 325.8 | 101.7 | 63.3 |
| N-tot. | 5.80 | 6.28 | 11.77 | 16.02 | 6.94 | 4.50 |
| C/N | 13.1 | 17.2 | 7.3 | 20.3 | 14.6 | 14.1 |
| Content (mg kg−1 d.m.) | ||||||
| Cu | 36.8 | 65.5 | 249.0 | 402.0 | 297.0 | 34.1 |
| Zn | 223.0 | 295 | 1360.0 | 980.0 | 831.0 | 133.0 |
| Mn | 334.0 | 210.6 | 300.5 | 228.0 | 273.6 | 326.8 |
| Pb | 5.50 | 24.9 | 20.3 | 15.8 | 178.0 | 29.1 |
| Cr | 10.20 | 15.3 | 33.20 | 56.90 | 53.7 | 19.8 |
| Ni | 6.64 | 17.7 | 36.6 | 28.0 | 35.2 | 15.5 |
| Fe | 3340.0 | 8050 | 11,260 | 15,590 | 16,200 | 5197 |
| Object | N-NH4 | N-NO3 | N-min. | N-eh. | N-hh. | N-nh. | N-Total | |
|---|---|---|---|---|---|---|---|---|
| C | f * | c | e | g | de | f | d | |
| NPK | a | d | d | f | b | e | c | |
| FYM | 1 × 10 t ha−1 | b | c | c | de | c | c | a |
| 2 × 5 t ha−1 | e | a | a | g | a | d | ab | |
| CSSS | 1 × 10 t ha−1 | cd | e | e | d | cd | c | a |
| 2 × 5 t ha−1 | f | f | h | e | e | c | ab | |
| DGSS | 1 × 10 t ha−1 | c | b | b | a | ef | b | a |
| 2 × 5 t ha−1 | e | c | d | b | d | cd | a | |
| CSS | 1 × 10 t ha−1 | e | d | f | c | cd | d | ab |
| 2 × 5 t ha−1 | c | b | b | c | f | a | a | |
| CUMW | 1 × 10 t ha−1 | g | e | g | d | d | b | a |
| 2 × 5 t ha−1 | e | c | d | d | d | b | a | |
| CMGW | 1 × 10 t ha−1 | d | f | g | f | g | g | b |
| 2 × 5 t ha−1 | ef | g | i | d | h | c | a | |
| Factor | N-NH4 | N-NO3 | N-min. | N-eh. | N-hh. | N-nh. | N-Total | |
|---|---|---|---|---|---|---|---|---|
| Waste organic materials | ||||||||
| FYM | a * | a | a | d | a | c | c | |
| CSSS | c | e | e | c | b | b | b | |
| DGSS | b | b | b | a | b | b | b | |
| CSS | b | c | c | b | b | b | b | |
| CUMW | d | d | d | c | b | a | a | |
| CMGW | c | e | f | c | c | c | d | |
| Application frequency | ||||||||
| 1 × 10 t ha−1 | a | b | ns | a | ns | b | ns | |
| 2 × 5 t ha−1 | b | a | ns | b | ns | a | ns | |
| Interaction | ||||||||
| FYM | 1 × 10 t ha−1 | d | a | a | h | a | f | f |
| 2 × 5 t ha−1 | a | c | c | f | b | c | bc | |
| CSSS | 1 × 10 t ha−1 | f | f | h | f | e | d | e |
| 2 × 5 t ha−1 | b | e | e | d | c | c | b | |
| DGSS | 1 × 10 t ha−1 | d | c | d | b | d | e | c |
| 2 × 5 t ha−1 | b | b | b | a | f | b | a | |
| CSS | 1 × 10 t ha−1 | b | b | b | c | g | a | a |
| 2 × 5 t ha−1 | de | d | f | c | c | f | d | |
| CUMW | 1 × 10 t ha−1 | d | c | d | e | d | b | ab |
| 2 × 5 t ha−1 | f | e | h | e | c | b | b | |
| CMGW | 1 × 10 t ha−1 | e | g | i | d | i | c | c |
| 2 × 5 t ha−1 | c | f | g | g | h | g | g | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbowska, J.; Sienkiewicz, S.; Załuski, D. Nitrogen Fractions in Soil Fertilized with Waste Organic Materials. Agronomy 2021, 11, 1474. https://doi.org/10.3390/agronomy11081474
Wierzbowska J, Sienkiewicz S, Załuski D. Nitrogen Fractions in Soil Fertilized with Waste Organic Materials. Agronomy. 2021; 11(8):1474. https://doi.org/10.3390/agronomy11081474
Chicago/Turabian StyleWierzbowska, Jadwiga, Stanisław Sienkiewicz, and Dariusz Załuski. 2021. "Nitrogen Fractions in Soil Fertilized with Waste Organic Materials" Agronomy 11, no. 8: 1474. https://doi.org/10.3390/agronomy11081474
APA StyleWierzbowska, J., Sienkiewicz, S., & Załuski, D. (2021). Nitrogen Fractions in Soil Fertilized with Waste Organic Materials. Agronomy, 11(8), 1474. https://doi.org/10.3390/agronomy11081474






