Alternative Strategies for Controlling the Brown Locust, Locustana pardalina (Walker)
Abstract
:1. Introduction
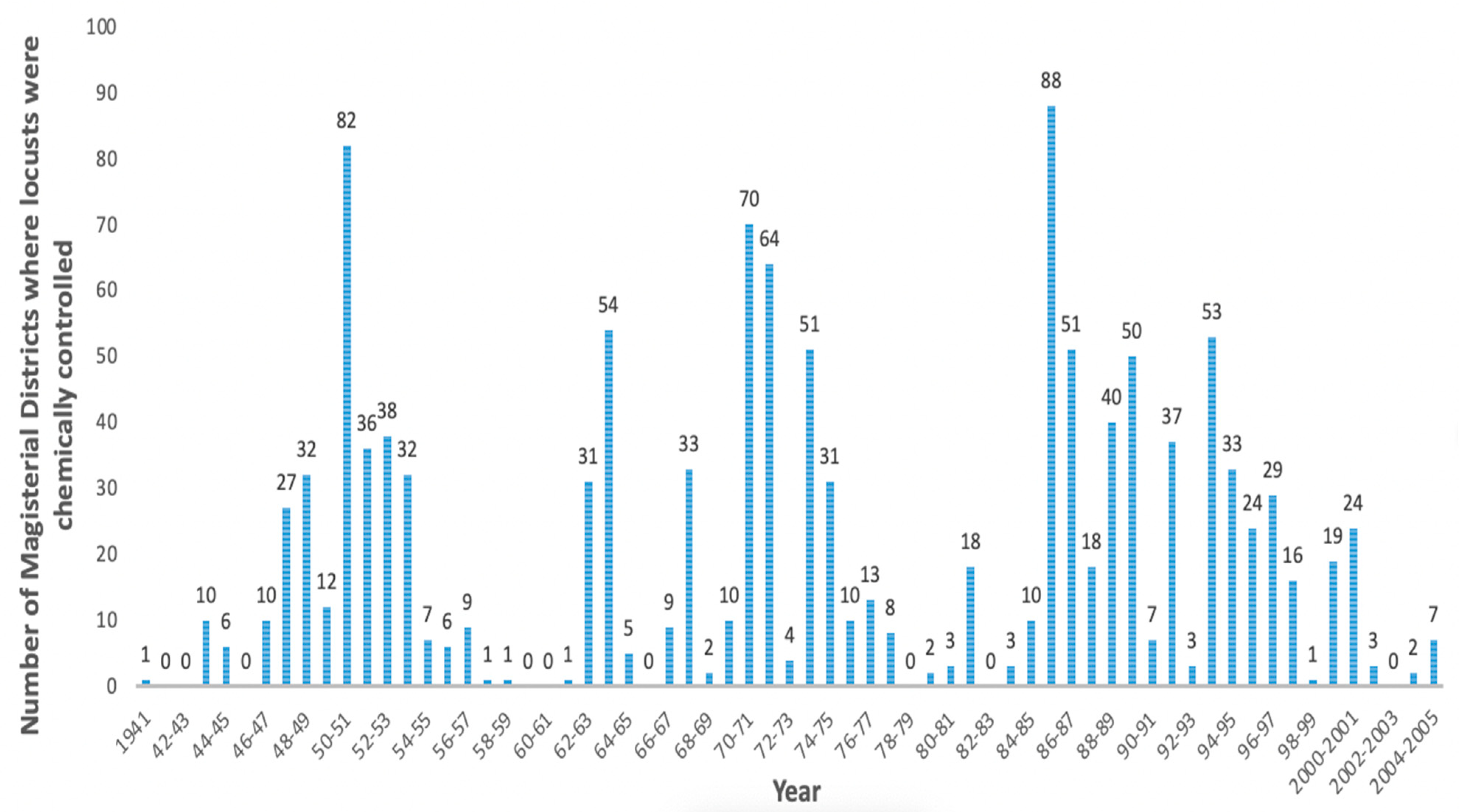
2. History of Brown Locust Outbreaks and Control
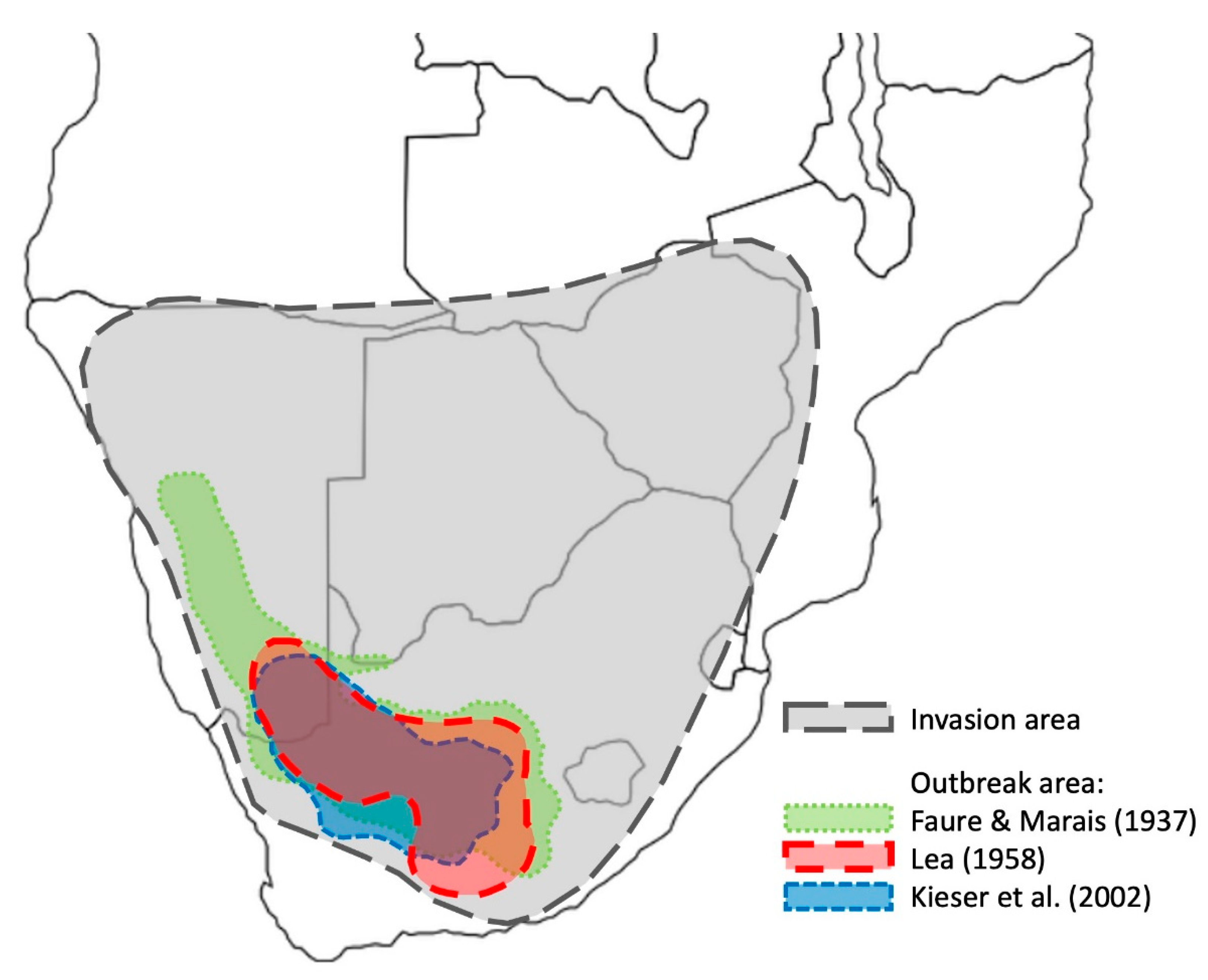
2.1. Outbreak Cycles of the Brown Locust
2.2. Control Strategy against the Brown Locust in South Africa
2.3. Outbreak Early Warning Systems
2.4. Environmental Impact of Synthetic Pyrethroid Insecticides
3. Alternative Control Methods
3.1. Natural Enemies
3.2. Mechanical Control
3.3. Insecticide Baits
3.4. Insecticide Barrier Treatments
3.5. Insect Growth Regulators (IGRs)
3.6. Metarhizium Myco-Insecticide
3.7. Pathogenic Micro-Organisms
4. Alternative Control Strategies
4.1. Abandoning Brown Locust Control Entirely
4.2. Update the Current “Commando System”
4.3. Abandon Hopper Control and Target Adult Swarms Only
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faure, J.C.; Marais, S.J.S. The control of Locustana pardalina in its outbreak centres. In Proceedings of the 4th International Locust Conference, Cairo, Egypt, 22 April 1936. 5p. [Google Scholar]
- Du Plessis, C. The Influence of Weather Conditions on the Incipient Swarming of the Brown Locust, Locustana Pardalina (Walker); Science Bulletin, Department of Agriculture and Forestry, Union of South Africa Government Printer: Pretoria, South Africa, 1938; Volume 186, pp. 1–51. [Google Scholar]
- Lea, A. Recent outbreaks of the brown locust with special reference to the influence of rainfall. J. Entmol. Soc. S. Afr. 1958, 21, 162–213. [Google Scholar]
- Kieser, M.E.; Thackrah, A.; Rosenberg, J. Changes in the outbreak region of the brown locust in Southern Africa. Grootfontein Agric. 2002, 4, 20–23. Available online: http://gadi.agric.za/articles/Kieser_M/kieser_vol4_2002_locust.php (accessed on 9 August 2021).
- Du Plessis, C. The Incipient Outbreaks of the Brown Locust in 1937–1938 with Special Reference to Biometrical Analysis; Science Bulletin, Department of Agriculture and Forestry, Union of South Africa Government Printer: Pretoria, South Africa, 1939; Volume 209, pp. 1–69. [Google Scholar]
- Faure, J.C. The phases of locusts in South Africa. Bull. Entmol. Res. 1932, 23, 293–405. [Google Scholar] [CrossRef]
- Uvarov, B.P. Grasshoppers and Locusts; Cambridge University Press: Cambridge, UK, 1966; Volume 2. [Google Scholar]
- Faure, J.C. The life history of the brown locust. J. Dept. Agric. Union S. Afr. 1923, 4, 205–224. [Google Scholar]
- Potgieter, J.T. A Contribution to the Biology of the Brown Swarm Locust and Its Natural Enemies; Science Bulletin, Department of Agriculture and Forestry, Union of South Africa Government Printer: Pretoria, South Africa, 1929; Volume 82, pp. 1–48. [Google Scholar]
- Smit, C.J.B. Field Observations on the Brown Locust in an Outbreak Centre; Science Bulletin, Department of Agriculture and Forestry, Union of South Africa Government Printer: Pretoria, South Africa, 1939; Volume 190, pp. 1–143. [Google Scholar]
- Smit, C.J.B. The behaviour of the brown locust in its solitary phase. In Technical Communication; Department of Agricultural and Technical Services: Pretoria, South Africa, 1960; Volume 1, pp. 1–132. [Google Scholar]
- Lea, A. Natural regulation and artificial control of brown locust numbers. J. Entomol. Soc. S. Afr. 1968, 31, 89–112. [Google Scholar]
- Lea, A. The distribution and abundance of brown locusts between 1954 and 1965. J. Entomol. Soc. S. Afr. 1969, 32, 367–398. [Google Scholar]
- Matthee, J.J. The Structure and Physiology of the Egg of Locustana pardalina (Walker); Science Bulletin, Department of Agriculture and Forestry, Union of South Africa Government Printer: Pretoria, South Africa, 1951; Volume 316, pp. 1–83. [Google Scholar]
- Botha, D.H. The viability of brown locust eggs, Locustana pardalina (Walker). S. Afr. J. Agric. Sci. 1967, 10, 445–460. [Google Scholar]
- De Wet, W.J.; Webb, D. Field observations on the behaviour of hoppers of the brown locust in the swarming phase; Science Bulletin, Department of Agriculture and Forestry, Union of South Africa. Government Printer: Pretoria, South Africa, 1951; Volume 337, pp. 1–38. [Google Scholar]
- Price, R.E. The life cycle of the brown locust, with reference to egg viability. In Proceedings of the Locust Symposium, MacGregor Museum, Kimberley, South Africa, 11–13 May 1987; Mckensie, B., Longridge, M., Eds.; South African Institute of Ecologists Bulletin, Special Issue: Rondebosch, South Africa, 1988; pp. 27–40. [Google Scholar]
- Lounsbury, C.P. The locust invasion. Agricultural Journal of the Cape of Good Hope; Department of Agriculture, Cape of Good Hope: Cape Town, South Africa, 1906; pp. 596–600. [Google Scholar]
- Price, R.E.; Brown, H.D. A century of locust control in South Africa. In Proceedings of the Workshop on Research Priorities for Migrant Pests of Agriculture in Southern Africa, PPRI, Pretoria, South Africa, 24–26 March 1999; Cheke, R.A., Rosenburg, L.J., Kieser, M.E., Eds.; Natural Resources Institute: Chatham, UK, 2000; pp. 37–50. [Google Scholar]
- Lounsbury, C.P. Some phases of the locust problem. S. Afr. J. Sci. 1915, 11, 33–45. [Google Scholar]
- Lea, A. Some major factors in the population dynamics of the brown locust, Locustana pardalina (Walker). Monogr. Biol. 1964, 14, 269–283. [Google Scholar]
- Lea, A. The plague dynamics of the brown locust, Locustana pardalina (Walk.). In Proceedings of the International Study Conference on Current and Future Problems of Acridology, London, UK, 6–16 July 1970; Hemming, C.F., Taylor, T.H.C., Eds.; Centre for Overseas Pest Research: London, UK, 1970; pp. 289–297. [Google Scholar]
- Lecoq, M. Recent progress in Desert and Migratory locust management in Africa. Are preventative actions possible? J. Orthoptera Res. 2001, 10, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.D.; Bashir, E.A. Criteria for classification of brown locust outbreaks. In Proceedings of the 4th Meeting of the Migratory Pests Sub-Committee of the Southern African Regional Commission for the Conservation and Utilization of the Soil (SARCCUS), Windhoek, Namibia, 4–6 June 1990. [Google Scholar]
- Mally, C.W. Arsenite of soda as a locust poison. J. Dept. Agric. Union. S. Afr. 1923, 13, 1–15. [Google Scholar]
- Policy for Managing the Locust Problem in South Africa. National Department of Agriculture. September 1988. Available online: https://www.nda.agric.za/docs/policy/policy98.htm (accessed on 9 August 2021).
- Peters, S.M.; Lindeman, H.; van der Westhuizen, M.C. Locust control data analysis: Environmental, manpower and financial analysis of locust control in South Africa. In Proceedings of the Workshop on Research Priorities for Migrant Pests of Agriculture in Southern Africa, PPRI, Pretoria, South Africa, 24–26 March 1999; Cheke, R.A., Rosenburg, L.J., Kieser, M.E., Eds.; Natural Resources Institute: Chatham, UK, 2000; pp. 75–87. [Google Scholar]
- Brown, H.D. Current pesticide application: Effectiveness and persistence. In Proceedings of the Locust Symposium, MacGregor Museum, Kimberley, South Africa, 11–13 May 1987; Mckensie, B., Longridge, M., Eds.; South African Institute of Ecologists Bulletin, Special Issue: Rondebosch, South Africa, 1988; pp. 101–117. [Google Scholar]
- Nailand, P.; Hanrahan, S.A. Modelling brown locust, Locustana pardalina (Walker), outbreaks in the Karoo. S. Afr. J. Sci. 1993, 89, 420–424. [Google Scholar]
- Todd, M.C.; Washington, R.; Cheke, R.A.; Kniverton, D. Brown locust outbreaks and climate variability in southern Africa. J. App. Ecol. 2002, 39, 31–42. [Google Scholar] [CrossRef]
- Riegert, P.W.; Ewen, A.B.; Lockwood, J.A. A history of chemical control of grasshoppers and locusts 1940–1990. In The Bionomics of Grasshoppers, Katydids and Their Kin; Gangwere, S.K., Muralirangan, M.C., Muralirangan, M., Eds.; CAB International: Wallingford, UK, 1997; pp. 385–405. [Google Scholar]
- Peveling, R. Environmental conservation and locust control—Possible conflicts and solutions. J. Orthoptera Res. 2001, 10, 171–187. [Google Scholar] [CrossRef] [Green Version]
- Stewart, D.A.B. Non-target grasshoppers as indicators of the side effects of chemical locust control in the Karoo, South Africa. J. Insect Conserv. 1998, 2, 263–276. [Google Scholar] [CrossRef]
- Price, R.E. Alternative Methods of Controlling the Brown Locust, Locustana pardalina (Walker). Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2003; pp. 1–152. Available online: http://hdl.handle.net/11660/6227 (accessed on 9 August 2021).
- Botha, D.H.; Lea, A. Do brown locust periods come in cycles? Farming S. Afr. 1970, 387, 1–5. [Google Scholar]
- Erasmus, B.H. Natural predators and parasites of the brown locust—an ecological appraisal. In Proceedings of the Locust Symposium, MacGregor Museum, Kimberley, South Africa, 11–13 May 1987; Mckensie, B., Longridge, M., Eds.; South African Institute of Ecologists Bulletin, Special Issue: Rondebosch, South Africa, 1988; pp. 118–137. [Google Scholar]
- Van Schalkwijk, H.A.D. The status of Wohlfahrtia euvitatta Vill. (Diptera, Sarcophagidae) as a parasite of the brown locust. J. Entmol. Soc. Sth. Afr. 1939, 2, 18–35. [Google Scholar]
- Price, R.E.; Brown, H.D. The status of the locust fly, Wohlfahrtia pachytyli (Diptera: Sarcophagidae), in the Karoo and the impact of locust control operations on its abundance. Afr. Entmol. 2006, 14, 35–43. [Google Scholar]
- Anonymous. First Report of the Committee of Control of the South African Central Locust Bureau; Fuller, C., Ed.; Republic of South Africa, Locust Control and Research, Department of Agriculture, Cape of Good Hope: Cape Town, South Africa, 1907; Volume 1. [Google Scholar]
- Du Plessis, C.; Botha, D.H. Preliminary field experiments on the attractiveness of certain chemicals and bait carriers to hoppers of the brown locust. J. Entomol. Soc. S. Afr. 1939, 2, 74–92. [Google Scholar]
- Price, R.E.; Brown, H.D. Locust control by means of selective baiting. In New Strategies for Locust Control; Krall, S., Peveling, R., Ba Diallo, D., Eds.; Birkhäuser: Basel, Switzerland, 1997; pp. 209–217. [Google Scholar]
- Bennett, L.V.; Symmons, P.M. A review of the effectiveness of certain control techniques against the Desert locust. Anti. Locust Bull. 1972, 50, 1–15. [Google Scholar]
- Brader, L. Control of grasshopper and migratory locusts. In Proceedings of the Crop Protection Conference (Pests and Diseases), Brighton, UK, 21–24 November 1988; pp. 283–288. [Google Scholar]
- Matthews, G.A. The Pesticide Referee Group of FAO and its contribution to locust control. J. Orthoptera Res. 2005, 14, 203–206. [Google Scholar] [CrossRef]
- Everts, J.W. Environmental Effects of Chemical Locust and Grasshopper Control. A Pilot Study Project ECLO/SEN/003/NET; FAO: Rome, Italy, 1990; 227p. [Google Scholar]
- Neuman, R.; Guyer, W. Biochemical and toxicological differences in the mode of action of the benzoylureas. Pest. Sci. 1987, 20, 147–156. [Google Scholar] [CrossRef]
- Bateman, R.P. The development of a mycoinsecticide for the control of locusts and grasshoppers. Outlook Agric. 1997, 26, 13–18. [Google Scholar] [CrossRef]
- Lomer, C.J.; Prior, C.; Kooyman, C. Development of Metarhizium spp. for the control of grasshoppers and locusts. Mem. Entomol. Soc. Canada 1997, 171, 265–286. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Evaluation of Field Trial Data on the Efficacy and Selectivity of Insecticides on Locusts and Grasshoppers. In Proceedings of the Report to FAO by the Pesticide Referee Group, 8th Meeting, Rome, Italy, 11–14 October 1999; Available online: http://www.fao.org/ag/locusts/common/ecg/800_en_PRG08e.pdf (accessed on 9 August 2021).
- Love, G.; Riwoe, D. Economic Costs and Benefits of Locust Control in Eastern Australia. ABARE eReport 05.14 Prepared for the Australian Plague Locust Commission; Australian Bureau of Agricultural and Resource Economics: Canberra, Australia, 2005. [Google Scholar]
- Bateman, R.P.; Price, R.E.; Müller, E.J.; Brown, H.D. Controlling brown locust hopper bands in South Africa with a myco-insecticide spray. In Proceedings of the Crop Protection Conference (Pests and Diseases), Brighton, UK, 21–24 November 1994; Volume 2, pp. 609–616. [Google Scholar]
- Price, R.E.; Bateman, R.P.; Brown, H.D.; Butler, E.T.; Müller, E.J. Aerial spray trials against brown locust, Locustana pardalina (Walker), nymphs in South Africa using an oil- formulation of Metarhizium flavoviride. Crop Prot. 1997, 16, 345–351. [Google Scholar] [CrossRef]
- Müller, E.J. Control of the brown locust, Locustana pardalina (Walker) (Orthoptera: Acrididae), using a myco-insecticide: Addressing the issues of speed of kill, dose rate, mortality and reduction in feeding. Afr. Entmol. 2001, 8, 217–221. [Google Scholar]
- Blanford, S.; Thomas, M.B. Thermal behaviour of two acridid species: Effects of habitat and season on body temperature and the potential impact on biocontrol with pathogens. Environ. Entmol. 2000, 29, 1060–1069. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Thomas, M.B. Effects of a mycoinsecticide on feeding and fecundity of the brown locust, Locustana pardalina. Biocontrol. Sci. Technol. 2000, 10, 321–329. [Google Scholar] [CrossRef]
- Streett, D.A.; McGuire, M.R. Pathogenic diseases of grasshoppers. In Biology of Grasshoppers; Chapman, R.F., Joern, A., Eds.; John Wiley & Sons: New York, NY, USA, 1990; pp. 483–516. [Google Scholar]
- Streett, D.A.; Woods, S.A.; Erlandson, M.A. Entomopoxviruses associated with grasshoppers and locusts: Biology and biological control potential. In Microbial Control of Grasshoppers and Locusts; Goettel, M.S., Johnson, D.L., Eds.; Memoirs of the Entomological Society of Canada: Ottawa, ON, Canada, 1997; Volume 171, pp. 115–130. [Google Scholar]
- Zelazny, B.; Goettel, M.S.; Keller, B. The potential of bacteria for the microbial control of grasshoppers and locusts. In Microbial Control of Grasshoppers and Locusts; Goettel, M.S., Johnson, D.L., Eds.; Memoirs of the Entomological Society of Canada: Ottawa, ON, Canada, 1997; Volume 171, pp. 147–156. [Google Scholar]
- Streett, D.A. Future prospect for microbial control of grasshoppers. In Integrated Pest Management on Rangeland; Capinera, J.L., Ed.; Westview Press: Boulder, CO, USA, 1987; pp. 205–218. [Google Scholar]
- Prior, C.; Greathead, D.J. Biological Control of Locusts: The Potential for the Exploitation of Pathogens. FAO Plant Prot. Bull. 1989, 37, 37–48. [Google Scholar]
- Cilliers, J.; van Someren-Greve, J.; Lea, A. A case of converging swarms of the brown locust and implications for control strategy. S. Afr. J. Agric. Sci. 1964, 7, 867–874. [Google Scholar]
- Steenkamp, D.; Botha, T.; van der Westhuizen, L. Cost benefit analysis of locust control in South Africa. In Insects in African Entomology and Environment, Proceedings of the Joint Conference Entomological Society of South Africa (11th Congress) and the Africa Association Insect Scientists (12th Congress), Stellenbosch, South Africa, 30 June–4 July 1997; Entomological Society of Southern Africa: Pretoria, South Africa, 1997; p. 81. [Google Scholar]
- Esler, K.J.; Milton, S.J.; Dean, W.R.J. Karoo Veld: Ecology and Management; Briza Publications: Pretoria, South Africa, 2010; 214p. [Google Scholar]
- Coetsee, J.C. Weidingsimpak van Die Bruinsprinkaan, Locustana pardalina (Walker) in Die Karoo-Uitbraakgebiet. Master’s Thesis, University of Free State, Bloemfontein, South Africa, 1994; 132p. [Google Scholar]
- Krall, S.; Herok, C. Economics of desert locust control. In New Strategies for Locust Control; Krall, S., Peveling, R., Ba Diallo, D., Eds.; Birkhäuser: Basel, Switzerland, 1997; pp. 401–414. [Google Scholar]
| Outbreak Season | No. Hopper Bands Controlled | No. Adults Swarms Controlled |
|---|---|---|
| 1983–1984 | 61 | 34 |
| 1984–1985 | 633 | 65 |
| 1985–1986 | 175,500 | 38,600 |
| 1986–1987 | 68,902 | 14 |
| 1987–1988 | 5618 | 1123 |
| 1988–1989 | 85,935 | 1642 |
| 1989–1990 | 36,553 | 1392 |
| 1990–1991 | 1142 | 357 |
| 1991–1992 | 18,131 | 1603 |
| 1992–1993 | 72 | 0 |
| 1993–1994 | 34,581 | 9565 |
| 1994–1995 | 20,895 | 663 |
| 1995–1996 | 24,489 | 6577 |
| 1996–1997 | 75,890 | 8081 |
| 1997–1998 | 1018 | 80 |
| 1998–1999 | 2 | 0 |
| 1999–2000 | 40,115 | 9021 |
| 2000–2001 | 28,642 | 1135 |
| 2001–2002 | 1905 | 137 |
| 2002–2003 | 0 | 0 |
| 2003–2004 | 128 | 154 |
| 2004–2005 | 1167 | 20 |
| Before 1906 | Mechanical and cultural control methods (trampling, beating, burning pastures, digging up of egg beds, collecting adult locusts in sacks, spraying soap solutions) |
| 1906–1934 | Application of sodium arsenite (supplied free of charge to farmers and applied as aqueous or dusting formulations using hand-operated pumps) |
| 1934–1944 | Sodium arsenite baits (moistened bran bait applied by hand to roosting hopper bands) |
| 1945–1986 | Benzene hexachloride (BHC) (applied first as a bait agent and as aqueous spray, but mainly as a dust formulation (mainly 7% gamma isomer, but also as Lindane dust with 99% gamma BHC formulation), applied as a dusting powder from hand-operated or motorised dusting machines at area application rates of 15–20 kg/ha) |
| 1975–1994 | Organophosphate insecticides (diazinon and fenitrothion applied as an ultra-low-volume (ULV) sprays from a range of motorised mist-blower and stacked-disc sprayers). A standard 400 g/ℓ fenitrothion formulation was applied at a volume rate of 2.5 ℓ/ha, giving an area dose rate of 1 kg a.i./ha |
| 1990–to date | Synthetic pyrethroid insecticides (deltamethrin and esfenvalerate applied as ULV spray at a volume rate of 2.5 ℓ/ha from motorised back-pack or vehicle-mounted sprayers). Esfenvalerate UL 8g a.i./ℓ formulation is now mainly employed in the Karoo |
| Future | New products already registered or trial work completed (e.g., alpha-cypermethrin, fipronil, Metarhizium myco-insecticide) |
| Control Strategies, Current and Alternatives | Benefits (pros) | Constraints (cons) |
|---|---|---|
| 0-‘Commando’ system | Current strategy. Long history, community-based system, local knowledge and communication networks, per diem payments to local people for operations, good control of small outbreaks and able to dampen large upsurges. Spray roosting swarms at night so more time to control large-size targets. | Not effective in some areas, weak reporting from remote farms, reduced access to all farms, mainly target L5 bands so not cost effective, not able to stop large upsurges or prevent plague cycles. No central coordination of operations or situation over-view, no early warning or mapping of outbreaks, sub-optimal use of strategic resources. |
| 1-Abandoning brown locust control entirely | With natural enemies and diseases, unfavourable climatic conditions, exodus of swarms into unsuitable areas, outbreaks will always end naturally. Ecologically sustainable. Money saved on control operations could be used to compensate for crop losses. No environmental damage from pesticides. | Large-scale outbreaks cause severe damage to Karoo grazing, widespread damage to commercial cereal crops outside the Karoo, threat of starvation amongst smallholder farmers and rural communities. Swarm invasions into neighbouring countries, political pressure on South Africa to contain locust outbreaks. |
| 2-Update the current “Commando system” | Adoption of modern GIS technology for target reporting and campaign management, more focus on control of aggregating swarms, more effective deployment of resources, better planning and direction of operations, more tactical use of spray aircraft when required. A viable and cost-effective strategy for brown locust control in some areas, such as the Upper and eastern Karoo. Will work well once the manpower and operational resources are in place. | Outdated and not currently effective over a vast area of the Central and Great Karoo and Bushmanland. Substantial investment necessary in new technologies. Will still have weak reporting of targets in remote areas, risk of sub-optimal adoption and use of technologies. Fewer locust control teams required – loss of income for communities. Availability, coordination and high costs of spray aircraft. |
| 3-Abandon hopper control and target adult swarms only | Adult swarms naturally coalesce to form large targets. Adult locusts are known to be more susceptible targets to kill with insecticides than late instar hoppers. Farmers and locust officers spot and report the swarms, limited number of small to medium capacity spray aircraft required. Sophisticated commercial aerial spray companies in South Africa. Reduced insecticide application and environmental impact. | During large outbreaks adult populations develop simultaneously over wide areas, swarm spotting capacity and spray aircraft resources overwhelmed, massed swarm escapes from the Upper and eastern Karoo. More locust damage to Karoo grazing and increased threat to cereal crops outside Karoo. Political pressure from commercial farmers and rural communities, swarm invasion of neighbouring countries. |
| 4-Necessity of an IPM approach Modernised and well-resourced Commando system in the Upper Karoo and eastern Karoo, spraying fledgling swarms and large hopper bands. + Stop using ground-based ‘Commando’ system in remote areas of central and western Karoo where ineffective and switch mainly to aircraft control of aggregating swarms. | Modernize and support Commando system in Upper and eastern Karoo. Stop wasting finances and resources with current ineffective system in remote areas. Locust officers now employed to spot and track aggregating swarms and to direct spray aircraft to targets. Use modern technology to map outbreaks and to direct ground operations and aircraft campaign. More effective allocation of manpower and resources. More effective kill of locusts (dead locusts per litre of insecticide). | Farmer political pressure that remote areas of Karoo are being ‘neglected’, loss of potential income for locust officers and spray teams. Manpower for spotting and tracking swarm targets in remote areas. Availability and coordination of aircraft for swarm control. High costs of hire aircraft capacity. New ULV formulations required for aircraft. Short window to spray roosting swarm targets in the morning. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, R. Alternative Strategies for Controlling the Brown Locust, Locustana pardalina (Walker). Agronomy 2021, 11, 2212. https://doi.org/10.3390/agronomy11112212
Price R. Alternative Strategies for Controlling the Brown Locust, Locustana pardalina (Walker). Agronomy. 2021; 11(11):2212. https://doi.org/10.3390/agronomy11112212
Chicago/Turabian StylePrice, Roger. 2021. "Alternative Strategies for Controlling the Brown Locust, Locustana pardalina (Walker)" Agronomy 11, no. 11: 2212. https://doi.org/10.3390/agronomy11112212
APA StylePrice, R. (2021). Alternative Strategies for Controlling the Brown Locust, Locustana pardalina (Walker). Agronomy, 11(11), 2212. https://doi.org/10.3390/agronomy11112212





