A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin
Abstract
1. Introduction
2. Fungal Diseases
2.1. Alternaria solani
2.2. Septoria lycopersici
2.3. Botrytis cinerea
2.4. Fusarium oxysporum
2.4.1. Fusarium oxysporum f. sp. lycopersici
2.4.2. Fusarium oxysporum f. sp. radicis-lycopersici
2.5. Verticillium dahliae
3. Bacterial Diseases
3.1. Clavibacter michiganensis subsp. michiganensis
3.2. Pseudomonas syringae pv. tomato
4. Phytoplasma Diseases
Candidatus Phytoplasma solani
5. Viral Diseases
5.1. Tomato Spotted Wilt Virus
5.2. Cucumber Mosaic Virus
5.3. Tomato Yellow Leaf Curl Virus and Tomato Yellow Leaf Curl Sardinia Virus
5.4. Tomato Brown Rugose Fruit Virus
5.5. Tomato Mosaic Virus
5.6. Parietaria Mottle Virus
5.7. Pepino Mosaic Virus
6. Viroid Diseases
Potato Spindle Tuber Viroid
7. Effect of Climate Change on Tomato Diseases
8. Future Prospects
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 September 2021).
- Davino, S.W.; Panno, S.; Parrella, G.; Davino, M.; Cocuzza, G.E.M.; Rapisarda, C.; Caruso, A.G.; Carpino, C. Viruses. In Tomato Diseases. Viruses and Soilborne Fungi; Davino, S.W., Ed.; Edizioni L’Informatore Agrario s.r.l.: Verona, Italy, 2018; pp. 11–137. [Google Scholar]
- Gilardi, G.; Matic, S.; Guarnaccia, V.; Garibaldi, A.; Gullino, M.L. First Report of Fusarium clavum Causing Leaf Spot and Fruit Rot on Tomato in Italy. Plant Dis. 2021. [Google Scholar] [CrossRef]
- Infantino, A.; Loreti, S. Malattie. In Il Pomodoro; Collana Coltura&Cultura, Bayer CropScience; Angelini, R., Ed.; Script: Bologna, Italy, 2010; pp. 194–219. [Google Scholar]
- Barba, M.; Martelli, G.; Tomassoli, L.; Galllitelli, D.; Di Serio, F.; Pasquini, G. Virosi e fitoplasmosi. In AA.VV. Il Pomodoro; Collana Coltura&Cultura; Bayer CropScience, Ed.; Script: Bologna, Italy, 2010. [Google Scholar]
- Zaagueri, T.; Mnari-Hattab, M.; Moussaoui, N.; Accotto, G.P.; Noris, E.; Marian, D.; Vaira, A.M. Chickpea chlorotic dwarf virus infecting tomato crop in Tunisia. Eur. J. Plant Pathol. 2019, 154, 1159–1164. [Google Scholar] [CrossRef]
- Williams, C.E.; Clair, D.A. Phenetic relationships and levels of variability detected by restriction fragment length polymorphism and random amplified polymorphic DNA analysis of cultivated and wild accessions of Lycopersicon esculentum. Genome 1993, 36, 619–630. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Blanca, J.; Montero-Pau, J.; Sauvage, C.; Bauchet, G.; Illa, E.; Díez, M.J.; Francis, D.; Causse, M.; van der Knaap, E.; Cañizares, J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genom. 2015, 16, 257. [Google Scholar] [CrossRef]
- King, K.C.; Lively, C.M. Does genetic diversity limit disease spread in natural host populations? Heredity 2012, 109, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, A.K.; Kumar, A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Oh, Y.; Panthee, D.R. Current Status of Early Blight Resistance in Tomato: An Update. Int. J. Mol. Sci. 2017, 18, 2019. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R. Agressividade de Alternaria tomatophila, A. grandis e A. solani em Batateira e Tomateiro. Ph.D. Thesis, Universidade Federal de Viçosa: Viçosa, Brazil, 2010. [Google Scholar]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Ferrandino, F.J.; Elmer, W.H. Reduction in tomato yield due to Septoria leaf spot. Plant Dis. 1992, 76, 208–211. [Google Scholar] [CrossRef]
- MacNeill, B.H. Studies in Septoria lycopersici Speg. Canadian J. Res. 1950, 28, 645–672. [Google Scholar] [CrossRef]
- Sohi, H.S.; Sokhi, S.S. Morphological physiological and pathological studies in Septoria lycopersici. Indian Phytopath. 1974, 26, 666–673. [Google Scholar]
- Utkhede, R.S.; Mathur, S. Preventive and curative biological treatments for control of Botrytis cinerea stem canker of greenhouse tomatoes. BioControl 2006, 51, 363–373. [Google Scholar] [CrossRef]
- Eden, M.A.; Hill, R.A.; Beresford, R.; Stewart, A. The influence of inoculum concentration, relative humidity, and temperature on infection of greenhouse tomatoes by Botrytis cinerea. Plant Pathol. 1996, 45, 795–806. [Google Scholar] [CrossRef]
- Carisse, O.; Van der Heyden, H. Relationship of airborne Botrytis cinerea conidium concentration to tomato flower and stem infections: A threshold for de-leafing operations. Plant Dis. 2015, 99, 137–142. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Zhou, Y.; Xia, X.; Yu, J.; Shi, K. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Khan, S.M. Effects of root-dip treatment with certain phosphate solubilizing microorganisms on the fusarial wilt of tomato. Bioresour. Technol. 2002, 85, 213–215. [Google Scholar] [CrossRef]
- Momol, T.; Ji, P.; Pernezny, K.; McGovern, R.; Olson, S. Three Soilborne Tomato Diseases Caused by Ralstonia and Fusarium Species and their Field Diagnostics. EDIS 2008, 205, 1–6. [Google Scholar] [CrossRef]
- Ramyabharathi, S.A.; Meena, B.; Raguchander, T. Induction of chitinase and β-1, 3-glucanase PR proteins in tomato through liquid formulated Bacillus subtilis EPCO 16 against Fusarium wilt. JTBSRR 2012, 1, 50–60. [Google Scholar]
- Singh, R.; Biswas, S.K.; Nagar, D.; Singh, J.; Singh, M.; Mishra, Y.K. Sustainable Integrated Approach for Management of Fusarium Wilt of Tomato Caused by Fusarium oxysporum f. sp. lycopersici (Sacc.) Synder and Hansen. Sustain. Agric. Res. 2015, 4, 138–147. [Google Scholar] [CrossRef][Green Version]
- Sharma, B.K.; Singh, R.P.; Saha, S.; Kumar, A.; Rai, A.B. Effect of temperature, pH and media on the growth and sporulation of Fusarium oxysporum f. sp. lycopersici causing wilt of tomato. Progress. Hortic. 2011, 43, 186–192. [Google Scholar]
- Katan, T.; Shlevin, E.; Katan, J. Sporulation of Fusarium oxysporum f. sp. lycopersici on stem surfaces of tomato plants and aerialdissemination of inoculum. Phytopathology 1997, 87, 712–719. [Google Scholar] [CrossRef]
- Minuto, A.; Clematis, F.; Gullino, M.L.; Garibaldi, A. Induced suppressiveness to Fusarium oxysporum f. sp. radicis lycopersici in rockwool substrate used in closed soilless systems. Phytoparasitica 2007, 35, 77–85. [Google Scholar] [CrossRef]
- Menzies, J.G.; Jarvis, W.R. The infestation of tomato seed by Fusarium oxysporum f. sp. radicis-lycopersici. Plant Pathol. 1994, 43, 378–386. [Google Scholar] [CrossRef]
- Kabaş, A.; Zengin, S.; Oğuz, A.; İlbi, H.; Ünlü, A. Improvement of new tomato varieties resistant to Fusarium oxysporum f. sp. radicis lycopersici. Acta Hortic. 2020, 1271, 427–434. [Google Scholar] [CrossRef]
- Rekah, Y.; Shtienberg, D.; Katan, J. Disease development following infection of tomato and basil foliage by airborne conidia of the soilborne pathogens Fusarium oxysporum f. sp. radicis-lycopersici and F. oxysporum f. sp. basilici. Phytopathology 2000, 90, 1322–1329. [Google Scholar] [CrossRef]
- Gullino, M.L.; Minuto, A.; Gilardi, G.; Garibaldi, A.; Ajwa, H.; Duafala, T. Efficacy of preplant soil fumigation with chloropicrin for tomato production in Italy. Crop Prot. 2002, 21, 741–749. [Google Scholar] [CrossRef]
- Papadaki, A.M.; Bletsos, F.A.; Eleftherohorinos, I.G.; Menexes, G.; Lagopodi, A.L. Effectiveness of seven commercial rootstocks against verticillium wilt and their effects on growth, yield, and fruit quality of tomato. Crop Prot. 2017, 102, 25–31. [Google Scholar] [CrossRef]
- Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Hibar, K.; Robb, J.; El Mahjoub, M. Effect of Temperature on Verticillium Wilts of Tomato in Tunisia. Plant Pathol. J. 2006, 5, 1–6. [Google Scholar] [CrossRef][Green Version]
- Chang, R.J.; Ries, S.M.; Pataky, J.K. Reductions in yield of processing tomatoes and incidence of bacterial canker. Plant Dis. 1992, 76, 805–809. [Google Scholar] [CrossRef]
- Poysa, V. Evaluation of tomato breeding lines resistant to bacterial canker. Can. J. Plant Pathol. 1993, 15, 301–304. [Google Scholar] [CrossRef]
- Hausbeck, M.K.; Bell, J.; Medina-Mora, C.; Podolsky, R.; Fulbright, D.W. Effect of Bactericides on Population Sizes and Spread of Clavibacter michiganensis subsp. michiganensis on Tomatoes in the Greenhouse and on Disease Development and Crop Yield in the Field. Phytopathology 2000, 90, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sharabani, G.; Manulis-Sasson, S.; Chalupowicz, L.; Borenstein, M.; Shulhani, R.; Lofthouse, M.; Sofer, M.; Frenkel, O.; Dror, O.; Shtienberg, D. Temperature at the early stages of Clavibacter michiganensis subsp. michiganensis infection affects bacterial canker development and virulence gene expression. Plant Pathol. 2014, 63, 1119–1129. [Google Scholar] [CrossRef]
- Xu, X.; Rajashekara, G.; Paul, P.A.; Miller, S.A. Colonization of tomato seedlings by bioluminescent Clavibacter michiganensis subsp. michiganensis under different humidity regimes. Phytopathology 2012, 102, 177–184. [Google Scholar] [CrossRef][Green Version]
- Yunis, H.; Bashan, Y.; Okon, Y.; Henis, Y. Weather dependence, yield losses, and control of bacterial speck of tomato caused by Pseudomonas tomato. Plant Dis. 1980, 64, 937–939. [Google Scholar] [CrossRef]
- Basim, H.; Basim, E.; Yilmaz, S.; Dickstein, E.R.; Jones, J.B. An outbreak of bacterial speck caused by Pseudomonas syringae pv. tomato on tomato transplants grown in commercial seedling companies located in the Western Mediterranean region of Turkey. Plant Dis. 2004, 88, 1050. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Z.; Shao, S.; Zhang, S.; Ahammed, G.J.; Zhang, G.; Jiang, Y.; Zhou, J.; Xia, X.; Zhou, Y.; et al. Tomato-Pseudomonas syringae interactions under elevated CO₂ concentration: The role of stomata. J. Exp. Bot. 2015, 66, 307–316. [Google Scholar] [CrossRef]
- Testi, V.; Delvago, C.; Mazzoli, G.L. Studio sulla diffusione e l’intensità di Stolbur su pomodoro da industria in provincia di Parma. Atti Giorn. Fitopatol. 2008, 2, 607–612. [Google Scholar]
- Rotter, A.; Nikolić, P.; Turnšek, N.; Kogovšek, P.; Blejec, A.; Gruden, K.; Dermastia, M. Statistical modeling of long-term grapevine response to ‘Candidatus Phytoplasma solani’ infection in the field. Eur. J. Plant Pathol. 2018, 150, 653–668. [Google Scholar] [CrossRef]
- Roselló, S.; Díez, M.J.; Nuez, F. Viral diseases causing the greatest economic losses to the tomato crop. I. The Tomato spotted wilt virus—A review. Sci. Hortic. 1996, 67, 117–150. [Google Scholar] [CrossRef]
- Sevik, M.A.; Arli-Sokmen, M. Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica 2012, 40, 87–93. [Google Scholar] [CrossRef]
- Riley, D.G.; Joseph, S.V.; Srinivasan, R. Temporal Relationship of Thrips Populations to Tomato Spotted Wilt Incidence in Tomato in the Field. J. Entomol. Sci. 2012, 47, 65–75. [Google Scholar] [CrossRef]
- Mahjabeen, K.P.A.; Sarwar, N.; Saleem, M.Y.; Asghar, M.; Iqbal, Q.; Jamil, F.F. Effect of cucumber mosaic virus infection on morphology, yield and phenolic contents of tomato. Arch. Phytopathol. Pflanzenschutz 2012, 45, 766–782. [Google Scholar] [CrossRef]
- White, J.L.; Tousignant, M.E.; Geletka, L.M.; Kaper, J.M. The replication of a necrogenic cucumber mosaic virus satellite is temperature sensitive in tomato. Arch. Virol. 1995, 140, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, M.; Friedmann, M.; Lachman, O.; Antignus, Y.; Nahon, S.; Cohen, S.; Pilowsky, M. Comparison of resistance level to tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Dis. 1997, 81, 1425–1428. [Google Scholar] [CrossRef]
- Valverde, R.A.; Lotrakul, P.; Landry, A.D.; Boudreaux, J.E. First Report of Tomato yellow leaf curl virus in Louisiana. Plant Dis. 2001, 85, 230. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, L.; Sun, Y.; Guo, H.; Ge, F. The Contrasting Effects of Elevated CO2 on TYLCV Infection of Tomato Genotypes with and without the Resistance Gene, Mi-1.2. Front. Plant Sci. 2016, 7, 1680. [Google Scholar] [CrossRef]
- Oladokun, J.O.; Halabi, M.H.; Barua, P.; Nath, P.D. Tomato brown rugose fruit disease: Current distribution, knowledge and future prospects. Plant Pathol. 2019, 68, 1579–1586. [Google Scholar] [CrossRef]
- Candilo, M.; di Faccioli, G.; Grassi, G.; Faeti, V. Effect of Tomato mosaic virus (ToMV) on yield of machine-harvested processing tomatoes. Phytopathol. Mediterr. 1992, 31, 32–36. [Google Scholar]
- Mohamed, E.F. Interaction Between Some Viruses Which Attack Tomato (Lycopersicon esculentum Mill.) Plants and Their Effect on Growth and Yield of Tomato Plants. J. Am. Sci. 2010, 6, 311–320. [Google Scholar]
- Imran, M.; Khan, M.A.; Fiaz, M.; Azeem, M.; Mustafa, M. Influence of environmental conditions on tomato mosaic virus disease development under natural condition. Pak. J. Phytopathol. 2013, 25, 117–122. [Google Scholar]
- Parrella, G. Sources of resistance in wild Solanum germplasm (section Lycopersicon) to parietaria mottle virus, an emerging virus in the Mediterranean basin. Plant Pathol. 2020, 69, 1018–1025. [Google Scholar] [CrossRef]
- Jordá, C.; Perez, A.L.; Martínez-Culebras, P.; Abad, P.; Lacasa, A.; Guerrero, M. First report of Pepino mosaic virus on tomato in Spain. Plant Dis. 2001, 85, 1292. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Mumford, R.; van der Vlugt, R.; Alfaro Fernandez, A.; Bese, G.; Glyn, J.; Lambourne, C.; Schenk, M. The Effect of Pepino mosaic virus on tomato yield. Acta Hortic. 2011, 914, 203–206. [Google Scholar] [CrossRef]
- Alcaide, C.; Sardanyés, J.; Elena, S.F.; Gómez, P. Increasing temperature alters the within-host competition of viral strains and influences virus genetic variability. Virus Evol. 2021, 7, veab017. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.E.; Barbetti, M.J.; Rodoni, B.; McKirdy, S.J.; Jones, R.A.C. Effects of a Potato Spindle Tuber Viroid Tomato Strain on the Symptoms, Biomass, and Yields of Classical Indicator and Currently Grown Potato and Tomato Cultivars. Plant Dis. 2019, 103, 3009–3017. [Google Scholar] [CrossRef]
- Harris, P.S.; Browning, I.A. The effects of temperature and light on the symptom expression and viroid concentration in tomato of a severe strain of potato spindle tuber viroid. Potato Res. 1980, 23, 85–93. [Google Scholar] [CrossRef]
- Verhoeven, J.T.J.; Hüner, L.; Virscek Marn, M.; Mavric Plesko, I.; Roenhorst, J.W. Mechanical transmission of Potato spindle tuber viroid between plants of Brugmansia suaveolens, Solanum jasminoides and potatoes and tomatoes. Eur. J. Plant Pathol. 2010, 128, 417–421. [Google Scholar] [CrossRef]
- EPPO. Potato Spindle Tuber Viroid. EPPO Datasheets on Pests Recommended for Regulation. 2021. Available online: https://gd.eppo.int/taxon/PSTVD0/datasheet (accessed on 27 September 2021).
- Woudenberg, J.H.; Truter, M.; Groenewald, J.Z.; Crous, P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014, 79, 1–47. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria Leaf Spot Caused by Alternaria Species: An Emerging Problem on Ornamental Plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.P.; Stall, R.E.; Zitter, T.A. Compendium of Tomato Diseases; APS Press: Saint Paul, MN, USA, 1991; p. 73. [Google Scholar]
- Bessadat, N.; Berruyer, R.; Hamon, B.; Bataille-Simoneau, N.; Benichou, S.; Kihal, M.; Henni, D.E.; Simoneau, P. Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in northwestern Algeria. Eur. J. Plant Pathol. 2017, 148, 181–197. [Google Scholar] [CrossRef]
- Strandberg, J.O. Alternaria species that attack vegetable crops. In Alternaria. Biology, Plant Diseases and Metabolites; Chelkowski, J., Visconti, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1992; pp. 175–208. [Google Scholar]
- Rotem, J. The Genus Alternaria: Biology, Epidemiology, and Pathogenicity; APS Press: Saint Paul, MN, USA, 1994; p. 326. [Google Scholar]
- Lawrence, C.B.; Singh, N.P.; Qiu, J.; Gardener, R.G.; Tuzun, S. Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as elicitor release mechanism. Physiol. Mol. Plant Pathol. 2000, 57, 211–220. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Ghoneem, K.M.; Rashad, Y.M.; Abdulkhair, W.M.; Hafez, E.E.; Shabana, Y.M.; Baka, Z.A. Occurrence and distribution of tomato seed-borne mycoflora in Saudi Arabia and its correlation with the climatic variables. Microb. Biotechnol. 2014, 7, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.L. Importance of chlamydospores as primary inoculum of Alternaria solani, incitant of collar rot and early blight on tomato. Plant Dis. 1991, 75, 274–278. [Google Scholar] [CrossRef]
- Lees, A.K.; Roberts, D.M.; Lynott, J.; Sullivan, L.; Brierley, J.L. Real-Time PCR and LAMP Assays for the Detection of Spores of Alternaria solani and Sporangia of Phytophthora infestans to Inform Disease Risk Forecasting. Plant Dis. 2019, 103, 3172–3180. [Google Scholar] [CrossRef]
- Ruszczak, B.; Smykała, K.; Dziubański, K. The detection of Alternaria solani infection on tomatoes using ensemble learning. J. Ambient Intell. Smart Environ. 2020, 12, 407–418. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kashyap, P.L.; Srivastava, A.K. Rapid detection and quantification of Alternaria solani in tomato. Sci. Hortic. 2013, 151, 184–189. [Google Scholar] [CrossRef]
- Olaya, G.; Stuerm, C.; Linley, R.; Edlebeck, K.; Torriani, S.F.F. Detection of the G143A Mutation that Confers Resistance to QoI Fungicides in Alternaria tomatophila from Tomatoes. In Modern Fungicides and Antifungal Compounds; Deising, H.B., Fraaije, B., Mehl, A., Oerke, E.C., Sierotzki, H., Stammler, G., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2017; Volume VIII, pp. 225–231. [Google Scholar]
- Malandrakis, A.A.; Apostolidou, Z.A.; Louka, D.; Markoglou, A.; Flouri, F. Biological and molecular characterization of field isolates of Alternaria alternata with single or double resistance to respiratory complex II and III inhibitors. Eur. J. Plant Pathol. 2018, 152, 199–211. [Google Scholar] [CrossRef]
- Pitblado, R.E. The Development and Implementation of TOMCAST—A Weather Timed Fungicide Spray Program for Field Tomatoes; Ministry of Agriculture and food, Ridgetown college of Agricultural technology: Ridgetown, ON, Canada, 1992; pp. 1–22.
- Foolad, M.R.; Heather, L.; Hamid, A. Genetics, Genomics and Breeding of Late Blight and Early Blight Resistance in Tomato. CRC Crit. Rev. Plant Sci. 2008, 27, 75–107. [Google Scholar] [CrossRef]
- Small, I.M.; Joseph, L.; Fry, W.E. Development and implementation of the BlightPro decision support system for potato and tomato late blight management. Comput. Electron. Agric. 2015, 115, 57–65. [Google Scholar] [CrossRef]
- Verkley, G.J.; Quaedvlieg, W.; Shin, H.D.; Crous, P.W. A new approach to species delimitation in Septoria. Stud. Mycol. 2013, 75, 213–305. [Google Scholar] [CrossRef]
- Elmer, W.H.; Ferrandino, F.J. Influence of spore density, leaf age, temperature, and dew periods on Septoria leaf spot of tomato. Plant Dis. 1995, 79, 287–290. [Google Scholar] [CrossRef]
- Parker, S.K.; Nutter, F.W., Jr.; Gleason, M.L. Directional spread of Septoria leaf spot in tomato rows. Plant Dis. 1997, 81, 272–276. [Google Scholar] [CrossRef]
- Das, S.; Pattanayak, S.; Bhargavi, B. Over view of Septoria Diseases on Different Crops and its Management. IJAEB 2020, 13, 351–370. [Google Scholar] [CrossRef]
- Cabral, R.N.; Marouelli, W.A.; Lage, D.A.C.; Café-Filho, A.C. Septoria leaf spot in organic tomatoes under diverse irrigation systems and water management strategies. Hortic. Bras. 2013, 31, 392–400. [Google Scholar] [CrossRef]
- Sanoubar, R.; Barbanti, L. Fungal diseases on tomato plant under greenhouse condition. Eur. J. Biol. Res. 2017, 7, 299–308. [Google Scholar] [CrossRef]
- Stevenson, W.R. Septoria leaf spot. In Compendium of Tomato Diseases; Jones, J.B., Jones, J.P., Stall, R.E., Zitter, T.A., Eds.; APS Press: Saint Paul, MN, USA, 1991; p. 22. [Google Scholar]
- Tokeshi, H.; Carvalho, P.C.T. Doenças do tomateiro—Lycopersicum esculentum Mill. In Manual de Fitopatologia—Doenças das Plantas Cultivadas; Galli, F., Ed.; Agronômica Ceres: São Paulo, Brazil, 1980; pp. 511–532. [Google Scholar]
- Ávila, M.C.R.; Lourenço, V., Jr.; Quezado-Duval, A.M.; Becker, W.F.; de Abreu-Tarazi, M.F.; Borges, L.C.; dos Reis Nascimento, A. Field validation of TOMCAST modified to manage Septoria leaf spot on tomato in the central-west region of Brazil. Crop Prot. 2020, 138, 105333. [Google Scholar] [CrossRef]
- Kumar, S.; Sugha, S.K. Role of cultural practices in the management of Septoria leaf spot of tomato. Indian Phytopathol. 2000, 53, 105–106. [Google Scholar]
- Staats, M.; van Baarlen, P.; van Kan, J.A.L. Molecular phylogeny of the plant pathogenic genus Botrytis and the evolution of host specificity. Mol. Biol. Evol. 2005, 22, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Gomez, V.; Urrutia, T.; Melgarejo, P. Survival of Botrytis cinerea in southeastern Spanish greenhouses. Eur. J. Plant Pathol. 2001, 107, 229–236. [Google Scholar] [CrossRef]
- Aissat, K.; Nicot, P.C.; Guechi, A.; Bardin, M.; Chibane, M. Grey mould development in greenhouse tomatoes under drip and furrow irrigation. Agron. Sustain. Dev. 2008, 28, 403–409. [Google Scholar] [CrossRef]
- Baptista, F.J.; Bailey, B.J.; Meneses, J.F. Effect of nocturnal ventilation on the occurrence of Botrytis cinerea in Mediterranean unheated tomato greenhouses. Crop Prot. 2012, 32, 144–149. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X. Effect of Six Fungicides against Botrytis cinerea on Protected Cultivation Tomato. In Proceedings of the Second International Conference on Digital Manufacturing and Automation, Zhangjiajie, China, 5–7 August 2011; IEEE Computer Society: Washington, DC, USA, 2011; pp. 489–491. [Google Scholar]
- Sutton, J.C.; Li, D.W.; Peng, G.; Yu, H.; Zhang, P.; Valdebenito-Sanhueza, R.M. Gliocladium roseum a versatile adversary of Botrytis cinerea in crops. Plant Dis. 1997, 81, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- O’Neill, T.M.; Shtienberg, D.; Elad, Y. Effect of some host and microclimate factors on infection of tomato stems by Botrytis cinerea. Plant Dis. 1997, 81, 36–40. [Google Scholar] [CrossRef]
- Nakajima, M.; Akutsu, K. Virulence factors of Botrytis cinerea. J. Gen. Plant Pathol. 2014, 80, 15–23. [Google Scholar] [CrossRef]
- Petrasch, S.; Silva, C.J.; Mesquida-Pesci, S.D.; Gallegos, K.; van den Abeele, C.; Papin, V.; Fernandez-Acero, F.J.; Knapp, S.J.; Blanco-Ulate, B. Infection Strategies Deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a Function of Tomato Fruit Ripening Stage. Front. Plant Sci. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Kotze, J.M.; Matthee, F.N. Development of a technique for the recovery of soilborne sclerotia of Botrytis cinerea. Phytopathology 1983, 73, 1374–1376. [Google Scholar] [CrossRef]
- Plesken, C.; Pattar, P.; Reiss, B.; Noor, Z.N.; Zhang, L.; Klug, K.; Huettel, B.; Hahn, M. Genetic diversity of Botrytis cinerea revealed by multilocus sequencing, and identification of B. cinerea populations showing genetic isolation and distinct host adaptation. Front. Plant Sci. 2021, 12, 663027. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.A.; Dickinson, M.J.; Boonham, N. Detection of Botrytis cinerea by loop-mediated isothermal amplification. Lett. Appl. Microbiol. 2010, 51, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Le Floch, G.; Rey, P.; Deéniel, F.; Benhamou, N.; Picard, K.; Tirilly, Y. Enhancement of development and induction of resistance in tomato plants by the antagonist, Pythium oligandrum. Agronomie 2003, 23, 455–460. [Google Scholar] [CrossRef]
- De Vega, D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 2021, 44, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Zhang, J.D.; Tang, J.Q.; Liu, Z.C.; Li, Y.Q.; Chen, J.; Zou, L.W. Combined Use of Trichoderma atroviride CCTCCSBW0199 and Brassinolide to Control Botrytis cinerea Infection in Tomato. Plant Dis. 2020, 104, 1298–1304. [Google Scholar] [CrossRef]
- Poveda, J.; Barquero, M.; González-Andrés, F. Insight into the Microbiological Control Strategies against Botrytis cinerea Using Systemic Plant Resistance Activation. Agronomy 2020, 10, 1822. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Baayen, R.P.; O’Donnell, K.; Bonants, P.J.M.; Cigelnik, E.; Kroon, L.P.N.M.; Roebroeck, E.J.A.; Waalwijk, C. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 2000, 90, 891–900. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sarver, B.A.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, C.; Nirmala Devi, D.; Narasimha Murthy, K.; Mohan, C.D.; Lakshmeesha, T.R.; Singh, B.; Kalagatur, N.K.; Niranjana, S.R.; Hashem, A.; Alqarawi, A.A.; et al. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—A review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Ö.; Siragusa, M.; Ikten, H.; Polat, I.; Gümrükcü, E.; Yigit, F.; Carimi, F.; Teixeira da Silva, J.A. Fusarium oxysporum f. sp. lycopersici races and their genetic discrimination by molecular markers in West Mediterranean region of Turkey. Physiol. Mol. Plant Pathol. 2009, 74, 68–75. [Google Scholar] [CrossRef]
- Gullino, M.L.; Katan, J.; Garibaldi, A. Fusarium Wilts of Greenhouse Vegetable and Ornamental Crops; APS Press: Saint Paul, MN, USA, 2012; pp. 1–243. [Google Scholar]
- Biju, V.C.; Fokkens, L.; Houterman, P.M.; Rep, M.; Cornelissen, B.J.C. Multiple Evolutionary Trajectories Have Led to the Emergence of Races in Fusarium oxysporum f. sp. lycopersici. Appl. Environ. Microbiol. 2017, 83, e02548-16. [Google Scholar] [CrossRef] [PubMed]
- Debbi, A.; Boureghda, H.; Monte, E.; Hermosa, R. Distribution and Genetic Variability of Fusarium oxysporum Associated with Tomato Diseases in Algeria and a Biocontrol Strategy with Indigenous Trichoderma spp. Front. Microbiol. 2018, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.J. BHRS 2–3 Fusarium wilt resistant tomato. HortScience 1988, 23, 1093–1094. [Google Scholar]
- Stravato, V.M.; Buonaurio, R.; Cappelli, C. First Report of Fusarium oxysporum f. sp. lycopersici Race 2 on Tomato in Italy. Plant Dis. 1999, 83, 967. [Google Scholar] [CrossRef] [PubMed]
- Khlode, R.S.A.; Abdel-Gawad, T.I.; El-Bana, A.A.; Galal, A.A. Fusarium oxysporum f. Sp. Lycopersici (fol) race 1 and 3 as wilt-incitants to tomato plants growing at el-minia governorate, Egypt. Minia J. Agric. Res. Develop. 2016, 36, 229–244. [Google Scholar]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Inami, K.; Kashiwa, T.; Kawabe, M.; Onokubo-Okabe, A.; Ishikawa, N.; Pérez, E.R.; Hozumi, T.; Caballero, L.A.; de Baldarrago, F.C.; Roco, M.J.; et al. The tomato wilt fungus Fusarium oxysporum f. sp. lycopersici shares common ancestors with nonpathogenic F. oxysporum isolated from wild tomatoes in the Peruvian Andes. Microbes Environ. 2014, 29, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A. Biology and Integrated Control of Tomato Wilt Caused by Fusarium oxysporum lycopersici: A Comprehensive Review under the Light of Recent Advancements. J. Bot. Res. 2020, 3, 84–99. [Google Scholar] [CrossRef]
- García-Enciso, E.L.; Benavides-Mendoza, A.; Flores-López, M.L.; Robledo-Olivo, A.; Juárez-Maldonado, A.; González-Morale, S. A Molecular Vision of the Interaction of Tomato Plants and Fusarium oxysporum f. sp. lycopersici. In Fusarium—Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Askun, T., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Minuto, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot. 2006, 25, 468–475. [Google Scholar] [CrossRef]
- Amini, J.; Sidovich, D. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with fusarium wilt of tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar] [CrossRef]
- Charoenporn, C.; Kanokmedhakul, S.; Lin, F.C.; Poeaim, S.; Soytong, K. Evaluation of bio-agent formulations to control Fusarium wilt of tomato. Afr. J. Biotechnol. 2010, 9, 5836–5844. [Google Scholar]
- Loganathan, M.; Garg, R.; Venkataravanappa, V.; Saha, S.; Rai, A.B. Plant growth promoting rhizobacteria (PGPR) induces resistance against Fusarium wilt and improves lycopene content and texture in tomato. Afr. J. Microbiol. Res. 2014, 8, 1105–1111. [Google Scholar] [CrossRef]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. An assessment of the modulation of the population dynamics of pathogenic Fusarium oxysporum f. sp. lycopersici in the tomato rhizosphere by means of the application of Bacillus subtilis QST 713, Trichoderma sp. TW2 and two composts. Biol. Control 2020, 142, 104158. [Google Scholar] [CrossRef]
- Hanaa, R.M.F.; Zeinab, A.A.; Dawlat, A.S.; Mervat, A.R.I.; Sror, H.A.M. Effect of neem and willow aqueous extracts on Fusarium wilt disease in tomato seedlings: Induction of antioxidant defensive enzymes. Arab Univ. J. Agric. Sci. 2011, 19, 131–140. [Google Scholar] [CrossRef]
- Almasi, M.A.; Dehabadi, S.M.H.; Moradi, A.; Eftekhari, Z.; Ojaghkandi, M.A.; Aghaei, S. Development and Application of Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Fusarium Oxysporum f. Sp. lycopersici. J. Plant Pathol. Microbiol. 2013, 4, 177. [Google Scholar] [CrossRef]
- Gonzalez-Cendales, Y.; Catanzariti, A.M.; Baker, B.; Mcgrath, D.J.; Jones, D.A. Identification of I-7 expands the repertoire of genes for resistance to Fusarium wilt in tomato to three resistance gene classes. Mol. Plant Pathol. 2016, 17, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil . J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Çolak, A.; Biçici, M. PCR detection of Fusarium oxysporum f. sp. radicis-lycopersici and races of F. oxysporum f. sp. lycopersici of tomato in protected tomato-growing areas of the eastern Mediterranean region of Turkey. Turk. J. Agric. For. 2013, 37, 457–467. [Google Scholar] [CrossRef]
- Boix-Ruiz, A.; Gómez-Tenorio, M.A.; Ruiz-Olmos, C.; Marín-Guirao, J.I.; Toresano-Sánchez, F.; Tello-Marquina, J.C.; Camacho-Ferre, F.; de Cara-García, M. Coconut fiber grow bags as a source of primary inoculum of Fusarium oxysporum f. sp. radicis-lycopersici in greenhouse tomato crops in Almeria (Spain). Acta Hortic. 2018, 1207, 275–280. [Google Scholar] [CrossRef]
- Katan, T.; Zamir, D.; Sarfatti, M.; Katan, J. Vegetative compatibility groups and subgroups in Fusarium oxysporum f. sp. radices-lycopersici. Phytopathology 1991, 81, 255–262. [Google Scholar] [CrossRef]
- Di Primo, P.; Cartia, G.; Katan, T. Vegetative compatibility and heterokaryon stability in Fusarium oxysporum f.sp. radicis-lycopersici from Italy. Plant Pathol. 2001, 50, 371–382. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Gautheron, N.; Steinberg, C. Genetic diversity of Fusarium oxysporum and related species pathogenic on tomato in Algeria and other Mediterranean countries. Plant Pathol. 2012, 61, 787–800. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Shoemaker, R.A. Taxonomic status of Fusarium oxysporum causing foot and root rot of tomato. Phytopathology 1978, 68, 1679–1680. [Google Scholar] [CrossRef]
- Menzies, J.G.; Koch, C.; Seywerd, F. Additions to the host range of Fusarium oxysporum f. sp. radicis-lycopersici. Plant Dis. 1990, 74, 569–572. [Google Scholar] [CrossRef]
- Szczechura, W.; Staniaszek, M.; Habdas, H. Fusarium oxysporum f. sp. radicis-lycopersici—The cause of Fusarium crown and root rot in tomato cultivation. J. Plant Prot. Res. 2013, 53, 172–176. [Google Scholar] [CrossRef]
- Benhamou, N.; Rey, P.; Cheérif, M.; Hockenhull, J.; Tirilly, Y. Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 1997, 87, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; van der Bij, A.J.; van der Drift, K.M.G.M.; Schripsema, J.; Kroon, B.; Scheffer, R.J.; Keel, C.; Bakker, P.A.H.M.; Tichy, H.-V.; et al. Biocontrol by phenazine- 1- carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f. sp. radicis-lycopersici. Mol. Plant Microbe Interact. 1998, 11, 1069–1077. [Google Scholar] [CrossRef]
- Kerkeni, A.; Daami-Remadi, M.; Tarchoun, N.; Khedher, M.B. In vitro and in vivo Suppression of Fusaruim oxysporum f. sp. radicis-lycopersici the Causal Agent of Fusarium Crown and Root Rot of Tomato by Some Compost Fungi. Int. J. Agric. Res. 2007, 2, 1022–1029. [Google Scholar] [CrossRef][Green Version]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G.S. Biocontrol potential and plant-growth-promoting effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on tomato. Acta Hortic. 2018, 1207, 139–146. [Google Scholar] [CrossRef]
- Gamliel, A.; Siti, M.; Arbel, A.; Katan, J. Soil solarization as a component in the integrated management of Fusarium crown and root rot in tomato. Acta Hortic. 2009, 808, 321–326. [Google Scholar] [CrossRef]
- Yuce, E.K.; Yigit, S.; Tosun, N. Efficacy of solarization combined with metam sodium and hydrogen peroxide in control of Fusarium oxysporum f. sp. radices lycopersici and Clavibacter michiganensis subsp. michiganensis in tomato greenhouse. Acta Hortic. 2011, 914, 385–391. [Google Scholar] [CrossRef]
- Nefzi, A.; Jabnoun-Khiareddine, H.; Aydi Ben Abdallah, R.; Ammar, N.; Medimagh-Saïdana, S.; Haouala, R.; Daami-Remadi, M. Suppressing Fusarium Crown and Root Rot infections and enhancing the growth of tomato plants by Lycium arabicum Schweinf. Ex Boiss. Extracts. S. Afr. J. Bot. 2017, 113, 288–299. [Google Scholar] [CrossRef]
- Besri, M. Verticillium wilt of tomato grown under plastic tunnel in Morocco. Acta Hortic. 1991, 287, 355–360. [Google Scholar] [CrossRef]
- Gao, Y.; Zitter, T.A.; Veilleux, R.E. Verticillium wilt in solanaceous crops. In Plant Breeding Reviews; Janick, J., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 115–144. [Google Scholar]
- EFSA PLH Panel. Scientific Opinion on the pest categorisation of Verticillium dahliae Kleb. EFSA J. 2014, 12, 3928. [Google Scholar] [CrossRef]
- Giraldo, A.; Crous, P.W. Inside Plectosphaerellaceae. Stud. Mycol. 2019, 92, 227–286. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, R.; van Esse, H.P.; Maruthachalam, K.; Bolton, M.D.; Santhanam, P.; Saber, M.K.; Zhang, Z.; Usami, T.; Lievens, B.; Subbarao, K.V.; et al. Tomato immune receptor Ve1 recognizes effector of multiple tomato fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 5110–5115. [Google Scholar] [CrossRef] [PubMed]
- Ligoxigakis, E.; Vakalounakis, D. Occurrence of race 2 of Verticillium dahliae on tomatoes in Crete. Plant Pathol. 1992, 41, 774–776. [Google Scholar] [CrossRef]
- Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Platt, H.W.; Ayed, F.; El Mahjoub, M. Variation in Aggressiveness of Tunisian Verticillium dahliae Races 1 and 2 Isolates and Response of Differential Tomato Cultivars to Verticillium Wilt. Int. J. Plant Breed. 2010, 4, 63–70. [Google Scholar]
- Jiménez-Díaz, R.M.; Olivares-García, C.; Trapero-Casas, J.L.; Jiménez-Gasco, M.M.; Navas-Cortés, J.A.; Landa, B.B.; Milgroom, M.G. Variation of pathotypes and races and their correlations with clonal lineages in Verticillium dahliae. Plant Pathol. 2017, 66, 651–666. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1955, 45, 180–181. [Google Scholar]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Short, D.P.G.; Gurung, S.; Maruthachalam, K.; Atallah, Z.; Subbarao, K.V. Verticillium dahliae race 2-specific PCR reveals a high frequency of race 2 strains in commercial spinach seed lots and delineates race structure. Phytopathology 2014, 104, 779–785. [Google Scholar] [CrossRef]
- Gullino, M.L.; Minuto, A.; Garibaldi, A. Soil fumigation with chloropicrin in Italy: Experimental results on melon, eggplant and tomato. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2002, 67, 171–180. [Google Scholar]
- Louws, F.J.; Ferguson, L.M.; Ivors, K.; Driver, J.; Jennings, K.; Milks, D.; Shoemaker, P.B.; Monks, D.W. Efficacy of methyl bromide alternatives for Verticillium and weed management in tomatoes. In Proceedings of the Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, Orlando, FL, USA, 31 October–3 November 2004; pp. 43-1–43-3. [Google Scholar]
- Hartz, T.K.; Johnstone, P.R.; Miyao, E.M.; Davis, R.M. Mustard cover crops are ineffective in suppressing soilborne disease or improving processing tomato yield. HortScience 2005, 40, 2016–2019. [Google Scholar] [CrossRef]
- Smolińska, U.; Kowalska, B. The Effect of Organic Amendments from Brassicaceae and Solanaceae Plants and Trichoderma harzianum on the Development of Verticillium dahliae Kleb. J. Fruit Ornam. Plant Res. 2008, 69, 93–104. [Google Scholar] [CrossRef]
- Abou Zeid, N.M.; Noher, A.M. Efficacy of DMDS as methyl bromide alternative in controlling soil borne diseases, root-knot nematode and weeds on pepper, cucumber and tomato in Egypt. Acta Hortic. 2014, 1044, 411–414. [Google Scholar] [CrossRef]
- Acharya, B.; Ingram, T.W.; Oh, Y.; Adhikari, T.B.; Dean, R.A.; Louws, F.J. Opportunities and Challenges in Studies of Host-Pathogen Interactions and Management of Verticillium dahliae in Tomatoes. Plants 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Ayed, F.; El Mahjoub, M. Biological Control of Tomato Verticillium Wilt by Using Indigenous Trichoderma spp. Afr. J. Plant Sci. Biotechnol. 2009, 3, 26–36. [Google Scholar]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yan, Z.C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Gartemann, K.H.; Kirchner, O.; Engemann, J.; Gräfen, I.; Eichenlaub, R.; Burger, A. Clavibacter michiganensis subsp. michiganensis: First steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 2003, 106, 179–191. [Google Scholar] [CrossRef] [PubMed]
- EPPO. EPPO A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests; EPPO: Paris, France, 2019. [Google Scholar]
- Bibi, A.; Ahmad, M.; Hussain, S. Prevalence of (Clavibacter michiganensis subsp. michiganensis) causal organism of bacterial canker in weed species in tomato fields of North West Pakistan. Sarhad J. Agric. 2018, 34, 123–129. [Google Scholar] [CrossRef][Green Version]
- Ignatov, A.N.; Spechenkova, N.A.; Taliansky, M.; Kornev, K.P. First Report of Clavibacter michiganensis subsp. michiganensis Infecting Potato in Russia. Plant Dis. 2019, 103, 147. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Balestra, G.M.; Varvaro, L. Severe Outbreak of Bacterial Canker Caused by Clavibacter michiganensis subsp. michiganensis on Tomato in Central Italy. Plant Dis. 2011, 95, 221. [Google Scholar] [CrossRef]
- Medina-Mora, C.M.; Hausbeck, M.K.; Fulbright, D.W. Bird’s eye lesions of tomato fruit produced by aerosol and direct application of Clavibacter michiganensis subsp. michiganensis. Plant Dis. 2001, 85, 88–91. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sen, Y.; van der Wolf, J.; Visser, R.G.F.; van Heusden, S. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Dis. 2015, 99, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Tsiantos, J. Transmission of Bacterium Corynebacterium michiganense pv. michiganense by Seeds. J. Phytopathol. 1987, 119, 142–146. [Google Scholar] [CrossRef]
- Fatmi, M.; Schaad, N. Semiselective agar medium for isolation of Clavibacter michiganense subsp. michiganense from tomato seed. Phytopathology 1988, 78, 121–126. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.; Landers, N.; Lebeis, A.; Fulbright, D.; Hausbeck, M. Genetic structure of Clavibacter michiganensis subsp. michiganensis populations in Michigan commercial tomato fields. Plant Dis 2012, 96, 788–796. [Google Scholar] [CrossRef] [PubMed]
- De León, L.; Siverio, F.; López, M.M.; Rodríguez, A. Clavibacter michiganesis subsp. michiganensis, a Seedborne Tomato Pathogen: Healthy Seeds Are Still the Goal. Plant Dis. 2011, 95, 1328–1338. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Kubota, R.; Jenkins, D.M.; Alvarez, A.M. Loop-mediated amplification of the Clavibacter michiganensis subsp. michiganensis micA gene is highly specific. Phytopathology 2013, 103, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara-Bell, J.; Baysal-Gurel, F.; Miller, S.A.; Alvarez, A.M. Utility of a loop-mediated amplification assay for detection of Clavibacter michiganensis subsp. michiganensis in seeds and plant tissues. Can. J. Plant Pathol. 2015, 37, 260–266. [Google Scholar] [CrossRef]
- Preston, G.M. Pseudomonas syringae pv. tomato: The right pathogen, of the right plant, at the right time. Mol. Plant Pathol. 2000, 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, C.G. The present status on bacterial diseases of tomatoes in Greece. In Progress on Pest Management in Field Vegetables, Proceedings of the CEC/IOBC Experts’ Group Meeting, Rennes, France, 20–22 November 1985; Cavallo, R., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 221–222. [Google Scholar]
- Cazorla, F.M.; Pérez-García, A.; Rivera, M.E.; Codina, J.C.; Torés, J.A.; de Vicente, A. Bacterial diseases of tomato in southern Spain: Application of a detached tissue assay to evaluate bacterial pathogenicity. Bull. OEPP 2000, 30, 351–356. [Google Scholar] [CrossRef]
- Şahin, F. Severe outbreak of bacterial speck, caused by Pseudomonas syringae pv. tomato, on field-grown tomatoes in the eastern Anatolia region of Turkey. Plant Pathol. 2001, 50, 799. [Google Scholar] [CrossRef]
- Cai, R.; Lewis, J.; Yan, S.; Liu, H.; Clarke, C.R.; Campanile, F.; Almeida, N.F.; Studholme, D.J.; Lindeberg, M.; Schneider, D.; et al. The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 2011, 7, e1002130. [Google Scholar] [CrossRef]
- Mensi, I.; Jabnoun-Khiareddine, H.; Zarrougui, N.E.; Ben Zahra, H.; Cesbron, S.; Jacques, M.A.; Daami-Remadi, M. First report of tomato bacterial speck caused by Pseudomonas syringae pv. tomato in Tunisia. New Dis. Rep. 2018, 38, 21. [Google Scholar] [CrossRef]
- Lawton, M.B.; MacNeill, B.H. Occurrence of race 1 of Pseudomonas syringae on field tomato in south western Ontario. Can. J. Plant Pathol. 1986, 8, 85–88. [Google Scholar] [CrossRef]
- Buonaurio, R.; Stravato, V.M.; Cappelli, C. Occurrence of Pseudomonas syringae pv. Tomato race 1 in Italy on Pto Gene-Bearing Tomato Plants. J. Phytopathol. 1996, 144, 437–440. [Google Scholar] [CrossRef]
- Cruz, L.; Cruz, J.; Eloy, M.; Oliveira, H.; Vaz, H.; Tenreiro, R. First report of bacterial speck of tomato caused by Pseudomonas syringae pv. tomato race 1 in Portugal. Plant Dis. 2010, 94, 1504–1505. [Google Scholar] [CrossRef] [PubMed]
- Goode, R.D.; Sasser, M. Prevention—The key to controlling bacterial spot and bacterial speck of tomato. Plant Dis. 1980, 64, 831–834. [Google Scholar] [CrossRef]
- Devash, Y.; Okon, Y.; Henis, Y. Survival of Pseudomonas tomato in soil and seeds. J. Phytopathol. 1980, 99, 175–185. [Google Scholar] [CrossRef]
- McCarter, S.M.; Jones, J.B.; Gitaitis, R.D.; Smithley, D.R. Survival of Pseudomonas syringae pv. tomato in association with tomato seed, soil, host tissue, and epiphytic weed hosts in Georgia. Phytopathology 1983, 73, 1393–1398. [Google Scholar] [CrossRef]
- Morris, C.E.; Sands, D.C.; Vinatzer, B.A.; Glaux, C.; Guilbaud, C.; Buffière, A.; Yan, S.; Dominguez, H.; Thompson, B.M. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J. 2008, 2, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.D.; Kang, H.J.; Chai, A.L.; Shi, Y.X.; Xie, X.W.; Li, L.; Li, B.J. Development of a loop-mediated isothermal amplification (LAMP) assay for rapid detection of Pseudomonas syringae pv. tomato in planta. Eur. J. Plant Pathol. 2020, 156, 739–750. [Google Scholar] [CrossRef]
- Chai, A.L.; Ben, H.Y.; Guo, W.T.; Shi, Y.X.; Xie, X.W.; Li, L.; Li, B.J. Quantification of Viable Cells of Pseudomonas syringae pv. tomato in Tomato Seed Using Propidium Monoazide and a Real-Time PCR Assay. Plant Dis. 2020, 104, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Pernezny, K.; Kůdela, V.; Kokošková, B.; Hládká, I. Bacterial diseases of tomato in the Czech and Slovak Republics and lack of streptomycin resistance among copper-tollerant bacterial strains. Crop Prot. 1995, 14, 267–270. [Google Scholar] [CrossRef]
- Shenge, K.C.; Wydra, K.; Mabagala, R.B.; Mortensen, C.N. Assessment of strains of Pseudomonas syringae pv. tomato from Tanzania for resistance to copper and streptomycin. Arch. Phytopathol. Plant Protect. 2008, 41, 572–585. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. Protection of tomato seedlings against infection by Pseudomonas syringae pv. tomato by using the plant growth-promoting bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 2002, 68, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Balestra, G.M.; Rossetti, A.; Quattrucci, A. Biological control of kiwifruit and tomato bacterial pathogens. In Cultivating the Future Based on Science, Proceedings of the 2nd Conference of the International Society of Organic Agriculture Research ISOFAR, Modena, Italy, 18–20 June 2008; ISOFAR: Modena, Italy, 2008. [Google Scholar]
- Sabir, A.; El-Khalfi, B.; Errachidi, F.; Chemsi, I.; Serrano, A.; Soukri, A. Evaluation of the Potential of Some Essential Oils in Biological Control against Phytopathogenic Agent Pseudomonas syringae pv. Tomato DC3000 Responsible for the Tomatoes Speck. J. Plant Pathol. Microbiol. 2017, 8, 420. [Google Scholar] [CrossRef]
- Wilson, M.; Campbell, H.L.; Ji, P.; Jones, J.B.; Cuppels, D.A. Biological control of bacterial speck of tomato under field conditions at several locations in North America. Phytopathology 2002, 92, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Campbell, H.L.; Kloepper, J.W.; Jones, J.B.; Suslow, T.V.; Wilson, M. Integrated biological control of bacterial speck and spot of tomato under Weld conditions using foliar biological control agents and plant growth-promoting rhizobacteria. Biol. Control 2006, 36, 358–367. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Senthilkumar, P.; Choi, K.M.; Lee, J.H.; Oh, B.T. Characterization and assessment of two biocontrol bacteria against Pseudomonas syringae wilt in Solanum lycopersicum and its genetic responses. Microbiol. Res. 2018, 206, 43–49. [Google Scholar] [CrossRef]
- Çemen, A. Possibilities of Using Bacteriophages for Biological Control of Tomato Bacterial Speck Disease (Pseudomonas syringae pv. Tomato); Ministry of Agriculture and Forestry, Department of Training and Publication, National AGRIS Center: Ankara, Turkey, 2017. [Google Scholar]
- Firrao, G.; Andersen, M.; Bertaccini, A.; Boudon, E.; Bové, J.M.; Daire, X.; Davis, R.E.; Fletcher, J.; Garnier, M.; Gibb, K.S.; et al. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. Int. J. Syst. Evol. Microbiol. 2004, 54, 1243–1255. [Google Scholar]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. “Candidatus Phytoplasma solani”, a novel taxon associated with stolbur and bois noir related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef]
- Buoso, S.; Pagliari, L.; Musetti, R.; Martini, M.; Marroni, F.; Schmidt, W.; Santi, S. ‘Candidatus Phytoplasma solani’ interferes with the distribution and uptake of iron in tomato. BMC Genom. 2019, 20, 703. [Google Scholar] [CrossRef]
- Favali, M.A.; Musetti, R.; Fossati, F.; Vighi, C. Association of stolbur phytoplasmas with diseased tomatoes in Italy. Bull. OEPP 2000, 30, 347–350. [Google Scholar] [CrossRef]
- Del Serrone, P.; Marzachì, C.; Bragaloni, M.; Galeffi, P. Phytoplasma infection of tomato in central Italy. Phytopathol. Mediterr. 2001, 40, 137–142. [Google Scholar] [CrossRef]
- Pracros, P.; Renaudin, J.; Eveillard, S.; Mouras, A.; Hernould, M. Tomato flower abnormalities induced by stolbur phytoplasma infection are associated with changes of expression of floral development genes. Mol. Plant Microbe Interact. 2006, 19, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Fos, A.; Danet, J.; Zreik, L.; Garnier, M.; Bove, J.M. Use of a monoclonal antibody to detect the Stolbur mycoplasmalike organism in plants and insects and to identify a vector in France. Plant Dis. 1992, 76, 1092–1096. [Google Scholar] [CrossRef]
- Maixner, M. Transmission of German grapevine yellows (Vergilbungskrankheit) by the planthopper Hyalesthes obsoletus (Auchenorrhyncha: Cixiidae). Vitis 1994, 33, 103–104. [Google Scholar] [CrossRef]
- Langer, M.; Maixner, M. Molecular characterisation of grapevine yellows associated phytoplasmas of the stolbur-group based on RFLP-analysis of non-ribosomal DNA. Vitis 2004, 43, 191–199. [Google Scholar] [CrossRef]
- Clair, D.; Larrue, J.; Aubert, G.; Gillet, J.; Cloquemin, G.; Boudon-Padieu, E. A multiplex nested-PCR assay for sensitive and simultaneous detection and direct identification of phytoplasma in the elm yellows group and stolbur group and its use in survey of grapevine yellows in France. Vitis 2003, 42, 151–157. [Google Scholar] [CrossRef]
- Kogovšek, P.; Mehle, N.; Pugelj, A.; Jakomin, T.; Schroers, H.-J.; Ravnikar, M.; Dermastia, M. Rapid loop-mediated isothermal amplification assays for grapevine yellows phytoplasmas on crude leaf-vein homogenate has the same performance as qPCR. Eur. J. Plant Pathol. 2017, 148, 75–84. [Google Scholar] [CrossRef]
- Bertaccini, A.; Duduk, B. Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol. Mediterr. 2009, 48, 355–378. [Google Scholar] [CrossRef]
- Zamorzaeva, I.; Bahsiev, A.; Mihnea, N. Spread of stolbur in some tomato varieties and indicators of their productivity. Sci. Pap. Ser. B Hortic. 2020, 64, 273–280. [Google Scholar]
- Feng, M.; Cheng, R.; Chen, M.; Guo, R.; Li, L.; Feng, Z.; Wu, J.; Xie, L.; Hong, J.; Zhang, Z.; et al. Rescue of tomato spotted wilt virus entirely from complementary DNA clones. Proc. Natl. Acad. Sci. USA 2020, 117, 1181–1190. [Google Scholar] [CrossRef]
- Roggero, P.; Pennazio, S. Thermal inactivation of tomato spotted wilt tospovirus in vivo. Physiol. Mol. Plant Pathol. 1997, 51, 35–40. [Google Scholar] [CrossRef]
- Feng, M.; Cheng, R.; Chen, M.; Guo, R.; Li, L.; Feng, Z.; Wu, J.; Xie, L.; Hong, J.; Zhang, Z.; et al. Rescue of Tomato spotted wilt tospovirus entirely from cDNA clones, establishment of the first reverse genetics system for a segmented (-) RNA plant virus. bioRxiv 2019, 680900. [Google Scholar]
- Pappu, H.R.; Whitfield, A.E.; de Oliveira, A.S. Tomato Spotted Wilt Virus (Tospoviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D., Zuckerman, M., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 761–767. [Google Scholar]
- EPPO Global Database. 2021. Available online: https://gd.eppo.int (accessed on 24 March 2021).
- Caciagli, P. Vegetable Viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; pp. 282–290. [Google Scholar]
- Aramburu, J.; Marti, M. The occurrence in north-east Spain of a variant of Tomato spotted wilt virus (TSWV) that breaks resistance in tomato (Lycopersicon esculentum) containing the Sw-5 gene. Plant Pathol. 2003, 52, 407. [Google Scholar] [CrossRef]
- Nagata, T.; Almeida, A.C.L.; Resende, R.O.; de Ávila, A.C. The competence of four thrips species to transmit and replicate four tospoviruses. Plant Pathol. 2004, 53, 136–140. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Rubio, L.; Rangel, E.; Davino, M.; García-Hernández, J.; Olmos, A. Simultaneous detection of the seven main tomato-infecting RNA viruses by two multiplex reverse transcription polymerase chain reactions. J. Virol. Methods 2012, 186, 152–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, X.; Chen, C.; Xiao, X.; Deng, M.J. Development of Reverse Transcription Thermostable Helicase-Dependent DNA Amplification for the Detection of Tomato Spotted Wilt Virus. J. AOAC Int. 2016, 99, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Davino, S.; Panno, S.; Rangel, E.A.; Davino, M.; Bellardi, M.G.; Rubio, L. Population genetics of cucumber mosaic virus infecting medicinal, aromatic and ornamental plants from northern Italy. Arch. Virol. 2012, 157, 739–745. [Google Scholar] [CrossRef]
- Diaz-Ruiz, J.R.; Kaper, J.M. Cucumber mosaic virus-associated RNA 5: III. Little nucleotide sequence homology between CARNA 5 and helper RNA. Virology 1977, 80, 204–213. [Google Scholar] [CrossRef]
- Palukaitis, P.; Zaitlin, M. Replicase-mediated resistance to plant virus disease. Adv. Virus Res. 1997, 48, 349–377. [Google Scholar] [CrossRef]
- Roossinck, M.J. Cucumber mosaic virus, a model for RNA virus evolution. Mol. Plant Pathol. 2001, 2, 59–63. [Google Scholar] [CrossRef]
- Lecoq, H.; Desbiez, C. Viruses of cucurbit crops in the Mediterranean region: An ever-changing picture. Adv. Virus Res. 2012, 84, 67–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kobayashi, M. Seed transmission of Cucumber mosaic virus in pepper. J. Virol. Methods 2010, 163, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Cillo, F.; Barbarossa, L.; Grieco, F.; Gallitelli, D. Lethal necrosis, fruit necrosis and top stunting: Molecular-biological aspects of three cucumber mosaic virus-induced diseases of processing tomatoes in Italy. Acta Hortic. 1994, 376, 369–376. [Google Scholar] [CrossRef]
- Mascia, T.; Cillo, F.; Fanelli, V.; Finetti-Sialer, M.M.; De Stradis, A.; Palukaitis, P.; Gallitelli, D. Characterization of the interactions between Cucumber mosaic virus and Potato virus Y in mixed infections in tomato. Mol. Plant Microbe Interact. 2010, 23, 1514–1524. [Google Scholar] [CrossRef]
- Fan, X.; Du, Y.; Cai, Y.; Zhang, Y.; Zhao, X.; Liang, J.; Yang, D.; Zhang, Q.; Zhang, X.; Zhang, W.; et al. Rapid and sensitive detection of cucumber mosaic virus by reverse transcription loop-mediated isothermal amplification. Acta Biochim. Biophys. Sin. 2019, 51, 223–226. [Google Scholar] [CrossRef]
- Sikora, E.J.; Murphy, J.F. Identification and management of Cucumber mosaic virus in Alabama. Acta Hortic. 2005, 695, 191–194. [Google Scholar] [CrossRef]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-elicited defense responses in Cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef]
- Davino, S.; Napoli, C.; Davino, M.; Accotto, G.P. Spread of Tomato yellow leaf curl virus in Sicily: Partial displacement of another geminivirus originally present. Eur. J. Plant Pathol. 2006, 114, 293–299. [Google Scholar] [CrossRef]
- Garcia-Andres, S.; Accotto, G.P.; Navas-Castillo, J.; Moriones, E. Founder effect, plant host, and recombination shape the emergent population of begomoviruses that cause the tomato yellow leaf curl disease in the Mediterranean basin. Virology 2007, 359, 302–312. [Google Scholar] [CrossRef]
- Davino, S.; Miozzi, L.; Panno, S.; Rubio, L.; Davino, M.; Accotto, G.P. Recombination profiles between Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus in laboratory and field conditions: Evolutionary and taxonomic implications. J. Gen. Virol. 2012, 93, 2712–2717. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Caruso, A.G.; Davino, S. The nucleotide sequence of a recombinant tomato yellow leaf curl virus strain frequently detected in Sicily isolated from tomato plants carrying the Ty-1 resistance gene. Arch. Virol. 2018, 163, 795–797. [Google Scholar] [CrossRef]
- Hasan, A.A.; Mouhanna, A.M. Detection of Tomato Yellow Leaf Curl Virus TYLCV in some vegetable crops in greenhouses and identify its strains in the Syrian Coast. Int. J. Chemtech Res. 2016, 9, 278–286. [Google Scholar]
- Glick, E.; Levy, Y.; Gafni, Y. The Viral Etiology of Tomato Yellow Leaf Curl Disease—A Review. Plant Prot. Sci. 2009, 45, 81–97. [Google Scholar] [CrossRef]
- Polston, E.G.; Anderson, P.K. The emergence of whitefly-transmitted geminiviruses in tomato in western Hemisphere. Plant Dis. 1997, 81, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Harpaz, I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol. Exp. Appl. 1964, 7, 155–160. [Google Scholar] [CrossRef]
- Cohen, S.; Nitzany, F.E. Transmission and host range of the tomato yellow leaf curl virus. Phtyopathology 1966, 56, 1127–1131. [Google Scholar]
- Sánchez-Campos, S.; Rodríguez-Negrete, E.A.; Cruzado, L.; Grande-Pérez, A.; Bejarano, E.R.; Navas-Castillo, J.; Moriones, E. Tomato yellow leaf curl virus: No evidence for replication in the insect vector Bemisia tabaci. Sci. Rep. 2016, 6, 30942. [Google Scholar] [CrossRef]
- Lee, W.; Park, J.; Lee, G.S.; Lee, S.; Akimoto, S. Taxonomic status of the Bemisia tabaci complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PLoS ONE 2013, 8, e63817. [Google Scholar] [CrossRef]
- Simón, B.; Cenis, J.L.; De La Rúa, P. Distribution patterns of the Q and B biotypes of Bemisia tabaci in the Mediterranean Basin based on microsatellite variation. Entomol. Exp. Appl. 2007, 124, 327–336. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Noris, E.; Miozzi, L. Real-Time PCR Protocols for the Quantification of the Begomovirus Tomato Yellow Leaf Curl Sardinia Virus in Tomato Plants and in Its Insect Vector. Methods Mol. Biol. 2015, 1236, 61–72. [Google Scholar] [CrossRef]
- Almasi, M.A.; Dehabadi, S.H.; Eftekhari, Z. Immunocapture Loop Mediated Isothermal Amplification for Rapid Detection of Tomato Yellow Leaf curl Virus (TYLCV) without DNA Extraction. J. Plant Pathol. Microb. 2013, 4, 185. [Google Scholar] [CrossRef]
- Ji, Y.; Scott, J.W.; Hanson, P.; Graham, E.; Maxwell, D.P. Sources of resistance, inheritance, and location of genetic loci conferring resistance to members of the tomato-infecting begomoviruses. In Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance; Czosnek, H., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 343–362. [Google Scholar] [CrossRef]
- Picó, B.; Díez, M.J.; Nuez, F. Viral disease causing the greatest economic losses to the tomato crop II. The Tomato yellow leaf curl virus: A review. Sci. Hortic. 1996, 67, 151–196. [Google Scholar] [CrossRef]
- Scott, J. Breeding for resistance to viral pathogens. Genet. Improv. Solanaceous Crops 2006, 2, 457–485. [Google Scholar]
- Gill, U.; Scott, J.W.; Shekasteband, R.; Ogundiwin, E.; Schuit, C.; Francis, D.M.; Sim, S.C.; Smith, H.; Hutton, S.F. Ty-6, a major begomovirus resistance gene on chromosome 10, is effective against Tomato yellow leaf curl virus and Tomato mottle virus. Theor. Appl. Genet. 2019, 132, 1543–1554. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A New Tobamovirus Infecting Tomato Crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-Y.; Ma, H.-Y.; Han, S.-L.; Geng, C.; Tian, Y.-P.; Li, X.-D. First report of Tomato brown rugose fruit virus infecting tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Davino, S. First Report of Tomato Brown Rugose Fruit Virus on Tomato Crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Alkowni, R.; Alabdallah, O.; Fadda, Z. Molecular identification of tomato brown rugose fruit virus in tomato in Palestine. J. Plant Pathol. 2019, 101, 719–723. [Google Scholar] [CrossRef]
- OEPP/EPPO. First Report of Tomato Brown Rugose Fruit Virus in Greece. EPPO Reporting Service. 2019. Available online: https://gd.eppo.int/reporting/article-6640 (accessed on 31 May 2021).
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.C.; Font, M.I. First report of Tomato brown rugose fruit virus in tomato in Spain. Plant Dis. 2020, PDIS-06. [Google Scholar] [CrossRef] [PubMed]
- OEPP/EPPO. First Report of Tomato Brown Rugose Fruit Virus in France. EPPO Reporting Service. 2020. Available online: https://gd.eppo.int/reporting/article-6668 (accessed on 31 May 2021).
- Salem, N.M.; Cao, M.J.; Odeh, S.; Turina, M.; Tahzima, R. First Report of Tobacco Mild Green Mosaic Virus and Tomato Brown Rugose Fruit Virus Infecting Capsicum annuum in Jordan. Plant Dis. 2020, 104, 601. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Blanco, G.; Davino, S. First report of Tomato brown rugose fruit virus infecting sweet pepper in Italy. New Dis. Rep. 2020, 41, 20. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of Tomato Brown Rugose Fruit Virus in Sicily and Evaluation of the Spatiotemporal Dispersion in Experimental Conditions. Agronomy 2020, 10, 834. [Google Scholar] [CrossRef]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato Brown Rugose Fruit Virus: Seed Transmission Rate and Efficacy of Different Seed Disinfection Treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of Tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 1. [Google Scholar] [CrossRef]
- Wilstermann, A.; Ziebell, H. Tomato brown rugose fruit virus (ToBRFV). JKI Data Sheets Plant Dis. Diagn. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef] [PubMed]
- Panno, S.; Ruiz-Ruiz, S.; Caruso, A.G.; Alfaro-Fernandez, A.; Font San Ambrosio, M.I.; Davino, S. Real-time reverse transcription polymerase chain reaction development for rapid detection of Tomato brown rugose fruit virus and comparison with other techniques. PeerJ. 2019, 7, e7928. [Google Scholar] [CrossRef]
- Sarkes, A.; Fu, H.; Feindel, D.; Harding, M.; Feng, J. Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of Tomato brown rugose fruit virus (ToBRFV). PLoS ONE 2020, 15, e0230403. [Google Scholar] [CrossRef]
- Zinger, A.; Lapidot, M.; Harel, A.; Doron-Faigenboim, A.; Gelbart, D.; Levin, I. Identification and Mapping of Tomato Genome Loci Controlling Tolerance and Resistance to Tomato Brown Rugose Fruit Virus. Plants 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Crescenzi, A. Tomato viruses in Italy: Evolution in the past few decades and present status. Acta Hortic. 2009, 808, 145–150. [Google Scholar] [CrossRef]
- Janssen, D.; García, C.; Ruiz, L.; de Cara-García, M.; Simon, A.; Martinez, A. Disease resistance in tomato crops produced in Spain. Acta Hortic. 2018, 1207, 63–68. [Google Scholar] [CrossRef]
- Rabie, M.; Ratti, C.; Calassanzio, M.; Aleem, E.A.; Fattouh, F.A. Phylogeny of Egyptian isolates of Cucumber mosaic virus (CMV) and Tomato mosaic virus (ToMV) infecting Solanum lycopersicum. Eur. J. Plant Pathol. 2017, 149, 219–225. [Google Scholar] [CrossRef]
- Moreira, S.R.; Eiras, M.; Chaves, A.L.; Galleti, S.R.; Colariccio, A. Caracterização de uma nova estirpe do Tomato mosaic virus isolada de tomateiro no Estado de São Paulo. Fitopatol. Bras. 2003, 28, 602–607. [Google Scholar] [CrossRef][Green Version]
- Broadbent, L. The epidemiology of tomato mosaic: XI. Seed-transmission of TMV. Ann. Appl. Biol. 1965, 56, 177–205. [Google Scholar] [CrossRef]
- Chitra, T.R.; Prakash, H.S.; Albrechtsen, S.E.; Shetty, H.S.; Mathur, S.B. Infection of tomato and bell pepper by ToMV and TMV at different growth stages and establishment of virus in seeds. J. Plant Pathol. 1999, 81, 123–126. [Google Scholar] [CrossRef]
- Jacobi, V.; Bachand, G.D.; Hamelin, R.C.; Castello, J.D. Development of a multiplex immunocapture RT-PCR assay for detection and differentiation of tomato and tobacco mosaic tobamoviruses. J. Virol. Methods 1998, 74, 167–178. [Google Scholar] [CrossRef]
- Hadas, R.; Pearlsman, M.; Gefen, T.; Lachman, O.; Hadar, E.; Sharabany, G.; Antignus, Y. Indexing System for Tomato Mosaic Virus (ToMV) in Commercial Tomato Seed Lots. Phytoparasitica 2004, 32, 421–424. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Smith, E. Seed transmission of Tobamoviruses: Aspects of global disease distribution. Adv. Seed Biol. Technol. 2017, 233–260. [Google Scholar] [CrossRef]
- Silva, P.P.; Freitas, R.A.; Nascimento, W.M. Detection of Tomato mosaic virus in tomato seed and treatment by thermotherapy. Acta Hortic. 2011, 917, 303–308. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of Tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef] [PubMed]
- Caciagli, P.; Boccardo, G.; Lovisolo, O. Parietaria mottle virus, a possible new ilarvirus from Parietaria officinalis (Urticaceae). Plant Pathol. 1989, 38, 577–584. [Google Scholar] [CrossRef]
- Roggero, P.; Ciuffo, M.; Katis, N.; Alioto, D.; Crescenzi, A.; Parrella, G.; Gallitelli, D. Necrotic disease in tomatoes in Greece and south Italy caused by tomato strain of Parietaria mottle virus. J. Plant Pathol. 2000, 82, 159. [Google Scholar]
- Galipienso, L.; Martínez, C.; Willemsen, A.; Alfaro-Férnandez, A.; Font-San Ambrosio, I.; Davino, S.; Rubio, L. Genetic variability and evolutionary analysis of parietaria mottle virus: Role of selection and genetic exchange. Arch. Virol. 2015, 160, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Galipienso, L.; Herranz, M.C.; Pallás, V.; Aramburu, J. Detection of a tomato strain of Parietaria mottle virus (PMoV-T) by molecular hybridization and RT-PCR in field samples from north-eastern Spain. Plant Pathol. 2005, 54, 29–35. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Bertacca, S.; Matić, S.; Davino, S.; Parrella, G. Detection of parietaria mottle virus by RT-qPCR: An emerging virus native of Mediterranean area that undermine tomato and pepper production in Southern Italy. Front. Plant Sci. 2021, 12, 698573. [Google Scholar] [CrossRef]
- Verdin, E.; Gognalons, P.; Cardin, L.; Moretti, A.; Parrella, G.; Cotillon, A.C.; Jacquemond, M.; Marchoux, G. Production d’un sérum polyclonal spécifique du Parietaria mottle ilarvirus (PMoV) et étude de sa transmission par le pollen. In Proceedings of the 10ème Rencontres de Virologie Végétale, Aussois, France, 6–10 March 2005. [Google Scholar]
- Aramburu, J.; Galipienso, L.; Aparicio, F.; Soler, S.; López, C. Mode of transmission of Parietaria mottle virus. J. Plant Pathol. 2010, 92, 679–684. [Google Scholar] [CrossRef]
- Davino, S.; Panno, S.; Iacono, G.; Sabatino, L.; D’Anna, F.; Iapichino, G.; Olmos, A.; Scuderi, G.; Rubio, L.; Tomassoli, L.; et al. Genetic variation and evolutionary analysis of Pepino mosaic virus in Sicily: Insights into the dispersion and epidemiology. Plant Pathol. 2017, 66, 368–375. [Google Scholar] [CrossRef]
- Spence, N.J.; Basham, J.; Mumford, R.A.; Hayman, G.; Edmondson, R.; Jones, D.R. Effect of Pepino mosaic virus on the yield and quality of glasshouse-grown tomatoes in the UK. Plant Pathol. 2006, 55, 595–606. [Google Scholar] [CrossRef]
- Bibi, I.; Djelouah, K.; Remah, A.; Afechtal, M. Pepino Mosaic Virus: A serious threat to tomato crops worldwide. Rev. Mar. Sci. Agron. Vét. 2017, 5, 231–237. [Google Scholar]
- Hanssen, I.; Paeleman, A.; Vandewoestijne, E.; Van Bergen, L.; Bragard, C.; Lievens, B.; Vanachter, A.; Thomma, B. Pepino mosaic virus isolates and differential symptomatology in tomato. Plant Pathol. 2009, 58, 450–460. [Google Scholar] [CrossRef]
- Salomone, A.; Roggero, P. Host range, seed transmission and detection by ELISA and lateral flow of an Italian isolate of Pepino mosaic virus. J. Plant Pathol. 2002, 84, 65–68. [Google Scholar] [CrossRef]
- Ling, K.-S.; Wechter, W.P.; Jordan, R. Development of a one-step immunocapture real-time TaqMan RTPCR assay for the broad spectrum detection of Pepino mosaic virus. J. Virol. Methods 2007, 144, 65–72. [Google Scholar] [CrossRef]
- Ling, K.-S.; Wintermantel, W.M.; Bledsoe, M. Genetic composition of Pepino mosaic virus population in North American greenhouse tomatoes. Plant Dis. 2008, 92, 1683–1688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gutiérrez-Aguirre, I.; Mehle, N.; Delić, D.; Gruden, K.; Mumford, R.; Ravnikar, M. Real-time quantitative PCR based sensitive detection and genotype discrimination of Pepino mosaic virus. J. Virol. Methods 2009, 162, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Warman, B. Development and Deployment of Genotype-Specific LAMP Assays for Monitoring Pepino Mosaic Virus (PepMV) in Tomato. Master’s Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Córdoba-Sellés, M.C.; García-Rández, A.; Alfaro-Fernández, A.; Jordá-Gutiérrez, C. Seed transmission of Pepino mosaic virus and efficacy of tomato seed disinfection treatments. Plant Dis. 2007, 91, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-S. Effectiveness of chemo- and thermotherapeutic treatments on Pepino mosaic virus in tomato seed. Plant Dis. 2010, 94, 325–328. [Google Scholar] [CrossRef][Green Version]
- Flores, R.; Randles, J.W.; Bar-Joseph, M.; Diener, T.O. A proposed scheme for viroid classification and nomenclature. Arch. Virol. 1998, 143, 623–629. [Google Scholar] [CrossRef]
- Owens, R.A.; Verhoeven, J.T.J. Potato spindle tuber. Plant Health Instr. 2009. [Google Scholar] [CrossRef]
- Ding, B. The biology of viroid-host interactions. Annu. Rev. Phytopathol. 2009, 47, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Grasmick, M.E.; Slack, S.A. Symptom expression enhanced and low concentrations of Potato spindle tuber viroid amplified in tomato with high light intensity and temperature. Plant Dis. 1985, 69, 49–51. [Google Scholar] [CrossRef]
- Mackie, A.E.; Rodoni, B.C.; Barbetti, M.J.; McKirdy, S.J.; Jones, R.A.C. Potato spindle tuber viroid: Alternative host reservoirs and strain found in a remote subtropical irrigation area. Eur. J. Plant Pathol. 2016, 145, 433–446. [Google Scholar] [CrossRef]
- Navarro, B.; Silletti, M.R.; Trisciuzzi, V.N.; Di Serio, F. Identification and characterization of Potato spindle tuber viroid infecting tomato in Italy. J. Plant Pathol. 2009, 91, 723–726. [Google Scholar] [CrossRef]
- EFSA PLH Panel. Scientific Opinion on the assessment of the risk of solanaceous pospiviroids for the EU territory and the identification and evaluation of risk management options. EFSA J. 2011, 9, 2330. [Google Scholar] [CrossRef]
- Singh, R.P. Seed transmission of potato spindle tuber virus in tomato and potato. Am. Potato J. 1970, 47, 225–227. [Google Scholar] [CrossRef]
- Kryczynski, S.; Paduch-Cichal, E.; Skrzeczkowski, L.J. Transmission of three viroids through seed and pollen of tomato plants. J. Phytopathol. 1988, 121, 51–57. [Google Scholar] [CrossRef]
- Salazar, L.F.; Querci, M.; Bartolini, I.; Lazarte, V. Aphid transmission of potato spindle tuber viroid assisted by potato leafroll virus. Fitopatología 1995, 30, 56–58. [Google Scholar]
- De Hoop, M.B.; Verhoeven, J.T.J.; Roenhorst, J.W. Phytosanitary measures in the European Union: A call for more dynamic risk management allowing more focus on real pest risks. Case study: Potato spindle tuber viroid (PSTVd) on ornamental Solanaceae in Europe. Bull. OEPP 2008, 38, 510–515. [Google Scholar] [CrossRef]
- Lenarčič, R.; Morisset, D.; Mehle, N.; Ravnikar, M. Fast real-time detection of Potato spindle tuber viroid by RT-LAMP. Plant Pathol. 2013, 62, 1147–1156. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Wang, H.; Fan, H.; Yao, H. Effects of Elevated CO2 on Tomato (Lycopersicon esculentum Mill.) Growth and Rhizosphere Soil Microbial Community Structure and Functionality. Agronomy 2020, 10, 1752. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Misra, A.K.; Yadav, S.B.; Mishra, S.K.; Tripathi, M.K. Impact of Meteorological Variables and Climate Change on Plant Diseases. In Plant Pathogens. Detection and Management for Sustainable Agriculture; Kumar, P., Tiwari, A.K., Kamle, M., Abbas, Z., Singh, P., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 313–327. [Google Scholar]
- Jones, R.A.C. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, M.; Nombela, G. Differential variation in development of the B- and Q-biotypes of Bemisia tabaci (homoptera: Aleyrodidae) on sweet pepper at constant temperatures. Environ. Entomol. 2001, 30, 720–726. [Google Scholar] [CrossRef]
- Anfoka, G.; Moshe, A.; Fridman, L.; Amrani, L.; Rotem, O.; Kolot, M.; Zeidan, M.; Czosnek, H.; Gorovits, R. Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Canto, T.; Aranda, M.A.; Freres, A. Climate change effects on physiology and population processes of hosts and vectors that influence the spread of hemipteran-borne plant viruses. Glob. Chang. Biol. 2009, 15, 1884–1894. [Google Scholar] [CrossRef]
- Hunjan, M.S.; Lore, J.S. Climate change: Impact on plant pathogens, diseases, and their management. In Crop Protection under Changing Climate; Jabran, K., Florentine, S., Chauhan, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 85–100. [Google Scholar]
- Uddin, M.N.; Bokelmann, W.; Entsminger, J.S. Factors affecting farmers’ adaptation strategies to environmental degradation and climate change effects: A farm level study in Bangladesh. Climate 2014, 2, 223–241. [Google Scholar] [CrossRef]
- Burdon, J.J.; Zhan, J. Climate change and disease in plant communities. PLoS Biol. 2020, 18, e3000949. [Google Scholar] [CrossRef]
- Gullino, M.L.; Pugliese, M.; Gilardi, G.; Garibaldi, A. Effect of increased CO2 and temperature on plant diseases: A critical appraisal of results obtained in studies carried out under controlled environmental facilities. J. Plant Pathol. 2018, 100, 371–389. [Google Scholar] [CrossRef]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant-pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Matić, S.; Garibaldi, A.; Gullino, M.L. Combined and single effects of elevated CO2 and temperatures on rice bakanae disease under controlled conditions in phytotrons. Plant Pathol. 2021, 70, 815–826. [Google Scholar] [CrossRef]
- Garbelotto, M.; Pautasso, M. Impacts of exotic forest pathogens on Mediterranean ecosystems: Four case studies. Eur. J. Plant Pathol. 2012, 133, 101–116. [Google Scholar] [CrossRef]
- Selvaraj, S.; Ganeshamoorthi, P.; Pandiaraj, T. Potential impacts of recent climate change on biological control agents in agro-ecosystem: A review. Int. J. Biodivers. Conserv. 2013, 5, 845–852. [Google Scholar]
- Litskas, V.D.; Migeon, A.; Navajas, M.; Tixier, M.-S.; Stavrinides, M.C. Impacts of climate change on tomato, a notorious pest and its natural enemy: Small scale agriculture at higher risk. Environ. Res. Lett. 2019, 14, 084041. [Google Scholar] [CrossRef]
- De Gelder, A.; Dieleman, J.A.; Bot, G.P.A.; Marcelis, L.F.M. An overview of climate and crop yield in closed greenhouses. J. Hortic. Sci. Biotechnol. 2012, 87, 193–202. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Parra, M.; Intrigliolo, D.S. Open field hydroponics in fruit crops: Developments and challenges. In Fruit Crops. Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 419–430. [Google Scholar]
- Quesada, N.; Iannetta, P.P.M.; White, P.J.; Tran, F.; Begg, G.S. What evidence exists on the effectiveness of the techniques and management approaches used to improve the productivity of field grown tomatoes under conditions of water-, nitrogen- and/or phosphorus-deficit? A systematic map protocol. Environ. Evid. 2019, 8, 26. [Google Scholar] [CrossRef]
- Burton, L.; Jayachandran, K.; Bhansali, S. Review—The “Real-Time” Revolution for In situ Soil Nutrient Sensing. J. Electrochem. Soc. 2020, 167, 037569. [Google Scholar] [CrossRef]
- Koukounaras, A. Advanced Greenhouse Horticulture: New Technologies and Cultivation Practices. Horticulturae 2021, 7, 1. [Google Scholar] [CrossRef]
- Balashova, I.; Sirota, S.; Pinchuk, Y. Vertical vegetable growing: Creating tomato varieties for multi-tiered hydroponic installations. IOP Conf. Ser. Earth Environ. Sci. 2019, 395, 012079. [Google Scholar] [CrossRef]
- Costes, E.; Khadari, B.; Zaher, H.; Moukhli, A.; Morillon, R.; Legave, J.-M.; Regnard, J.-L. Adaptation of Mediterranean fruit tree cultivation to climate change. In The Mediterranean Region under Climate Change; Moatti, J.-P., Thiébault, S., Eds.; IRD Éditions: Marseille, France, 2016; pp. 503–510. [Google Scholar]
- Ntinas, G.K.; Kadoglidou, K.; Tsivelika, N.; Krommydas, K.; Kalivas, A.; Ralli, P.; Irakli, M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae 2019, 5, 42. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Influence of climate change on protected cultivation: Impacts and sustainable adaptation strategies—A review. J. Clean. Prod. 2019, 225, 481–495. [Google Scholar] [CrossRef]
- Ikeda, T.; Ishigami, Y.; Goto, E. The effect of CO2 enrichment in a closed greenhouse equipped with NIR-reflecting film and EHP cooling on the yield and quality of tomato fruits during the summer season. J. Agric. Meteorol. 2020, 76, 104–110. [Google Scholar] [CrossRef]
- Islam, S.M.; Matsui, T.; Yoshida, Y. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. Sci. Hortic. 1996, 65, 137–149. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Zhang, M.; Zhang, Y.; Wang, Q. Effect of carbon dioxide enrichment on health-promoting compounds and organoleptic properties of tomato fruits grown in greenhouse. Food Chem. 2014, 153, 157–163. [Google Scholar] [CrossRef]
- Stein, E.W. The Transformative Environmental Effects Large-Scale Indoor Farming May Have On Air, Water, and Soil. Air Soil Water Res. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Porter, J.R.; Challinor, A.J.; Henriksen, C.B.; Howden, S.M.; Martre, P.; Smith, P. Invited review: Intergovernmental Panel on Climate Change, agriculture, and food—A case of shifting cultivation and history. Glob. Chang. Biol. 2019, 25, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Hikosaka, S.; Kobayashi, M.; Nishizawa, T.; Saito, K.; Goto, E.; Kusano, M. A Systems Analysis With “Simplified Source-Sink Model” Reveals Metabolic Reprogramming in a Pair of Source-to-Sink Organs During Early Fruit Development in Tomato by LED Light Treatments. Front. Plant Sci. 2018, 9, 1439. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Lemgo, G.N.; Sabbadini, S.; Pandolfini, T.; Mezzetti, B. Biosafety considerations of RNAi-mediated virus resistance in fruit-tree cultivars and in rootstock. Transgenic Res. 2013, 22, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Sabbadini, S.; Miozzi, L.; Mezzetti, B.; Noris, E. Host-Induced Gene Silencing and Spray-Induced Gene Silencing for Crop Protection Against Viruses. In RNAi for Plant Improvement and Protection; Mezzetti, B., Sweet, J., Burgos, L., Eds.; CABI: Wallingford, UK, 2021. [Google Scholar]
- Tenllado, F.; Díaz-Ruíz, J.R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Green, J.C.; Hu, J.S. Editing plants for virus resistance using CRISPR-Cas. Acta Virol. 2017, 61, 138–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borrelli, V.; Brambilla, V.; Rogowsky, P.; Marocco, A.; Lanubile, A. The Enhancement of Plant Disease Resistance Using CRISPR/Cas9 Technology. Front. Plant Sci. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Makarova, S.S.; Khromov, A.V.; Spechenkova, N.A.; Taliansky, M.E.; Kalinina, N.O. Application of the CRISPR/Cas System for Generation of Pathogen-Resistant Plants. Biochemistry 2018, 83, 1552–1562. [Google Scholar] [CrossRef]
- Wolter, F.; Puchta, H. The CRISPR/Cas revolution reaches the RNA world: Cas13, a new Swiss Army knife for plant biologists. Plant J. 2018, 94, 767–775. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Q.; Yi, X.; An, H.; Zhao, Y.; Ma, S.; Zhou, G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018, 16, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Qiu, J.L. Genome editing for plant disease resistance: Applications and perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2020, 29, 207–221. [Google Scholar] [CrossRef]
- Schenke, D.; Cai, D. Applications of CRISPR/Cas to Improve Crop Disease Resistance: Beyond Inactivation of Susceptibility Factors. iScience 2020, 23, 101478. [Google Scholar] [CrossRef]
- Tyagi, S.; Kumar, R.; Kumar, V.; Won, S.Y.; Shukla, P. Engineering disease resistant plants through CRISPR-Cas9 technology. GM Crops Food 2021, 12, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cobine, P.A.; Coleman, J.J. Efficient genome editing in Fusarium oxysporum based on CRISPR/ Cas9 ribonucleoprotein complexes. Fungal Genet. Biol. 2018, 117, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Prihatna, C.; Barbetti, M.J.; Barker, S.J. A novel tomato fusarium wilt tolerance gene. Front. Microbiol. 2018, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion OPEN. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Zhao, R.; Yu, W.; Li, R.; Li, Y.; Sheng, J.; Shen, L. Knockout of SlMAPK3 reduced disease resistance to botrytis cinerea in tomato plants. J. Agric. Food Chem. 2018, 66, 8456–8949. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef]
- Ali, Z.; Ali, S.; Tashkandi, M.; Zaidi, S.S.; Mahfouz, M.M. CRISPR/Cas9-Mediated Immunity to Geminiviruses: Differential Interference and Evasion. Sci. Rep. 2016, 6, 26912. [Google Scholar] [CrossRef]
- Tashkandi, M.; Ali, Z.; Aljedaani, F.; Shami, A.; Mahfouz, M.M. Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant Signal. Behav. 2018, 13, e1525996. [Google Scholar] [CrossRef]
- Thomazella, D.P.D.T.; Brail, Q.; Dahlbeck, D.; Staskawicz, B. CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. BioRxiv 2016, 064824. [Google Scholar] [CrossRef]
- Campos, M.D.; do Rosário Félix, M.; Patanita, M.; Materatski, P.; Varanda, C. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic. Res. 2021, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Alon, D.M.; Hak, H.; Bornstein, M.; Pines, G.; Spiegelman, Z. Differential Detection of the Tobamoviruses Tomato Mosaic Virus (ToMV) and Tomato Brown Rugose Fruit Virus (ToBRFV) Using CRISPR-Cas12a. Plants 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Mahas, A.; Hassan, N.; Aman, R.; Marsic, T.; Wang, Q.; Ali, Z.; Mahfouz, M.M. LAMP-Coupled CRISPR-Cas12a Module for Rapid and Sensitive Detection of Plant DNA Viruses. Viruses 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Karthika, S.; Varghese, S.; Jisha, M.S. Exploring the efficacy of antagonistic rhizobacteria as native biocontrol agents against tomato plant diseases. Biotech 2020, 10, 320. [Google Scholar] [CrossRef]
- Choi, K.; Choi, J.; Lee, P.A.; Roy, N.; Khan, R.; Lee, H.J.; Weon, H.Y.; Kong, H.G.; Lee, S.W. Alteration of Bacterial Wilt Resistance in Tomato Plant by Microbiota Transplant. Front. Plant Sci. 2020, 11, 1186. [Google Scholar] [CrossRef]
- Vannier, N.; Agler, M.; Hacquard, S. Microbiota-mediated disease resistance in plants. PLoS Pathog. 2019, 15, e1007740. [Google Scholar] [CrossRef]










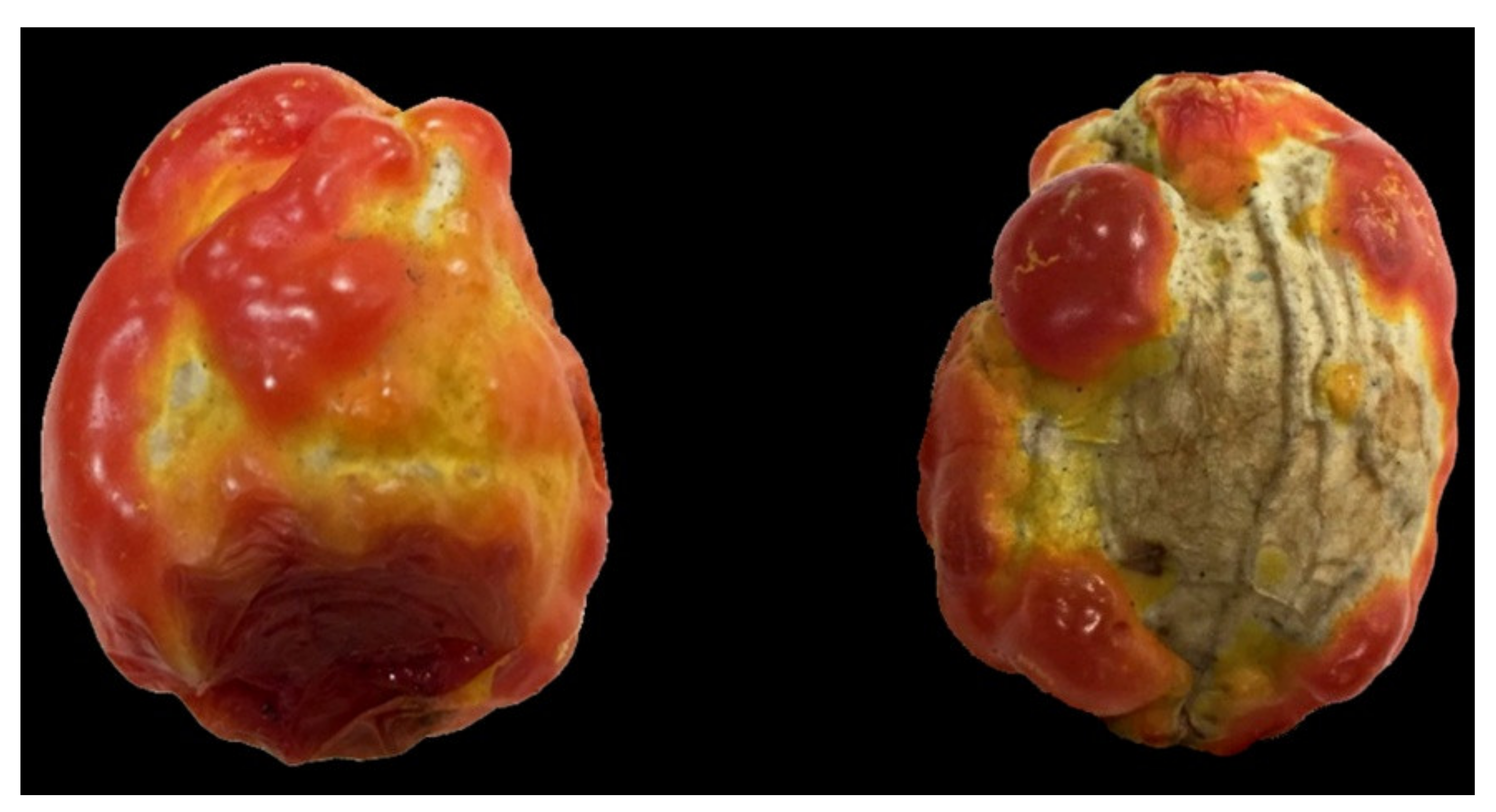






| Pathogen Group | Pathogen Name | Reference |
|---|---|---|
| Fungi | Alternaria solani, Botrytis cinerea, Cladosporium fulvum, Colletotrichum coccodes, Fusarium oxysporum, Fusarium clavum, Leveillula taurica, Oidium lycopersici, Pseudoidium neolycopersici, Pyrenochaeta lycopersici, Rhizoctonia solani, Septoria lycopersici, Sclerotinia sclerotiorum, Sclerotium rolfsii, Stemphylium spp., Verticillium dahliae | [2,3] |
| Oomycetes | Phytophthora infestans, Phytophthora nicotianae, Phytophtora cryptogea, Pythium debaryanum, Pythium sylvaticum | [4] |
| Bacteria | Clavibacter michiganensis subsp. michiganensis, Erwinia carotovora subsp. carotovora, Pseudomonas corrugata, Pseudomonas mediterranea, Pseudomonas syringae pv. tomato, Ralstonia solanacearum, Xanthomonas axonopodis pv. vesicatoria | [4] |
| Phytoplasma | Candidatus Phytoplasma solani | [5] |
| Viruses | Alfalfa mosaic virus (AMV), Chickpea chlorotic dwarf virus (CpCDV), Cucumber mosaic virus (CMV), Eggplant mottled dwarf virus (EMDV), Parietaria mottle virus (PMoV), Pelargonium zonate spot virus (PZSV), Pepino mosaic virus (PepMV), Potato virus Y (PVY), Southern tomato virus (STV), Tobacco mosaic virus (TMV), Tomato brown rugose fruit virus (ToBRFV), Tomato chlorosis virus (ToCV), Tomato infectious chlorosis virus (TICV), Tomato leaf curl New Delhi virus (ToLCNDV), Tomato mosaic virus (ToMV), Tomato spotted wilt virus (TSWV), Tomato torrado virus (ToTV), Tomato yellow leaf curl virus (TYLCV), Tomato yellow leaf curl Sardinia virus (TYLCSV) | [2,6] |
| Viroids | Potato spindle tuber viroid (PSTVd), Tomato apical stunt viroid (TASVd) | [5] |
| Tomato Pathogen | Tomato Disease | Geographical Distribution * | Maximal Yield Loss (%) | Impact of Climate Change on Disease Severity on Tomato | ||
|---|---|---|---|---|---|---|
| Temperature and CO2 Concentration | Relative Humidity | |||||
| Fungi | Alternaria solani | Early blight of tomato | Portugal, Spain, France, Italy, Greece, Turkey, Cyprus, Malta, Israel, Lebanon, Egypt, Lybia, Morocco | 80 [12] | Decrease at low and high T; Optimal T at 25 °C [13] | Increase with free moisture or near-saturated RH [14] |
| Septoria lycopersici | Septoria leaf spot | Portugal, Spain, France, Italy, Greece, Turkey, Cyprus, Israel, Lebanon, Lybia, Morocco | 50 [15] | No germination of conidia at ≥30 °C [16,17] | Increase with high RH [16] | |
| Botrytis cinerea | Grey mould | Spain, Italy, Turkey, Egypt | 40 [18] | Increase at high T (25 °C) [19,20]; Decrease at high CO2 (800 ppm) [21] | Increase with nearly 90% RH [18] | |
| Fusarium oxysporum f. sp. lycopersici | Fusarium wilt of tomato | Spain, France, Italy, Albania, Turkey, Cyprus, Israel, Egypt, Lybia, Morocco | 70 [22,23,24,25] | Conidial germination decreases at low T (<20 °C) and high T (>35 °C) [26] | Increase with high RH [27] | |
| Fusarium oxysporum f. sp. radicis-lycopersici | Crown and root rot | Spain, Italy, Greece, Turkey, Israel, Egypt, Tunisia, Malta | 90 [28,29,30] | Increase at high T (27 °C) [31] | Decrease at high humidity in first stages of the disease [31] | |
| Verticillium dahliae | Verticillium wilt of tomato | Portugal, Spain, France, Italy, Greece, Turkey, Cyprus, Malta, Israel, Lebanon, Syria, Egypt, Algeria, Morocco | 50 [32,33] | Increase at medium to high T (21–30 °C) [34] | n.a. | |
| Bacteria | Clavibacter michiganensis subsp. michiganensis | Bacterial canker | Portugal, Spain, France, Italy, Greece, Turkey, Cyprus, Malta, Israel, Lebanon, Syria, Egypt, Tunisia, Morocco | 84 [35,36,37] | Decrease at low T (<18 °C) and high T (>28 °C) [38] | Increase with high RH (80%) [39] |
| Pseudomonas syringae pv. tomato | Bacterial speck | Portugal, Spain, France, Italy, Greece, Turkey, Israel, Lebanon, Tunisia, Morocco | 75 [40,41] | Decrease at high T (28 °C) [42]; Decrease at high CO2 (800 ppm) [43] | Increase with high RH [40] | |
| Phytoplasma | Candidatus Phytoplasma solani | Stolbur | Spain, France, Italy, Greece, Albania, Montenegro, Croatia, Turkey, Israel, Lebanon, Syria | 80 [5,44] | n.a. | Increase with high RH [45] |
| Viruses | Tomato spotted wilt virus | Spotted wilt disease of tomato | Portugal, Spain, France, Italy, Greece, Croatia, Albania, Montenegro, Cyprus, malta, Turkey, Israel, Lebanon, Egypt, Lybia, Algeria, Tunisia | 95 [46,47] | Increase at medium to high T [48] | n.a. |
| Cucumber mosaic virus | Tomato fern leaf | Portugal, Spain, France, Italy, Greece, Croatia, Albania, Montenegro, Cyprus, Malta, Turkey, Israel, Lebanon, Egypt, Algeria, Tunisia, Morocco | 100 [49] | Decrease at high T (32 °C) [50] | n.a. | |
| Tomato yellow leaf curl virus | Tomato yellow leaf curl disease | Portugal, Spain, France, Italy, Greece, Cyprus, Malta, Turkey, Turkey, Israel, Lebanon, Egypt, Algeria, Lybia, Tunisia, Morocco | 100 [51,52] | Decrease at elevated CO2 (750 ppm) [53] | n.a. | |
| Tomato yellow leaf curl Sardinia virus | Tomato yellow leaf curl disease | Spain, Italy, Greece, Tunisia, Morocco | 100 [51,52] | n.a. | n.a. | |
| Tomato brown rugose fruit virus | Tomato brown rugose fruit disease | Spain, Italy, Greece, Cyprus, Malta, Turkey, Israel, Palestine, Egypt | 100 [54] | n.a. | n.a. | |
| Tomato mosaic virus | Tomato mosaic disease | Spain, France, Italy, Turkey, Syria, Egypt, Algeria | 70 [55,56] | Increase at medium to high T (20–31 °C) [57] | Decrease with high RH (>70%) [57] | |
| Parietaria mottle virus | n.a. | Spain, France, Italy, Greece | 30 [58] | n.a. | n.a. | |
| Pepino mosaic virus | n.a. | Spain, France, Italy, Greece, Cyprus, Turkey, Israel, Syria, Egypt, Morocco | 80 [59,60] | Increase or decrease at high T (30 °C) depending on the virus genotype [61] | n.a. | |
| Viroids | Potato spindle tuber viroid | Bunchy top of tomato | Spain, Italy, Croatia, Montenegro, Greece, Malta, Turkey, Israel, Egypt | 90 [62] | Decrease at low T (15 °C); Increase at high T (31 °C) [63,64] | Increase with dry climatic conditions (RH 30–40%) [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin. Agronomy 2021, 11, 2188. https://doi.org/10.3390/agronomy11112188
Panno S, Davino S, Caruso AG, Bertacca S, Crnogorac A, Mandić A, Noris E, Matić S. A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin. Agronomy. 2021; 11(11):2188. https://doi.org/10.3390/agronomy11112188
Chicago/Turabian StylePanno, Stefano, Salvatore Davino, Andrea Giovanni Caruso, Sofia Bertacca, Ana Crnogorac, Ana Mandić, Emanuela Noris, and Slavica Matić. 2021. "A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin" Agronomy 11, no. 11: 2188. https://doi.org/10.3390/agronomy11112188
APA StylePanno, S., Davino, S., Caruso, A. G., Bertacca, S., Crnogorac, A., Mandić, A., Noris, E., & Matić, S. (2021). A Review of the Most Common and Economically Important Diseases That Undermine the Cultivation of Tomato Crop in the Mediterranean Basin. Agronomy, 11(11), 2188. https://doi.org/10.3390/agronomy11112188








