Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. The Extracts Preparation
2.3. Experimental Design and Treatments
2.4. Growth Performance
2.5. Leaf Pigments
2.6. Proximate Analysis of Quinoa Seeds
2.7. Total Saponins
2.8. Tannin Content
2.9. Total Phenolic and Flavonoids Content
2.10. Statistical Analysis
3. Results
3.1. Analysis of Variance
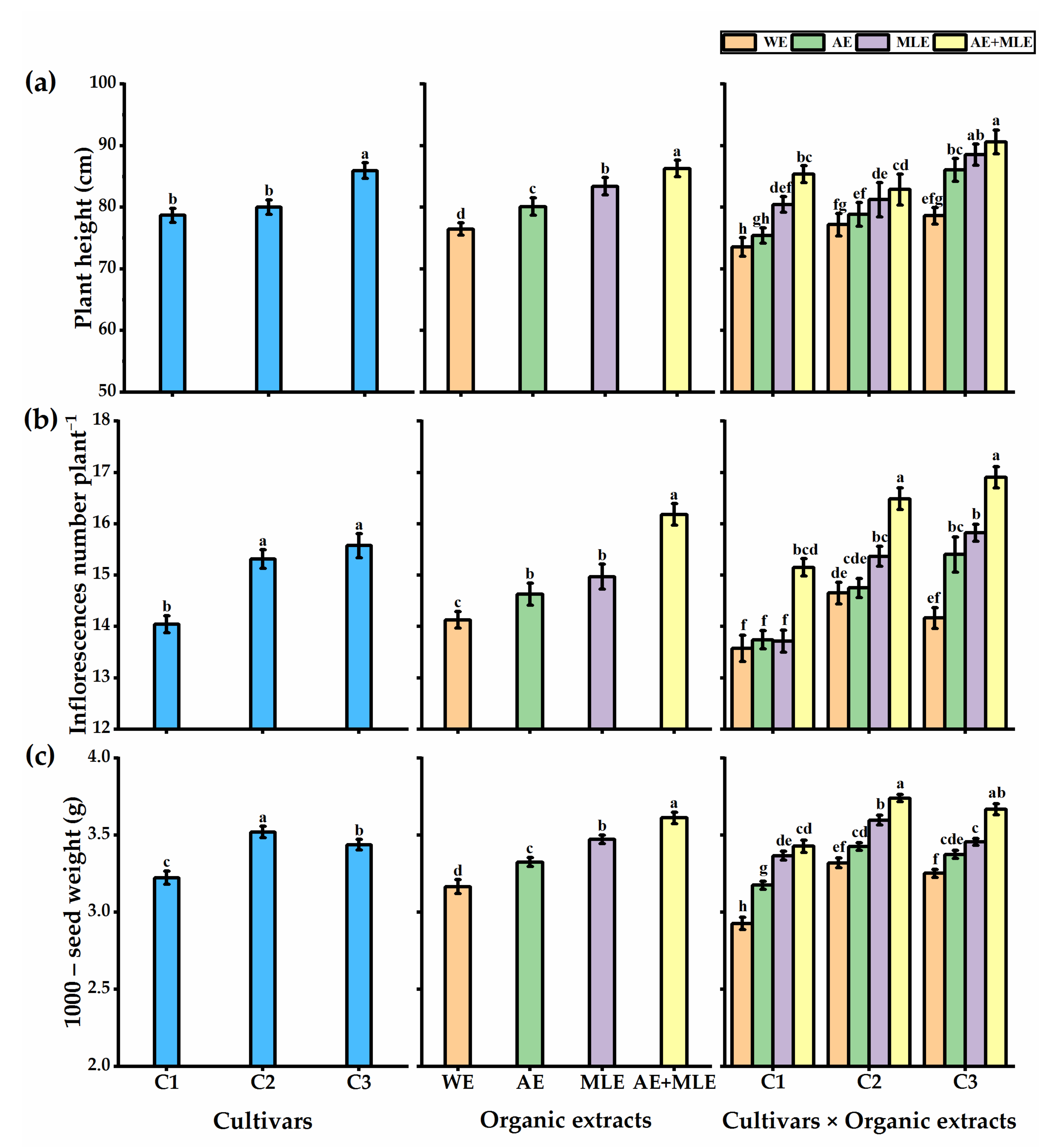
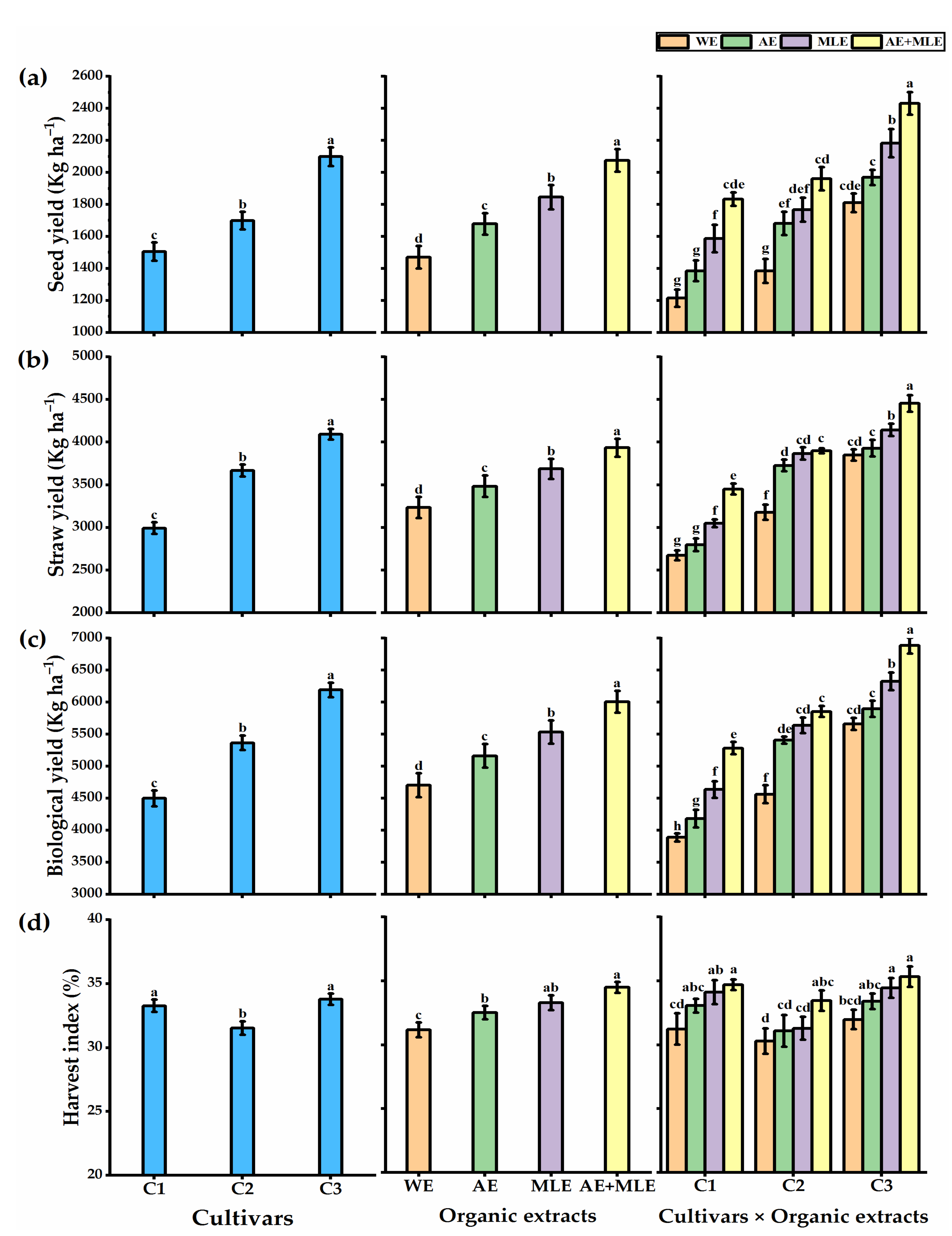
3.2. Growth Performance and Yields
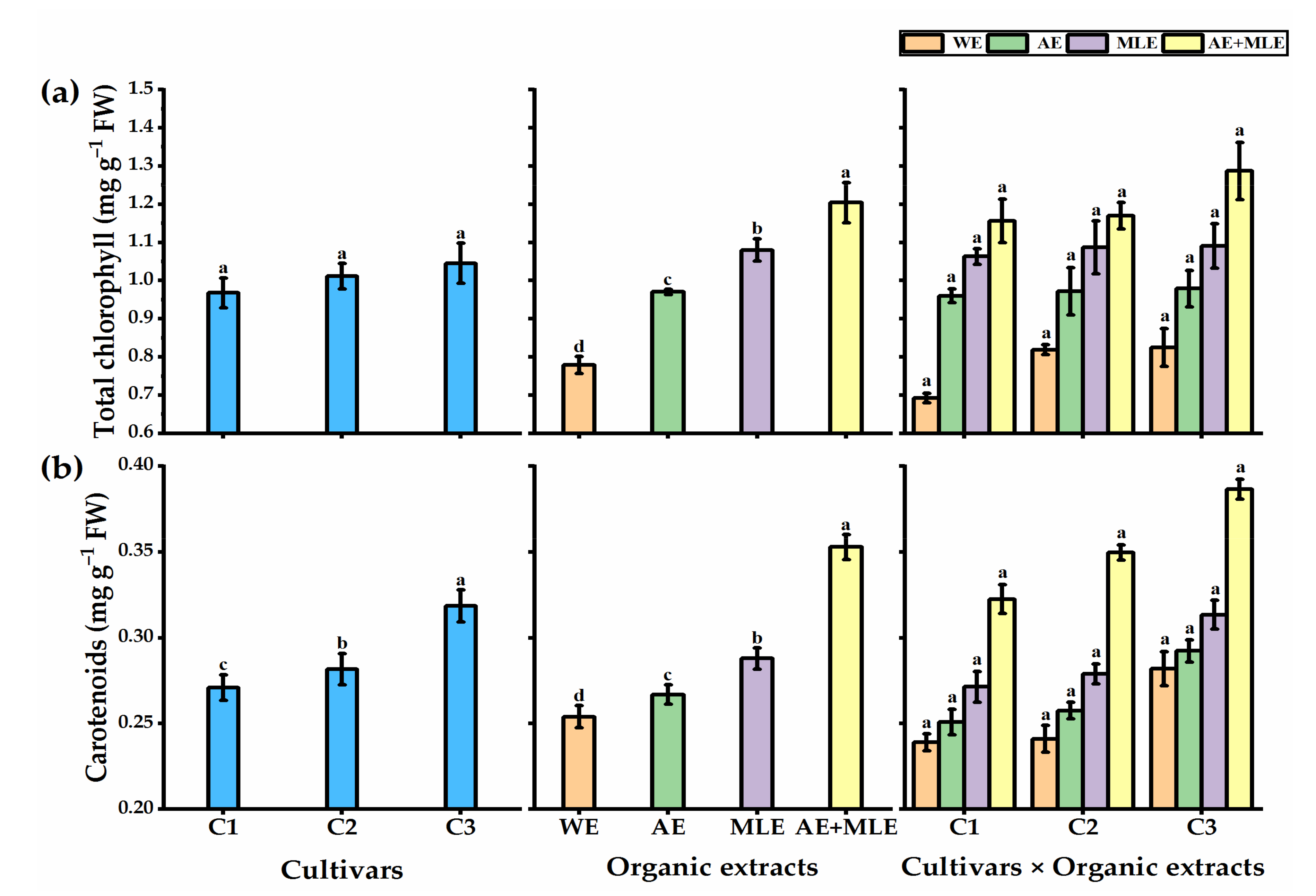
3.3. Leaf Pigments
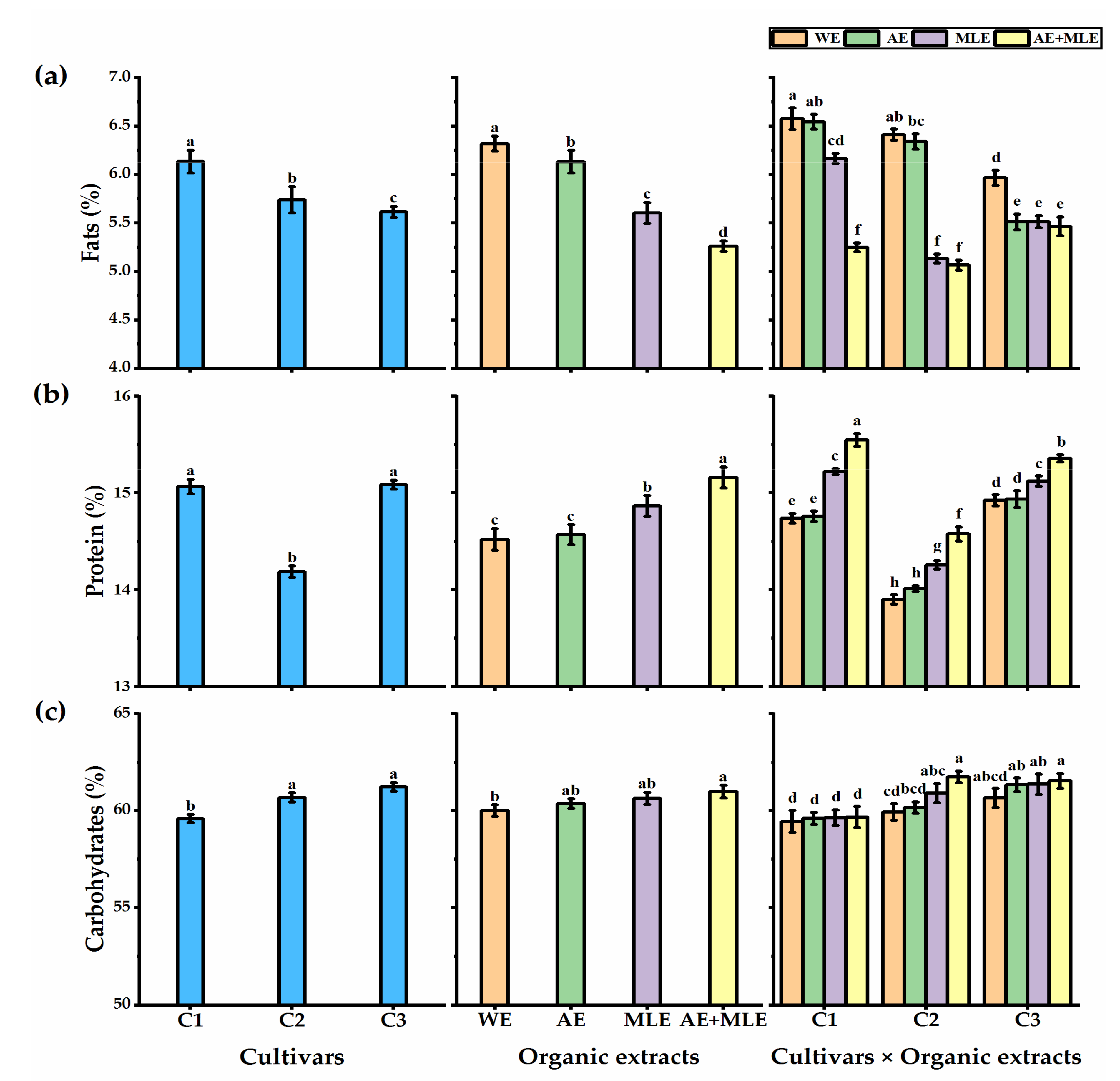
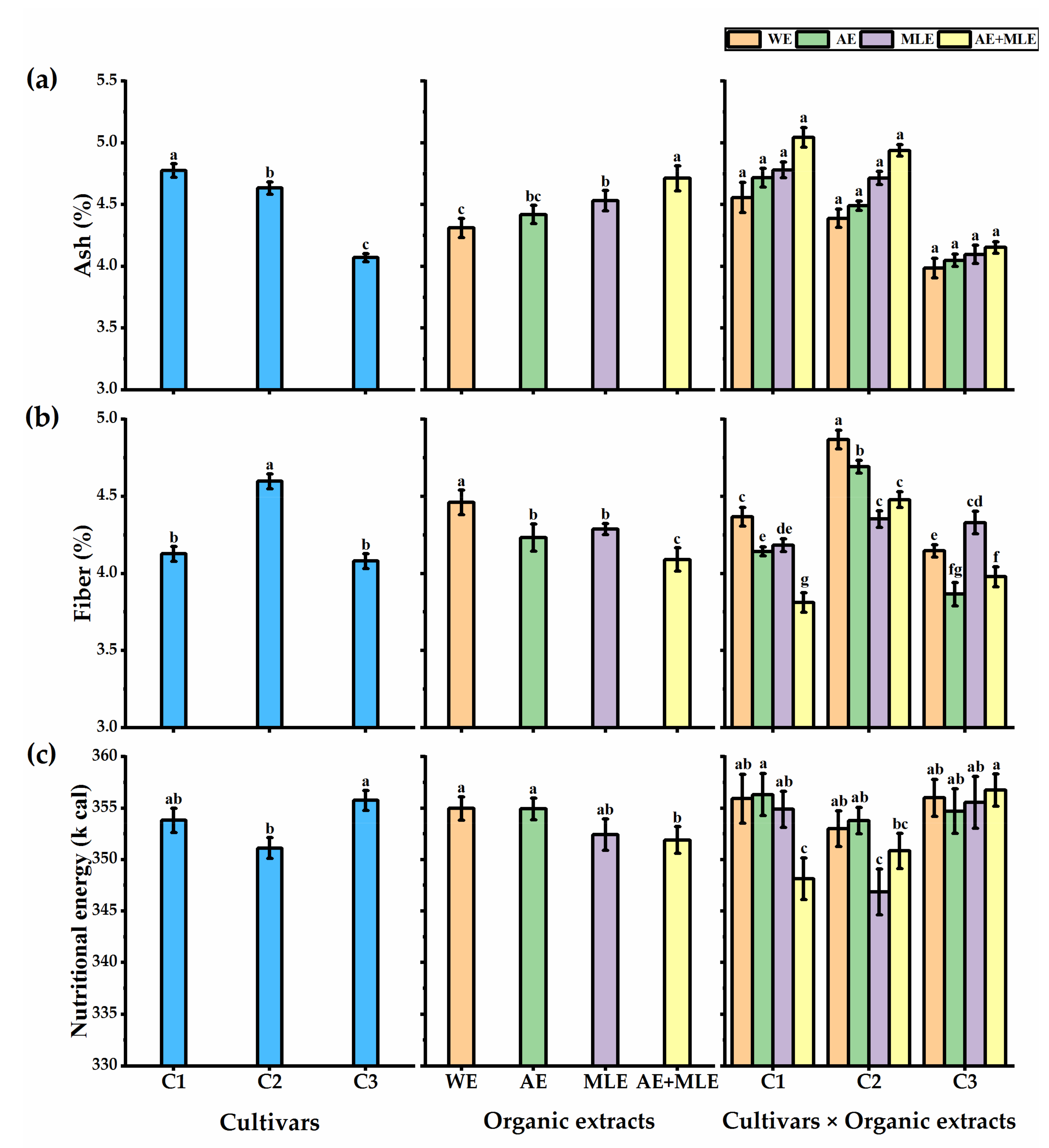
3.4. Proximate Analysis of Quinoa Seeds
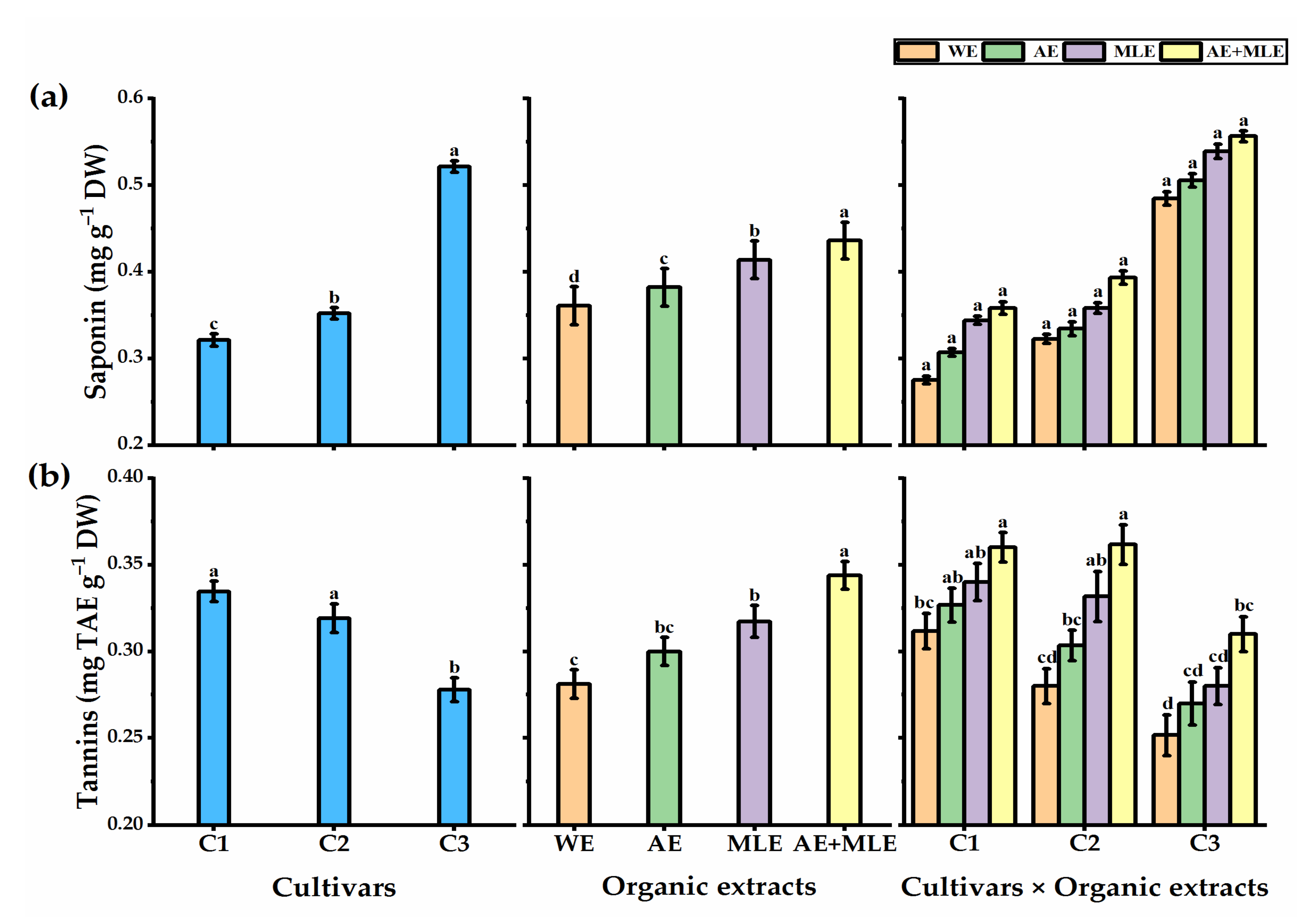
3.5. Saponin Content
3.6. Tannin Content
3.7. Total Phenols and Flavonoids Content
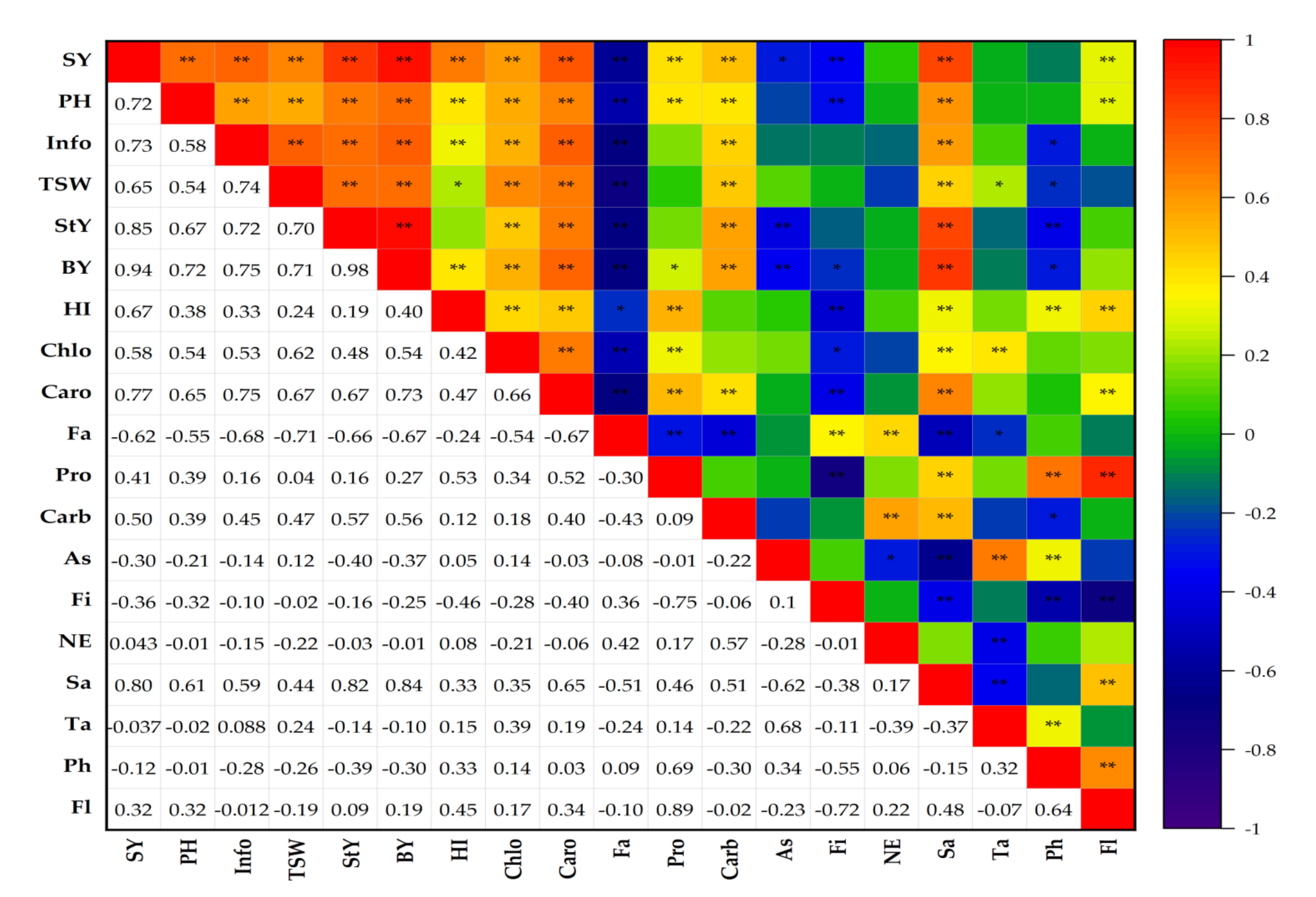
3.8. The Correlation Coefficients Analyses
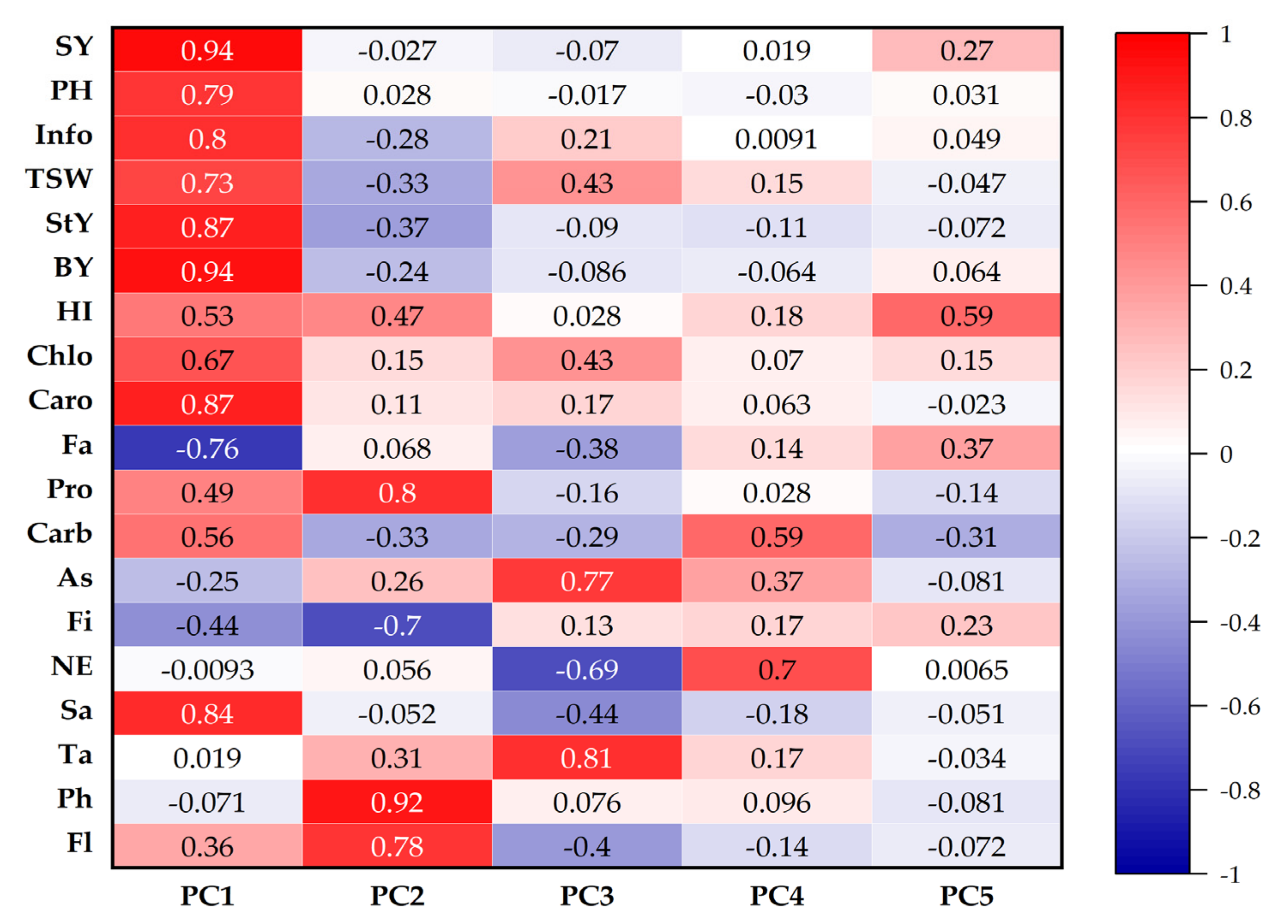
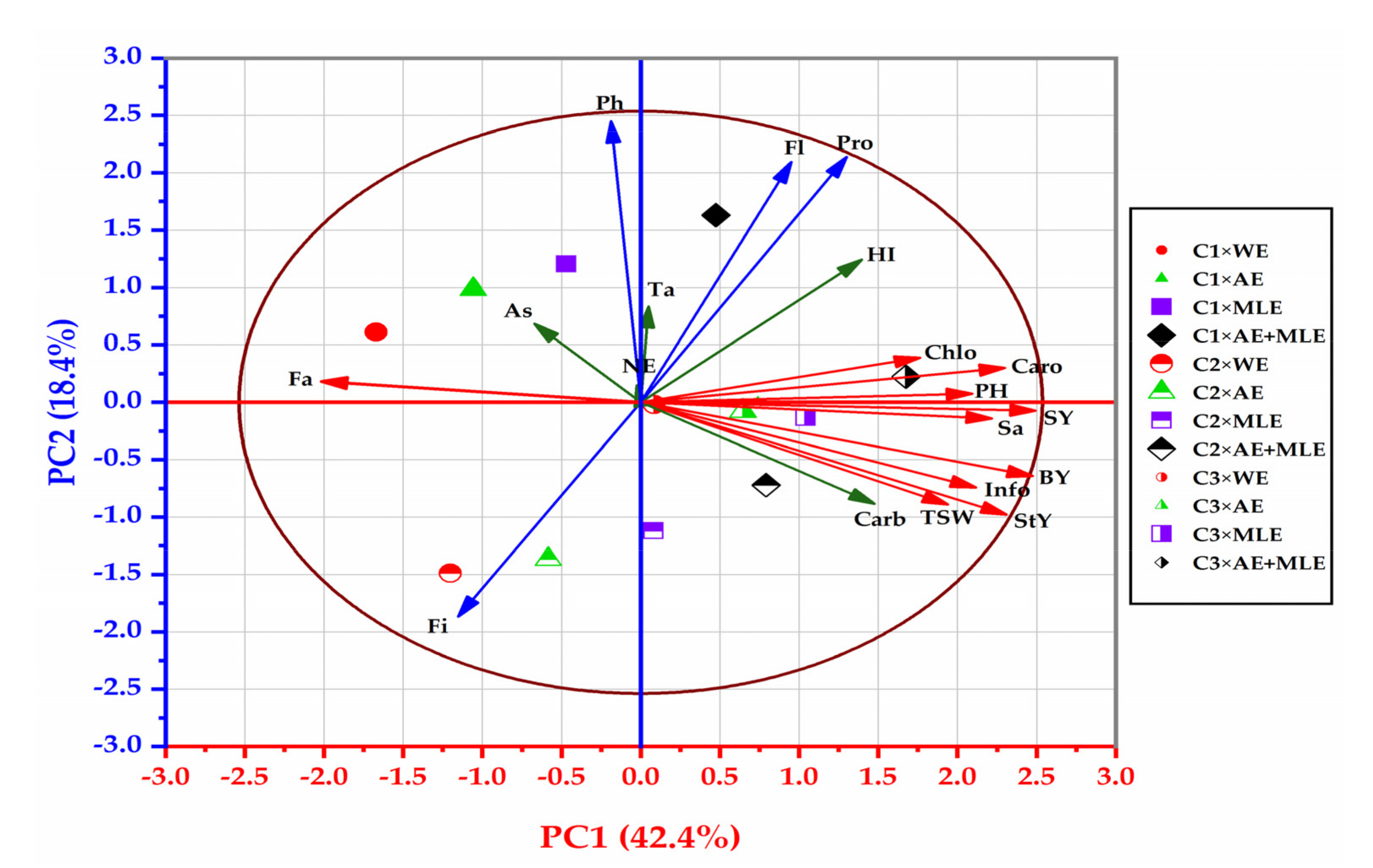
3.9. Principal Component Analysis
4. Discussion
4.1. Plant Growth and Yield
4.2. Proximate Analysis
4.3. Photochemical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prakash, D.; Pal, M. Chenopodium: Seed protein, fractionation and amino acid composition. Int. J. Food Sci. Nutr. 1998, 49, 271–275. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and heritability of selected traits during different cuttings of vegetable Chenopodium. Ind. J. Genet. Plant Breed. 2003, 63, 359–360. [Google Scholar]
- James, A.L.E. Quinoa (Chenopodium quinoa Willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kaniwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Stewart, L.K.; Soileau, J.L.; Ribnicky, D.; Wang, Z.Q.; Raskin, I.; Poulev, A.; Majewski, M.; Cefalu, W.T.; Gettys, T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism 2008, 57, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spedding, G.; Ratty, A.; Middleton, E., Jr. Inhibition of reverse transcriptases by flavonoids. Antivir. Res. 1989, 12, 99–110. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266–273. [Google Scholar] [CrossRef]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten-free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Functional components and anti-nutritional factors in gluten-free grains: A focus on quinoa seeds. Foods 2021, 10, 351. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A. Quinoa. In Caballero B.: Encyclopedia of Food Science and Nutrition; Academic Press: Amsterdam, The Netherlands, 2003; Volume 8, pp. 4895–4902. [Google Scholar]
- Güçlü-Üstündag, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Kuljanabhagavad, T.; Thongphasuk, P.; Chamulitrat, W.; Wink, M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry 2008, 69, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, S.E. The worldwide potential of quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A. Effect of nitrogen fixing bacteria and moringa leaf extract on fruit yield, estragole content and total phenols of organic fennel. Sci. Hortic. 2020, 265, 109209. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Dahab, A.A.; Al-Ashkar, I. Can yeast extract and chitosan-oligosaccharide improve fruit yield and modify the pharmaceutical active ingredients of organic fennel? Ind. Crops Prod. 2021, 173, 114130. [Google Scholar] [CrossRef]
- Malik, A.; Mor, V.S.; Tokas, J.; Punia, H.; Malik, S.; Malik, K.; Sangwan, S.; Tomar, S.; Singh, P.; Singh, N.; et al. Biostimulant-treated seedlings under sustainable agriculture: A global perspective facing climate change. Agronomy 2021, 11, 14. [Google Scholar] [CrossRef]
- El-Serafy, R.S. Phenotypic plasticity, biomass allocation, and biochemical analysis of cordyline seedlings in response to oligo-chitosan foliar spray. J. Soil Sci. Plant Nutr. 2020, 20, 1503–1514. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Atteya, A.K.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, Z.; Lin, X.; Zhao, F.; Wang, B.; Lin, F.; Ge, Y.; Eissa, M.A. Biochar impacts on NH3-volatilization kinetics and growth of sweet basil (Ocimum basilicum L.) under saline conditions. Ind. Crops Prod. 2020, 157, 11290–12903. [Google Scholar] [CrossRef]
- Shaltout, K.H.; El-Komi, T.M.; Eid, E.M. Seasonal variation in the phytomass, chemical composition and nutritional value of Azolla filiculoides Lam. along the water courses in the Nile Delta. Egypt Feddes Reper. 2012, 123, 37–49. [Google Scholar] [CrossRef]
- Bocchi, S.; Malgioglio, A. Azolla-anabaena as a biofertilizer for rice paddy fields in the Po Valley, a Temperate Rice Area in Northern Italy. Int. J. Agron. 2010, 2010, 152158. [Google Scholar] [CrossRef] [Green Version]
- Awodun, M.A. Effect of Azolla (Azolla species) on physiomineral properties of the soil. World J. Agric. Sci. 2008, 4, 157–160. [Google Scholar]
- Kassem, A.H.M.; Abd El-Aal, A.M. Minimizing the effect of soil salinity on fennel plant using cyanobacteria and compost. J. Product. Dev. 2016, 21, 153–178. [Google Scholar]
- Setiawati, M.R.; Damayani, M.; Herdiyantoro, D.; Suryatmana, P.; Anggraini, D.; Khumairah, F.H. The application dosage of Azolla pinnata in fresh and powder form as organic fertilizer on soil chemical properties, growth and yield of rice plant. AIP Conf. Proc. 2018, 1927, 030017. [Google Scholar]
- Bindhu, K.B. Effect of azolla extract on growth performance of Pisum Sativum. Int. Res. J. Biol. Sci. 2013, 2, 88–90. [Google Scholar]
- Ripley, B.S.; Kiguli, L.N.; Barker, N.P. Azolla fliculoides as a biofertilizer of wheat under dry-land soil conditions. South Afr. J. Bot. 2003, 69, 295–300. [Google Scholar] [CrossRef]
- De Bever, A.; Ndakidemi, P.A.; Laubscher, C.P. Efects of different combinations of Hoagland’s solution and Azolla fliculoides on photosynthesis and chlorophyll content in Beta vulgaris subsp. Cycla ‘fordhook giant’ grown in hydroponic cultures. Afr. J. Biotechnol. 2013, 12, 2006–2012. [Google Scholar]
- Petruccelli, R.; Bati, C.B.; Carlozzi, P.; Padovani, G.; Vignozzi, N.; Bartolini, G. Use of Azolla as a growing medium component in the nursery production of olive trees. Int. J. Basic Appl. Sci. 2015, 4, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Musse, A.A.; El-Sheshtawy, A.A.; Massoud, O.N.; Ghanem, K.M. Response of eggplant to irrigation water quality, fertilization and organic extracts under urban agriculture system. Al-Azhar J. Agric. Res. 2021, 46, 74–90. [Google Scholar]
- Youssef, M.A.; AL-Huqail, A.A.; Ali, E.F.; Majrashi, A. Organic amendment and mulching enhanced the growth and fruit quality of squash plants (Cucurbita pepo L.) grown on silty loam soils. Horticulturae 2021, 7, 269. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Kumar, S.S.; Fagodiya, R.K.; Pugazhendhi, A.; Duc, P.A. Mitigation of greenhouse gas intensity by supplementing with Azolla and moderating the dose of nitrogen fertilizer. Biocatal. Agric. Biotechnol. 2019, 20, 101266. [Google Scholar] [CrossRef]
- Maswada, H.F.; Abd El-Razek, U.A.; El-Sheshtawy, A.A.; Mazrou, Y.S.A. Effect of Azolla filiculoides on growth, physiological and yield attributes of maize grown under water and nitrogen deficiencies. J. Plant Growth Regul. 2021, 40, 558–573. [Google Scholar] [CrossRef]
- Rady, M.M.; Varma, B.C.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Yasmeen, A.; Nouman, W.; Basra, S.M.A.; Wahid, A.; Hussain, N.; Afzal, I. Morphological and physiological response of tomato (Solanum lycopersicum L.) to natural and synthetic cytokinin sources: A comparative study. Acta Physiol. Plant 2014, 36, 3147–3155. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch. Agron. Soil Sci. 2017, 63, 687–699. [Google Scholar] [CrossRef]
- Atteya, A.K.G.; Sami, R.; Al-Mushhin, A.A.M.; Ismail, K.A.; Genaidy, E.A.E. Response of seeds, oil yield and fatty acids percentage of Jojoba Shrub strain EAI to mycorrhizal fungi and moringa leaves extract. Horticulturae 2021, 7, 395. [Google Scholar] [CrossRef]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar]
- Iqbal, M.A. Improving the growth and yield of canola (Brassica napus L.) with seed treatment and foliar sprays of brassica (Brassica naups L.) and moringa (Moringa oleifera L.) leaf extracts. Am.-Eurasian J. Agric. Environ. Sci. 2014, 14, 1067–1073. [Google Scholar]
- Rashid, N.; Basra, S.M.; Shahbaz, M.; Iqbal, S.; Hafeez, M.B. Foliar applied moringa leaf extract induces terminal heat tolerance in quinoa. Int. J. Agric. Biol. 2018, 20, 157–164. [Google Scholar]
- Yasmeen, A.; Basra, S.M.A.; Farooq, M.; Rehman, H.; Hussain, N.; Athar, H.R. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 2013, 69, 225–233. [Google Scholar] [CrossRef]
- Howladar, S.M. A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef]
- Maswada, H.F.; Abd El-Razek, U.A.; El-Sheshtawy, A.A.; Elzaawely, A.A. Morpho-physiological and yield responses to exogenous moringa leaf extract and salicylic acid in maize (Zea mays L.) under water stress. Arch. Agron. Soil Sci. 2018, 64, 994–1010. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous applications of moringa leaf extract effect on retro transposon, ultra structural and biochemical contents of common bean plants under environmental stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Aslam, M.; Sultana, B.; Anwar, F.; Munir, H. Foliar spray of selected plant growth regulators affected the biochemical and antioxidant attributes of spinach in a field experiment. Turk. J. Agric. For. 2016, 40, 136–145. [Google Scholar] [CrossRef]
- Prabhu, M.; Kumar, A.R.; Rajamani, K. Influence of different organic substances on growth and herb yield of sacred basil (Ocimum sanctum L.). Indian J. Agric. Res. 2010, 44, 48–52. [Google Scholar]
- Nasir, M.; Khan, A.S.; Basra, S.A.; Malik, A.U. Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of ‘Kinnow’mandarin. Sci. Hortic. 2016, 210, 227–235. [Google Scholar]
- Yasmeen, A.; Basra, S.M.A.; Wahid, A.; Nouman, W.; Rehman, H. Exploring the potential of Moringa oleifera leaf extract (MLE) as seed priming agent in improving wheat performance. Turk. J. Bot. 2012, 37, 512–520. [Google Scholar]
- Dere, Ş.; Güneş, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Tr. J. Botany 1998, 22, 13–17. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Weinmann, H. Determination of total available carbohydrates in plants. Plant Physiol. 1947, 22, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.A.; Veas, E.; Jorquera, C.; San Martın, R.; Jara, P. Re-Introduction of quinoa into Arid Chile: Cultivation of two lowland races under extremely low irrigation. J. Agron. Crop Sci. 2009, 195, 1–10. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2004, 92, 491–497. [Google Scholar] [CrossRef]
- Rebaya, A.; Souad, I.B.; Béatrice, B.; Valérie, M.L.; Fathi, M.; Evelyne, O.; Jamila, K.C.; Malika, T.A. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2014, 5, 52–57. [Google Scholar]
- Boateng, J.; Verghese, M.; Walker, L.T.; Ogutu, S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT-Food Sci. Technol. 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Talukdar, D. Flavonoid-deficient mutants in grass pea (Lathyrus sativus L.): Genetic control, linkage relationships, and mapping with aconitase and S-nitrosoglutathione reductase isozyme loci. Sci. World J. 2012, 2012, 345983. [Google Scholar] [CrossRef] [Green Version]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Razavipour, T.; Moghaddam, S.S.; Doaei, S.; Noorhosseini, S.A.; Damalas, C.A. Azolla (Azolla filiculoides) compost improves grain yield of rice (Oryza sativa L.) under different irrigation regimes. Agric. Water Manag. 2018, 209, 1–10. [Google Scholar]
- Sharifi, P.; Shorafa, M.; Mohammadi, M.H. Comparison of the effect of cow manure, vermicompost, and azolla on safflower growth in a saline-sodic soil. Commun. Soil Sci. Plant Anal. 2019, 50, 1417–1424. [Google Scholar]
- Maham, S.G.; Rahimi, A.; Subramanian, S.; Smith, D.L. The environmental impacts of organic greenhouse tomato production based on the nitrogen-fixing plant (Azolla). J. Clean. Prod. 2020, 245, 118679. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Occurrence of cytokinin-like compounds in two aquatic ferns and their exudates. Environ. Exp. Bot. 2003, 49, 77–85. [Google Scholar] [CrossRef]
- De Vries, S.; De Vries, J.; Teschke, H.; von Dahlen, J.K.; Rose, L.E.; Gould, S.B. Jasmonic and salicylic acid response in the fern Azolla filiculoides and its cyanobiont. Plant Cell Environ. 2018, 41, 2530–2548. [Google Scholar] [CrossRef]
- Maham, S.G.; Rahimi, A.; Smith, D.L. Environmental assessment of the essential oils produced from dragonhead (Dracocephalum moldavica L.) in conventional and organic farms with different irrigation rates. J. Clean. Prod. 2018, 204, 1070–1086. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Atteya, A.K.G.; Albalwa, A.N.; El-Serafy, R.S.; Albalwa, K.; Bayomy, H.M.; Genaidy, E.A.E. Response of Moringa oleifera seeds and fixed oil production to vermicompost and NPK under calcareous soil conditions. Plants 2021, 10, 1998. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates, Inc., Publishers Sunderland: Sunderland, MA, USA, 2010; p. 782. [Google Scholar]
- Fuglie, L.J. The Miracle Tree: Moringa Oleifera: Natural Nutrition for the Tropics. The Miracle Tree: The Multiple Attributes of Moringa; Harborne, J.B., Ed.; Introduction to Ecological Biochemistry; Academic Press: New York, NY, USA, 2000; p. 172. [Google Scholar]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. Hortorum Cultus 2013, 12, 3–16. [Google Scholar]
- Ashfaq, M.; Basra, S.; Ashfaq, U. Moringa: A miracle plant of agro-forestry. J. Agric. Soc. Sci. 2012, 8, 115–122. [Google Scholar]
- Marti, A.; Ulrici, A.; Foca, G.; Quaglia, L.; Pagani, M.A. Characterization of common wheat flours (Triticum aestivum L.) through multivariate analysis of conventional rheological parameters and gluten peak test indices. LWT-Food Sci. Technol. 2015, 64, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Kunkulberga, D.; Linina, A.; Ruza, A. Effect of nitrogen fertilization on protein content and rheological properties of winter wheat wholemeal. Foodbalt 2019, 2019, 88–92. [Google Scholar]
- Emongor, V.E. Effects of moringa (Moringa oleifera) leaf extract on growth, yield and yield components of snap beans (Phaseolus vulgaris). Br. J. Appl. Sci. Technol. 2015, 6, 114–122. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994; p. 476. [Google Scholar]
- Filho, M.M.; Destro, D.; Miranda, L.A.; Spinosa, W.A.; CarrãoPanizzi, M.C.; Montalván, R. Relationships among oil content, protein content and seed size in soybeans. Braz. Arch. Biol. Technol. 2001, 44, 23–33. [Google Scholar] [CrossRef] [Green Version]
- El-Serafy, R.S.; El-Sheshtawy, A.A.; Ali, H.E. Phenology, architecture, yield and fatty acid content of chia in response to sowing date and plant spacing. Fayoum J. Agric. Res. Dev. 2020, 34, 314–331. [Google Scholar]
- Verza, S.G.; Silveira, F.; Cibulski, S.; Kaiser, S.; Ferreira, F.; Gosmann, G.; Roehe, P.M.; Ortega, G.G. Immunoadjuvant activity, toxicity assays, and determination by UPLC/Q-TOFMS of triterpenic saponins from Chenopodium quinoa seeds. J. Agric. Food Chem. 2012, 60, 3113–3118. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, 1018–1023. [Google Scholar]
- Noratto, G.; Carrión-Rabanal, R.; Medina, G.; Mencia, A.; Mohanty, I.; González, D.A.; Murphy, K. Quinoa protective effects against obesity-induced intestinal inflammation. FASEB J. 2015, 29, 602–609. [Google Scholar] [CrossRef]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity-associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Shi, J.; Arunasalam, K.; Yeung, D.; Kakuda, Y.; Mittal, G.; Jiang, Y. Saponins from edible legumes: Chemistry, processing, and health benefits. J. Med. Food 2004, 7, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ye, C.; Huang, H.; Yang, M.; Mei, X.; Du, F.; He, X.; Zhu, S.; Liu, Y. Appropriate nitrogen application enhances saponin synthesis and growth mediated by optimizing root nutrient uptake ability. J. Ginseng Res. 2020, 44, 627–636. [Google Scholar] [CrossRef]
- Bilalis, D.; Kakabouki, I.; Karkanis, A.; Travlos, I.; Triantafyllidis, V.; Hela, D. Quinoa seed and saponin production of organic quinoa (Chenopodium quinoa Willd.) for different tillage and fertilization. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Aslam, M.; Sultana, B.; Ali, S.; Ur-Rehman, K. Modification in antioxidant potential of Coriandum sativum using selected plant growth regulators. Asian J. Chem. 2014, 26, 5105–5109. [Google Scholar] [CrossRef]
- Rabie, K.A.E.; El-Sherif, M.H.; El-Shahat, R.M.; Ali, F.S.I. Azolla as an alternative of mineral nitrogen for chamomile plant (Matricaria chamomilla L.) fertilization in sandy soil. Asian J. Soil Sci. Plant Nutr. 2020, 5, 1–13. [Google Scholar]
- El-Sadek, A.N. Multi-environmental evaluation for grain yield and its components of quinoa genotypes across the northwestern coast of Egypt. Egypt. J. Desert Res. 2017, 67, 65–82. [Google Scholar] [CrossRef]


| Season | Temperature (°C) | RH (%) | Rainfall | WS (m s−1) | ||
|---|---|---|---|---|---|---|
| Maximum | Minimum | Average | (mm/day) | |||
| Season 2018/2019 | ||||||
| 2018 year | ||||||
| November | 26.13 | 14.62 | 20.37 | 64.32 | 0.52 | 0.24 |
| December | 21.21 | 11.13 | 16.17 | 65.12 | 0.56 | 0.28 |
| 2019 year | ||||||
| January | 19.21 | 8.19 | 13.71 | 50.90 | 0.44 | 0.33 |
| February | 22.15 | 8.25 | 15.20 | 56.82 | 0.66 | 0.32 |
| March | 23.73 | 12.33 | 18.03 | 56.29 | 0.85 | 0.64 |
| April | 27.39 | 14.83 | 21.11 | 48.43 | 0.15 | 0.65 |
| Season 2019/2020 | ||||||
| 2019 year | ||||||
| November | 27.70 | 16.03 | 18.36 | 57.09 | 0.21 | 0.32 |
| December | 21.48 | 11.72 | 16.6 | 61.65 | 0.67 | 0.43 |
| 2020 year | ||||||
| January | 18.50 | 9.00 | 13.75 | 62.74 | 0.87 | 0.57 |
| February | 20.94 | 10.11 | 15.52 | 64.18 | 0.96 | 0.40 |
| March | 23.79 | 12.45 | 18.12 | 63.56 | 1.89 | 0.65 |
| April | 27.83 | 15.32 | 21.57 | 54.35 | 0.15 | 0.68 |
| N | P | K | Ca | Mg | Zn | |
|---|---|---|---|---|---|---|
| Azolla | 19.6 | 1.91 | 11.3 | 20.6 | 7.9 | 0.17 |
| Moringa | 23.6 | 3.8 | 14.1 | 16.3 | 9.3 | 1.5 |
| Traits | S | R (S) | C | S × C | Error (I) | OE | S × OE | C × OE | S × C × OE | Error (II) |
|---|---|---|---|---|---|---|---|---|---|---|
| df | 1 | 4 | 2 | 2 | 8 | 3 | 3 | 6 | 6 | 36 |
| SY | 35,793 | 83,800 | 2,196,667 ** | 3680 | 11,522 | 1,182,889 ** | 22,876 | 56,415 * | 21,800 | 20,624 |
| PH | 126.9 | 162.4 | 358.7 ** | 2.1 | 19.0 | 323.3 ** | 4.5 | 24.2 ** | 2.7 | 6.9 |
| Info | 0.004 | 0.325 | 16.08 ** | 0.411 | 0.24 | 13.7 ** | 0.161 | 0.777 * | 0.274 | 0.30 |
| TSW | 0.031 | 0.011 | 0.562 ** | 0.013 | 0.005 | 0.664 ** | 0.001 | 0.017 ** | 0.004 | 0.004 |
| StY | 136,643 | 106,569 | 7,396,168 ** | 17,198 | 14,597 | 1,596,958 ** | 69,064 * | 109,584 ** | 47,788 * | 17,819 |
| BY | 312,310 ** | 261,231 ** | 17,224,209 ** | 6577 | 18,885 | 5,517,462 ** | 271,247 ** | 177,146 * | 45,715 | 53,809 |
| HI | 0.15 | 13.49 | 28.62 ** | 2.12 | 3.52 | 24.86 ** | 6.88 | 7.85 * | 6.25 | 3.45 |
| Chlo | 0.01 | 0.015 | 0.036 | 0.04 | 0.012 | 0.586 ** | 0.019 | 0.01 | 0.012 | 0.021 |
| Caro | 0.002 | 0.000 | 0.015 ** | 0.000 | 0.000 | 0.035 ** | 0.000 | 0.000 | 0.001 * | 0.000 |
| Fa | 0.027 | 0.092 | 1.77 ** | 0.008 | 0.018 | 4.24 ** | 0.052 | 0.832 ** | 0.027 | 0.029 |
| Pro | 0.004 | 0.064 | 6.31 ** | 0.025 | 0.032 | 1.50 ** | 0.008 | 0.059 ** | 0.009 | 0.013 |
| Carb | 0.26 | 3.36 | 11.72 ** | 3.03 | 1.27 | 4.07 ** | 1.19 | 2.13 * | 0.497 | 0.87 |
| As | 0.035 | 0.093 | 3.33 ** | 0.007 | 0.06 | 0.530 ** | 0.004 | 0.054 | 0.013 | 0.029 |
| Fi | 0.008 | 0.045 | 1.96 ** | 0.03 | 0.028 | 0.422 ** | 0.005 | 0.228 ** | 0.008 | 0.017 |
| NE | 0.69 | 111.68 * | 129.91 * | 44.51 | 19.57 | 47.29 * | 35.34 | 51.54 ** | 7.44 | 14.99 |
| Sa | 0.00002 | 0.00008 | 0.139 ** | 0.00010 | 0.00002 | 0.0067 ** | 0.00011 | 0.00005 | 0.00005 | 0.00001 |
| Ta | 0.0013 | 0.0000 | 0.0201 ** | 0.0012 | 0.0016 | 0.0101 ** | 0.0001 | 0.0017 * | 0.0009 | 0.0006 |
| Ph | 0.190 | 0.050 | 5.52 ** | 0.025 | 0.023 | 0.373 ** | 0.050 | 0.137 ** | 0.022 | 0.031 |
| Fl | 0.0001 | 0.0022 | 11.412 ** | 0.0134 | 0.042 | 0.405 ** | 0.0120 | 0.11 * | 0.021 | 0.041 |
| Traits | S | R (S) | C | S × C | Error (I) | OE | S × OE | C × OE | S × C × OE | Error (II) |
|---|---|---|---|---|---|---|---|---|---|---|
| df | 1 | 4 | 2 | 2 | 8 | 3 | 3 | 6 | 6 | 36 |
| SY | 0.37 | 3.46 | 44.29 | 0.08 | 0.95 | 36.61 | 0.71 | 3.49 | 1.35 | 8.69 |
| PH | 4.17 | 21.36 | 23.58 | 0.14 | 5.01 | 31.87 | 0.44 | 4.76 | 0.53 | 8.14 |
| Info | 0.00 | 1.37 | 33.84 | 0.86 | 2.02 | 43.40 | 0.51 | 4.91 | 1.73 | 11.36 |
| TSW | 0.85 | 1.13 | 31.70 | 0.85 | 1.13 | 56.33 | 0.08 | 2.83 | 0.85 | 4.25 |
| StY | 0.62 | 1.93 | 66.96 | 0.16 | 0.53 | 21.68 | 0.94 | 2.98 | 1.30 | 2.90 |
| BY | 0.55 | 1.85 | 60.85 | 0.02 | 0.27 | 29.24 | 1.44 | 1.88 | 0.48 | 3.42 |
| HI | 0.03 | 12.05 | 12.78 | 0.94 | 6.29 | 16.66 | 4.61 | 10.52 | 8.37 | 27.75 |
| Chlo | 0.33 | 1.98 | 2.31 | 2.64 | 3.30 | 58.09 | 1.98 | 1.98 | 2.31 | 25.08 |
| Caro | 1.56 | 0.75 | 19.40 | 0.96 | 1.11 | 65.66 | 0.59 | 2.54 | 2.18 | 5.25 |
| Fa | 0.13 | 1.60 | 15.28 | 0.09 | 0.65 | 54.86 | 0.69 | 21.52 | 0.69 | 4.49 |
| Pro | 0.02 | 1.38 | 67.10 | 0.27 | 1.33 | 25.22 | 0.16 | 1.86 | 0.27 | 2.39 |
| Carb | 0.22 | 11.54 | 20.14 | 5.21 | 8.74 | 10.50 | 3.08 | 10.99 | 2.56 | 27.02 |
| As | 0.29 | 3.59 | 64.53 | 0.10 | 2.03 | 15.41 | 0.10 | 3.10 | 0.77 | 10.08 |
| Fi | 0.13 | 2.34 | 51.04 | 0.78 | 2.86 | 16.54 | 0.13 | 17.85 | 0.65 | 7.68 |
| NE | 0.00 | 21.30 | 12.30 | 4.30 | 7.50 | 6.80 | 5.10 | 14.80 | 2.10 | 25.80 |
| Sa | 0.00 | 0.20 | 87.90 | 0.10 | 0.20 | 9.30 | 0.20 | 0.30 | 0.30 | 1.50 |
| Ta | 1.05 | 0.13 | 32.38 | 1.86 | 10.11 | 24.49 | 0.28 | 8.33 | 4.31 | 17.06 |
| Ph | 1.27 | 1.33 | 73.55 | 0.33 | 1.20 | 7.46 | 1.00 | 5.46 | 0.87 | 7.53 |
| Fl | 0.00 | 0.03 | 85.50 | 0.10 | 1.24 | 4.55 | 0.14 | 2.46 | 0.47 | 5.51 |
| Season | 2018/2019 | 2019/2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sources | R | C | Error (I) | OE | C× OE | Error (II) | R | C | Error (I) | OE | C× OE | Error (II) |
| df | 2 | 2 | 4 | 3 | 6 | 18 | 2 | 2 | 4 | 3 | 6 | 18 |
| SY | 72,350.09 | 1,182,504 ** | 16,468.03 | 808,294 ** | 6892.302 | 26,352.84 | 95,249 * | 1,017,843 ** | 6575 | 454,092 ** | 26,346 | 20,452 |
| PH | 527.6 ** | 1938 ** | 13.07 | 1633 ** | 664.8 ** | 14.06 | 278.43 * | 154.80 * | 18.75 | 174.65 ** | 19.96 * | 7.24 |
| Info | 0.137 | 8.687 ** | 0.264 | 7.568 ** | 0.578 * | 0.215 | 0.514 | 7.591 ** | 0.218 | 6.328 ** | 0.872 * | 0.273 |
| TSW | 0.0124 | 0.371 ** | 0.007 | 0.361 ** | 0.022 * | 0.004 | 0.007 | 0.205 ** | 0.003 | 0.305 ** | 0.010 * | 0.004 |
| StY | 19,984 | 3,575,937 ** | 11,080 | 944,563 ** | 88,362 * | 24,615 | 193,156 * | 3,837,433 ** | 18,113 | 721,460 ** | 69,009 ** | 11,024 |
| BY | 72,761 * | 8,212,878 ** | 8858 | 3,485,758 ** | 199,944 * | 74,031 | 449,700 * | 8,667,902 ** | 28,912 | 2,302,954 ** | 139,583 ** | 33,587. |
| HI | 15.94 | 44.187 ** | 5.42 | 14.52 ** | 7.57 * | 3.14 | 11.04 | 10.59 * | 1.12 | 13.55 ** | 5.85 * | 2.04 |
| Chlo | 0.003 | 0.0003 | 0.002 | 0.255 ** | 0.008 | 0.007 | 0.027 | 0.076 | 0.023 | 0.349 ** | 0.014 | 0.035 |
| Caro | 0.0004 | 0.0064 ** | 0.0002 | 0.0187 ** | 0.0002 | 0.0002 | 0.0002 | 0.0089 ** | 0.0002 | 0.0163 ** | 0.0006 | 0.0003 |
| Fa | 0.052 | 1.004 ** | 0.018 | 1.924 ** | 0.388 ** | 0.309 | 0.132 * | 0.775 ** | 0.019 | 2.364 ** | 0.471 ** | 0.027 |
| Pr | 0.12 * | 3.57 ** | 0.008 | 0.81 ** | 0.04 * | 0.013 | 0.009 | 2.77 ** | 0.054 | 0.778 ** | 0.033 * | 0.012 |
| Carb | 4.72 * | 13.59 ** | 0.39 | 2.82 ** | 1.49 * | 0.56 | 1.99 | 8.30 * | 0.90 | 5.10 ** | 1.09 * | 0.35 |
| As | 0.176 ** | 1.625 ** | 0.009 | 0.281 ** | 0.02 | 0.012 | 0.009 | 1.711 ** | 0.043 | 0.253 ** | 0.046 | 0.045 |
| Fi | 0.066 * | 0.761 ** | 0.008 | 0.179 ** | 0.137 ** | 0.017 | 0.024 | 1.234 ** | 0.048 | 0.247 ** | 0.099 ** | 0.016 |
| NE | 143.82 ** | 142.01 ** | 6.42 | 42.37 * | 34.79 * | 10.489 | 54.56 | 100.42 * | 11.25 | 116.93 ** | 24.18 * | 6.71 |
| Sa | 0.0004 | 0.1426 ** | 0.0003 | 0.0116 ** | 0.0002 | 0.0002 | 0.0002 | 0.1365 ** | 0.0001 | 0.0087 ** | 0.0004 | 0.0004 |
| Ta | 0.000002 | 0.014836 ** | 0.001665 | 0.00526 ** | 0.001103 * | 0.000404 | 0.00008 | 0.00792 * | 0.00080 | 0.00602 ** | 0.00167 * | 0.00061 |
| Ph | 0.038 | 2.312 ** | 0.027 | 0.159 ** | 0.108 ** | 0.024 | 0.060 | 3.184 ** | 0.019 | 0.277 ** | 0.083 * | 0.031 |
| Fl | 0.004 | 6.019 ** | 0.050 | 0.266 ** | 0.115 * | 0.041 | 0.001 | 5.309 ** | 0.033 | 0.151 * | 0.105 * | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Abd El-Razek, U.A.; Abd El-Hakim, A.F.; Hasham, M.M.A.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions. Agronomy 2021, 11, 2186. https://doi.org/10.3390/agronomy11112186
El-Serafy RS, El-Sheshtawy A-NA, Abd El-Razek UA, Abd El-Hakim AF, Hasham MMA, Sami R, Khojah E, Al-Mushhin AAM. Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions. Agronomy. 2021; 11(11):2186. https://doi.org/10.3390/agronomy11112186
Chicago/Turabian StyleEl-Serafy, Rasha S., Abdel-Nasser A. El-Sheshtawy, Usama A. Abd El-Razek, Ahmed F. Abd El-Hakim, Mostafa M. A. Hasham, Rokayya Sami, Ebtihal Khojah, and Amina A. M. Al-Mushhin. 2021. "Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions" Agronomy 11, no. 11: 2186. https://doi.org/10.3390/agronomy11112186
APA StyleEl-Serafy, R. S., El-Sheshtawy, A.-N. A., Abd El-Razek, U. A., Abd El-Hakim, A. F., Hasham, M. M. A., Sami, R., Khojah, E., & Al-Mushhin, A. A. M. (2021). Growth, Yield, Quality, and Phytochemical Behavior of Three Cultivars of Quinoa in Response to Moringa and Azolla Extracts under Organic Farming Conditions. Agronomy, 11(11), 2186. https://doi.org/10.3390/agronomy11112186







