Maceration Time Affects the Efficacy of Borage Extracts as Potential Biostimulant on Rocket Salad
Abstract
:1. Introduction
2. Materials and Methods
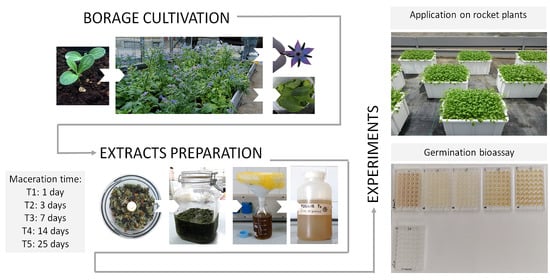
2.1. Plant Material, Growth Conditions, and Experimental Design
2.1.1. Preparation of Borage Extracts
2.1.2. Chemical Characterization of the Extracts
2.1.3. Rocket Cultivation
2.2. Non-Destructive Analyses
2.2.1. Chlorophyll
2.2.2. Chlorophyll a Fluorescence
2.3. Destructive Analyses
2.3.1. Total Fresh Biomass and Dry Matter
2.3.2. Chlorophylls and Carotenoids
2.3.3. Spectrophotometric Phenol and Anthocyanin Determination
2.3.4. Nitrate Concentration
2.3.5. Sucrose and Total Sugars
2.4. Germination Bioassay
2.5. Statistical Analysis
3. Results
3.1. Total Fresh Biomass and Dry Matter Percentage
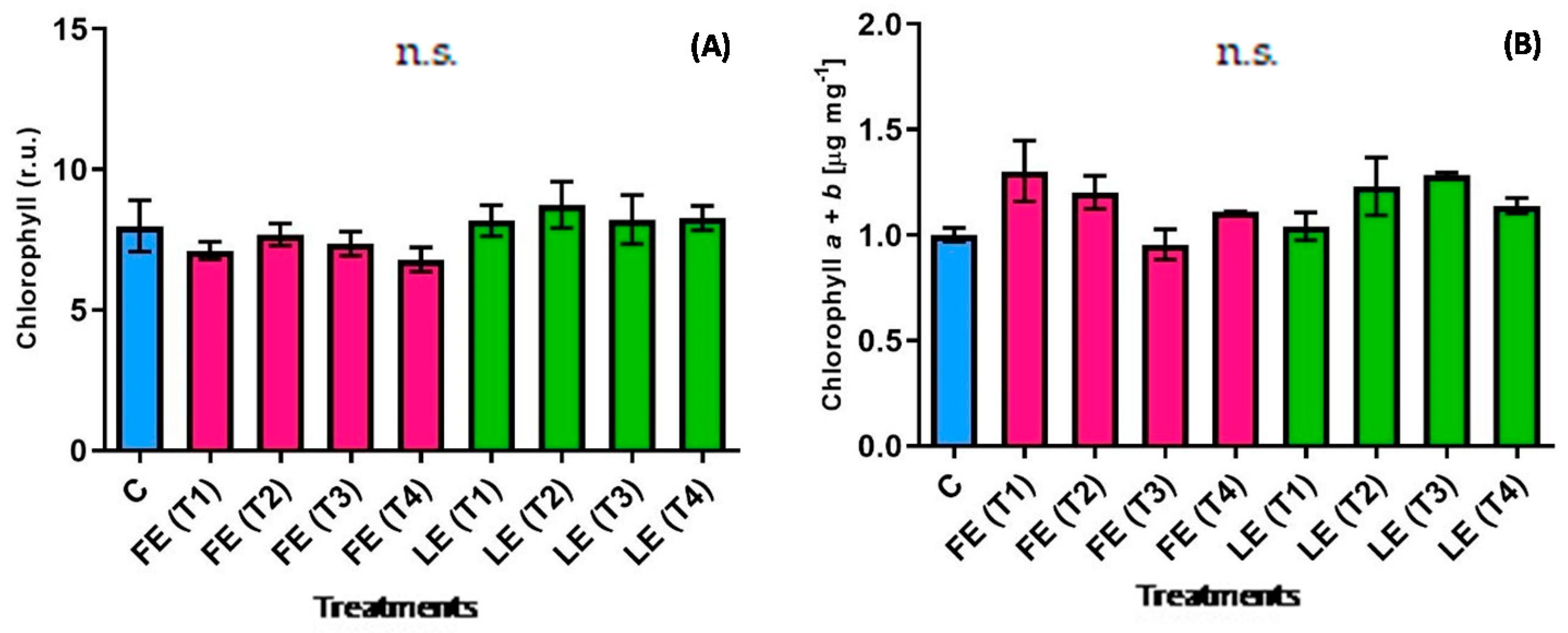
3.2. Chlorophyll
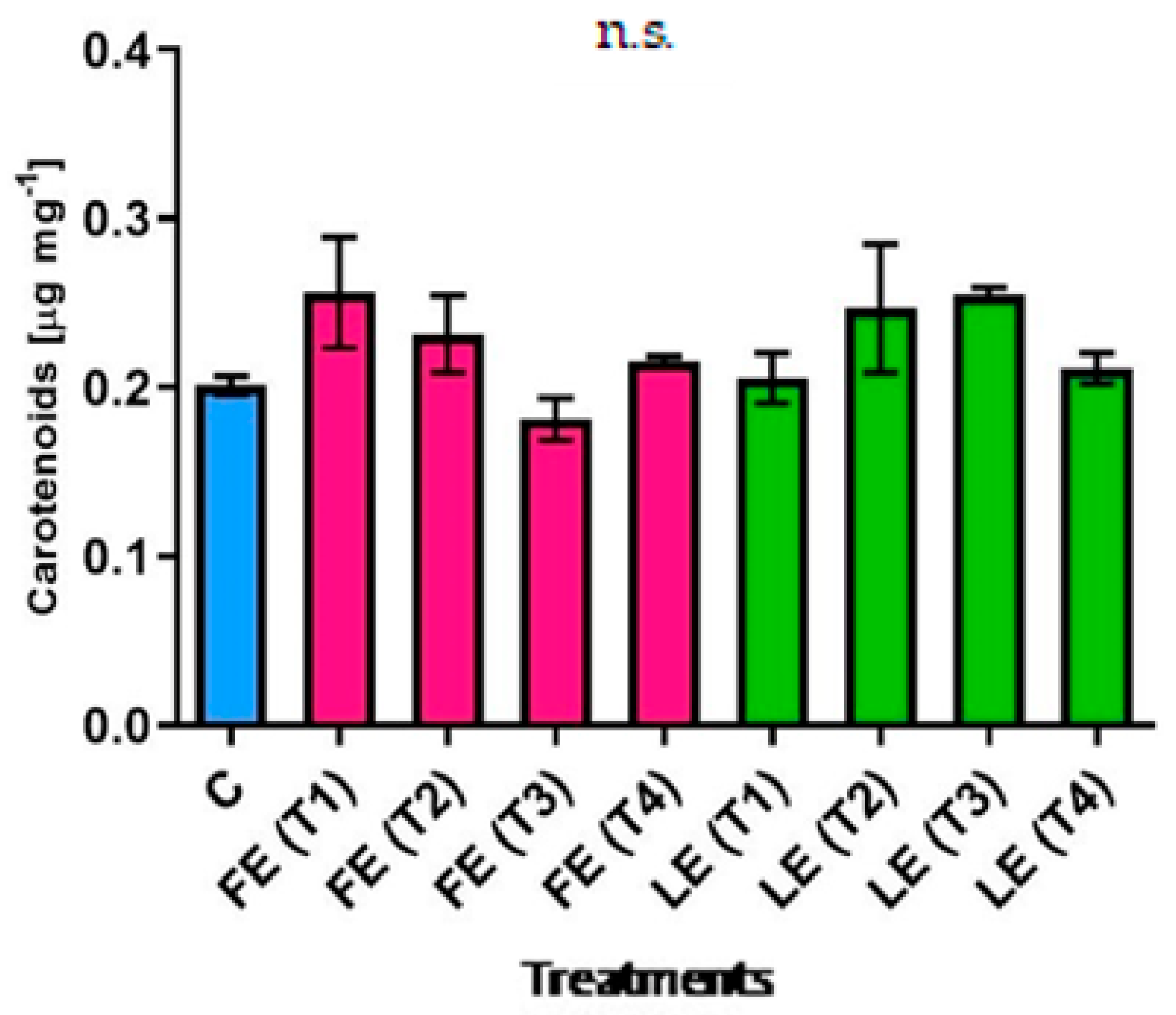
3.3. Total Carotenoids
3.4. Phenols and Anthocyanin
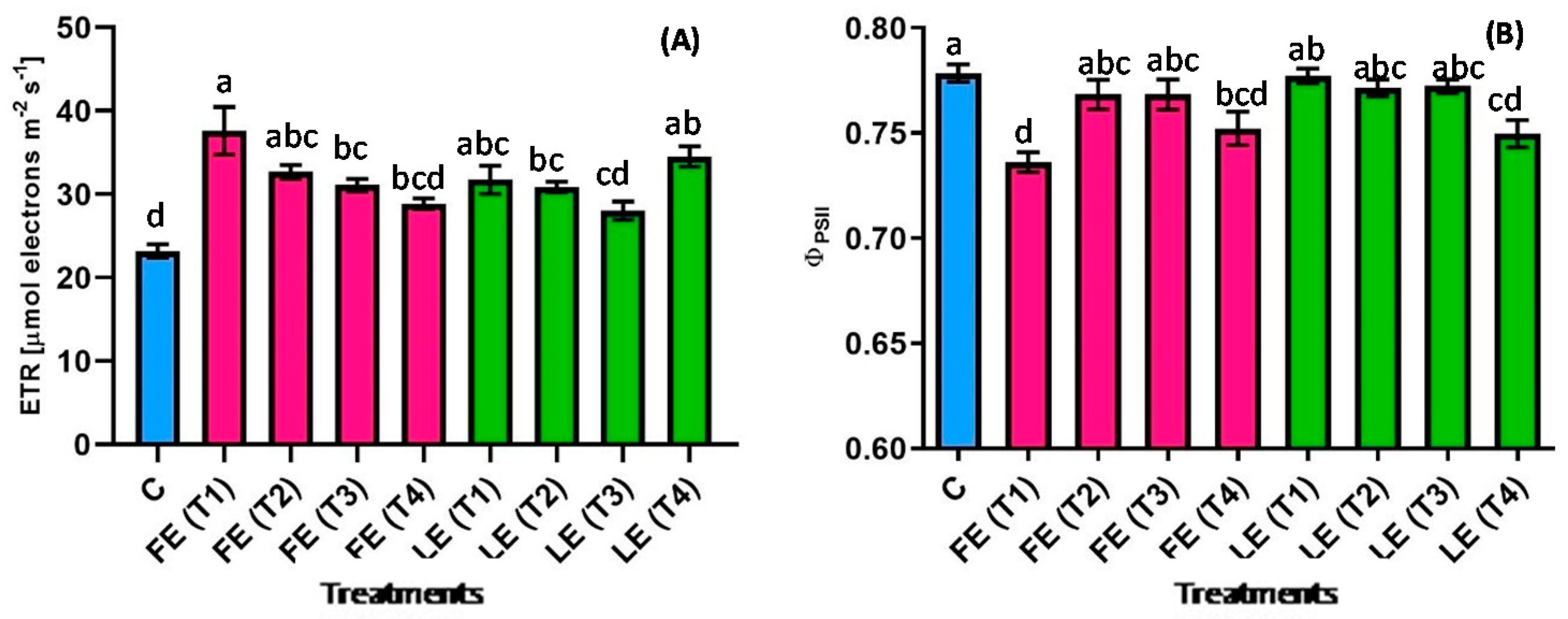
3.5. Chlorophyll a Fluorescence
3.6. Nitrate Concentration
3.7. Sucrose and Total Sugar Concentration
3.8. Seed Germination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metabolism 2008, 57, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Ben Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Trivellini, A.; Bulgari, R.; Cocetta, G.; Ferrante, A. Bioactive Molecules as Regulatory Signals in Plant Responses to Abiotic Stresses. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–182. [Google Scholar]
- Dias, L.S.; Dias, A.S. Secondary metabolites as sources of bioherbicides: Present situation and perspectives. Rev. Ciênc. Agrár. 2007, 30, 510–517. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Handa, S.S.; Khanuja, S.P.S.; Longo, G.; Rakesh, D.D. Extraction Technologies for Medicinal and Aromatic Plants; Earth, Environmental and Marine Sciences and Technologies: Trieste, Italy, 2008. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Raynie, D.E. Extraction. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 118–128. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crop. Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Sithisarn, P.; Supabphol, R.; Gritsanapan, W. Comparison of free radical scavenging activity of Siamese neem tree (Azadirachta indica A. Juss var. siamensis Valeton) leaf extracts prepared by different methods of extraction. Med. Princ. Pract. 2006, 15, 219–222. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.S.A.; Abdel-Kader, A.A.S. Response of Fennel (Foeniculum vulgare, Mill) plants to foliar application of moringa leaf extract and benzyladenine (BA). S. Afr. J. Bot. 2020, 129, 113–122. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.; Matter, F.M. Using moringa leaf extract as biostimulant and giberrellic acid for enhancing fennel (Foeniculum vulgare var. azoricum Mill.) growth and oil yield. Acta Sci. Intellectus 2016, 2, 7–20. [Google Scholar]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Howladar, S.M. A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Culver, M.; Fanuel, T.; Chiteka, A.Z. Effect of Moringa Extract on Growth and Yield of Tomato. Greener J. Agric. Sci. 2012, 2, 207–211. [Google Scholar]
- Rady, M.M.; Desoky, E.S.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R.; et al. Evaluation of Borage Extracts as Potential Biostimulant Using a Phenomic, Agronomic, Physiological, and Biochemical Approach. Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Ferrante, A. Borage extracts affect wild rocket quality and influence nitrate and carbon metabolism. Physiol. Mol. Biol. Plants 2020, 26, 649–660. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Ferrante, A. Transcriptional Regulation in Rocket Leaves as Affected by Salinity. Plants 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, M.; Amiri, M.S.; Zibaee, E.; Boghrati, Z.; Ayati, Z.; Sahebkar, A.; Emami, S.A. A Review on the Phytochemistry, Ethnobotanical Uses and Pharmacology of Borago Species. Curr. Pharm. Des. 2019, 26, 110–128. [Google Scholar] [CrossRef]
- Garcia-Herreros, C.; Garcia-Iñiguez-de-Ciriano, M.; Astiasarán, I.; Ansorena-Artieda, D. Antioxidant activity and phenolic content of water extracts of Borago officinalis L: Influence of plant part and cooking procedures. Ital. J. Food Sci. 2010, 22, 156–164. [Google Scholar]
- Salem, N.; Msaada, K.; Hammami, M.; Jday, A.; Salem, S.; Limam, F.; Marzouk, B. Variations in Tunisian borage essential oil profiles and their antioxidant activities during flowering. Nat. Prod. Res. 2014, 28, 1919–1922. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Hammami, M.; Limam, F.; Vasapollo, G.; Marzouk, B. Variation in anthocyanin and essential oil composition and their antioxidant potentialities during flower development of Borage (Borago officinalis L.). Plant Biosyst. 2014, 148, 444–459. [Google Scholar] [CrossRef]
- Bandoniene, D.; Murkovic, M.; Venskutonis, P.R. Determination of rosmarinic acid in sage and borage leaves by high-performance liquid chromatography with different detection methods. J. Chromatogr. Sci. 2005, 43, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Wettasinghe, M.; Shahidi, F.; Amarowicz, R.; Abou-Zaid, M.M. Phenolic acids in defatted seeds of borage (Borago officinalis L.). Food Chem. 2001, 75, 49–56. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. J. Food Meas. Charact. 2018, 12, 826–838. [Google Scholar] [CrossRef]
- Herrmann, M.; Joppe, H.; Schmaus, G. Thesinine-4′-O-β-D-glucoside the first glycosylated plant pyrrolizidine alkaloid from Borago officinalis. Phytochemistry 2002, 60, 399–402. [Google Scholar] [CrossRef]
- Vacillotto, G.; Favretto, D.; Seraglia, R.; Pagiotti, R.; Traldi, P.; Mattoli, L. A rapid and highly specific method to evaluate the presence of pyrrolizidine alkaloids in Borago officinalis seed oil. J. Mass Spectrom. 2013, 48, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Bell, L. Rocket Science: Phytochemical, Postharvest, Shelf-Life & Sensory Attributes of Rocket Species. Ph.D. Thesis, University of Reading, Reading, UK, 2016. [Google Scholar]
- Bell, L.; Lignou, S.; Wagstaff, C. High Glucosinolate Content in Rocket Leaves (Diplotaxis tenuifolia and Eruca sativa) after Multiple Harvests Is Associated with Increased Bitterness, Pungency, and Reduced Consumer Liking. Foods 2020, 9, 1799. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Ferrante, A. Effect of glutamic acid foliar applications on lettuce under water stress. Physiol. Mol. Biol. Plants 2021, 27, 1059–1072. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
- Klein, A.O.; Hagen, C.W. Anthocyanin production in detached petals of impatiens balsamina L. Plant Physiol. 1961, 36, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Rorem, E.S.; Walker, H.G.; McCready, R.M. Biosynthesis of Sucrose and Sucrose-Phosphate by Sugar Beet Leaf Extracts. Plant Physiol. 1960, 35, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [Green Version]
- Pardo-García, A.I.; Martínez-Gil, A.M.; Cadahía, E.; Pardo, F.; Alonso, G.L.; Salinas, M.R. Oak extract application to grapevines as a plant biostimulant to increase wine polyphenols. Food Res. Int. 2014, 55, 150–160. [Google Scholar] [CrossRef]
- Szparaga, A.; Czerwińska, E.; Piskier, T. The effect of treating the seeds of Brassica oleracea L. with aqueous extracts on the germination capacity and seed healthiness. J. Res. Appl. Agric. Eng. 2017, 62, 162–167. [Google Scholar]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2019, 25, 27–36. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing Biostimulants from Agro-Food and Industrial By-Products. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 13, 932–937. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Naboulsi, I.; Aboulmouhajir, A.; Kouisni, L.; Bekkaoui, F.; Yasri, A. Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. Acad. J. Med. Plants 2018, 6, 223–240. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.; Mohammad, F. Role of Secondary Metabolites in Chemical Defence Mechanisms in Plants. Front. Life Sci. 2011, 3, 232–249. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piaggesi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A. Application of Biostimulants in Floating System for Improving Rocket Quality. J. Food Agric. Environ. 2005, 3, 86–88. [Google Scholar]
- Bulgari, R.; Podetta, N.; Cocetta, G.; Piaggesi, A.; Ferrante, A. The effect of a complete fertilizer for leafy vegetables production in family and urban gardens. Bulg. J. Agric. Sci. 2014, 20, 1361–1367. [Google Scholar]
- Abbas, S.M.; Akladious, S.A. Application of carrot root extract induced salinity tolerance in cowpea (Vigna sinensis L.) seedlings. Pak. J. Bot. 2013, 45, 795–806. [Google Scholar]
- Ferrante, A.; Incrocci, L.; Maggini, R.; Serra, G.; Tognoni, F. Colour changes of fresh-cut leafy vegetables during storage. J. Food. Agric. Environ. 2004, 22, 40–44. [Google Scholar]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.; Hassan, N.M.; Hatta, F.A.M. Influence of Environmental Stress toward Carotenogenesis Regulatory Mechanism through In Vitro Model System. In Carotenoids; InTech: Rijeka, Croatia, 2017; Volume i, p. 13. [Google Scholar]
- Morosinotto, T.; Caffarri, S.; Dall’Osto, L.; Bassi, R. Mechanistic aspects of the xanthophyll dynamics in higher plant thylakoids. Physiol. Plant. 2003, 119, 347–354. [Google Scholar] [CrossRef]
- Kocira, S.; Sujak, A.; Oniszczuk, T.; Szparaga, A.; Szymanek, M.; Karakuła-Juchnowicz, H.; Krawczuk, A.; Kupryaniuk, K. Improvement of the photosynthetic activity of Moldavian dragonhead (Dracocephalum moldavica L.) through foliar application of a nitrophenolate–based biostimulant. BIO Web Conf. 2018, 10, 01009. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Trivellini, A.; Ferrante, A. Effects of Two Doses of Organic Extract-Based Biostimulant on Greenhouse Lettuce Grown Under Increasing NaCl Concentrations. Front. Plant Sci. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Qiu, W.; Wang, Z.; Huang, C.; Chen, B.; Yang, R. Nitrate accumulation in leafy vegetables and its relationship with water. J. Soil Sci. Plant Nutr. 2014, 14, 761–768. [Google Scholar] [CrossRef]
- Bosnir, D.B.J.; Bevardi, M.; Boskovic, A.G.; Lasic, S.M.D.; Krivohlavek, A.; Racs, A.; Mojosovic-Cuic, A.; Trstenjak, N.U. Nitrate in Leafy Green Vegetables and Estimated Intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [Green Version]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The Effect of Biostimulant and Fertilizer on “Low Input” Lettuce Production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, E.; Disciglio, G.; Frabboni, L.; Libutti, A.; Gatta, G.; Gagliaridi, A.; Tarantino, A. Effects of Biostimulant Application on Quali-Quantitative Characteristics of Cauliflower, Pepper and Fennel Crops under Organic and Conventional Fertilization. WASET Int. J. Agric. Biosyst. Eng. 2015, 9, 712–716. [Google Scholar]
- Amanda, A.; Ferrante, A.; Valagussa, M.; Piaggesi, A. Effect of biostimulants on quality of baby leaf lettuce grown under plastic tunnel. Acta Hortic. 2009, 807, 407–412. [Google Scholar] [CrossRef]
- Turk, M.A.; Tawaha, A.M. Allelopathic effect of black mustard (Brassica nigra L.) on germination and growth of wild oat (Avena fatua L.). Crop Prot. 2003, 22, 673–677. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowsk, A.; Zakrzewska, W.; Oracz, K.; Gawroński, S.W. Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth. Biol. Plant. 2006, 50, 156–158. [Google Scholar] [CrossRef]
- Islam, A.; KatoNoguchi, H. Plant growth inhibitory activity of medicinal plant Hyptis suaveolens: Could allelopathy be a cause? Emirates J. Food Agric. 2013, 25, 692. [Google Scholar] [CrossRef] [Green Version]
- Baziar, M.R.; Farahvash, F.; Mirshekari, B.; Rashidi, V. Allelopathic effect of ryegrass (Lolium persicum) and wild mustard (Sinapis arvensis) on barley. Pak. J. Bot. 2014, 46, 2069–2075. [Google Scholar]
- Bhowmik, P.C. Inderjit Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003, 22, 661–671. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Findura, P.; Hara, P.; Szparaga, A.; Kocira, S.; Czerwińska, E.; Bartoš, P.; Nowak, J.; Treder, K. Evaluation of the Effects of Allelopathic Aqueous Plant Extracts, as Potential Preparations for Seed Dressing, on the Modulation of Cauliflower Seed Germination. Agriculture 2020, 10, 122. [Google Scholar] [CrossRef] [Green Version]
- Azania, A.A.P.M.; Azania, C.A.M.; Alves, P.L.C.A.; Palaniraj, R.; Kadian, H.S.; Sati, S.C.; Rawat, L.S.; Dahiya, D.S.; Narwal, S.S. Allelopathic plants. 7. Sunflower (Helianthus annuus L.). Allelopath. J. 2003, 11, 1–20. [Google Scholar]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. CRC Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Chon, S.-U.; Jang, H.-G.; Kim, D.-K.; Kim, Y.-M.; Boo, H.-O.; Kim, Y.-J. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic. 2005, 106, 309–317. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Tajik, H. Antioxidant and antibacterial activities of various extracts of borago officinalis flowers. J. Food Process. Preserv. 2012, 36, 539–544. [Google Scholar] [CrossRef]

| Treatment | Yield (g m−2) | Dry Matter (%) |
|---|---|---|
| Control | 2771 | 15 |
| FE (T1) | 2343 | 16 |
| FE (T2) | 2760 | 14 |
| FE (T3) | 2386 | 16 |
| FE (T4) | 1947 | 18 |
| LE (T1) | 3027 | 14 |
| LE (T2) | 2559 | 15 |
| LE (T3) | 2662 | 15 |
| LE (T4) | 2749 | 15 |
| Treatment | Phenol Index [Abs320 nm g−1] | Anthocyanin [Cyanidin eq. mg/100 g] |
|---|---|---|
| Control | 19.7 ± 0.66 b | 16.6 ± 0.35 b |
| FE (T1) | 24 ± 0.83 ab | 20.3 ± 0.77 ab |
| FE (T2) | 26.9 ± 0.17 a | 21.7 ± 0.25 a |
| FE (T3) | 20.2 ± 2.24 b | 18.2 ± 1.18 b |
| FE (T4) | 22.9 ± 1.20 ab | 19.7 ± 0.67 ab |
| LE (T1) | 22.6 ± 1.57 ab | 20 ± 1.25 ab |
| LE (T2) | 27.3 ± 1.88 a | 23.6 ± 1.64 a |
| LE (T3) | 21.6 ± 0.07 ab | 18.7 ± 0.16 b |
| LE (T4) | 22.5 ± 0.86 ab | 19.5 ± 0.35 ab |
| Treatment | Nitrate [mg kg−1 FW] | Sucrose [mg kg−1 FW] | Total Sugars [mg kg−1 FW] |
|---|---|---|---|
| Control | 6056.3 ± 673.67 ab | 414.3 ± 35.43 a | 2901.4 ± 199.14 |
| FE (T1) | 4509.9 ± 246.38 b | 303.5 ± 9.60 bcd | 1227.9 ± 345.42 |
| FE (T2) | 4842.8 ± 407.35 ab | 326.5 ± 14.38 bcd | 1787.4 ± 460.18 |
| FE (T3) | 4577.9 ± 207.07 b | 354.3 ± 13.73 ad | 1891.6 ± 485.37 |
| FE (T4) | 7550.8 ± 728.49 a | 318.8 ± 1.26 bcd | 2641.1 ± 288.75 |
| LE (T1) | 2764.0 ± 1335.32 b | 332.5 ± 4.68 bcd | 2151.3 ± 255.18 |
| LE (T2) | 4316.2 ± 159.04 b | 321.3 ± 3.15 bcd | 1372.2 ± 261.03 |
| LE (T3) | 4151.0 ± 976.44 b | 364.5 ± 11.22 ab | 1642.1 ± 114.20 |
| LE (T4) | 5236.6 ± 800.75 ab | 358.7 ± 5.31 ac | 2749.5 ± 703.27 |
| Treatment | Germination Percentage (%) |
|---|---|
| Control | 58.3 |
| FE (T1) | 0.0 |
| FE (T2) | 0.0 |
| FE (T3) | 0.0 |
| FE (T4) | 0.0 |
| LE (T1) | 25.0 |
| LE (T2) | 4.2 |
| LE (T3) | 0.0 |
| LE (T4) | 0.0 |
| FE (T5) | 33.3 |
| LE (T5) | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzoni, G.; Bulgari, R.; Ferrante, A. Maceration Time Affects the Efficacy of Borage Extracts as Potential Biostimulant on Rocket Salad. Agronomy 2021, 11, 2182. https://doi.org/10.3390/agronomy11112182
Franzoni G, Bulgari R, Ferrante A. Maceration Time Affects the Efficacy of Borage Extracts as Potential Biostimulant on Rocket Salad. Agronomy. 2021; 11(11):2182. https://doi.org/10.3390/agronomy11112182
Chicago/Turabian StyleFranzoni, Giulia, Roberta Bulgari, and Antonio Ferrante. 2021. "Maceration Time Affects the Efficacy of Borage Extracts as Potential Biostimulant on Rocket Salad" Agronomy 11, no. 11: 2182. https://doi.org/10.3390/agronomy11112182