Application of Barley Tweaky Spike Mutants for the Study of Effects of Plant Immunity-Related Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Mouldy Germinating Grain (MGG) Test
2.3. Identification of Internal Fungi in Barley Grains
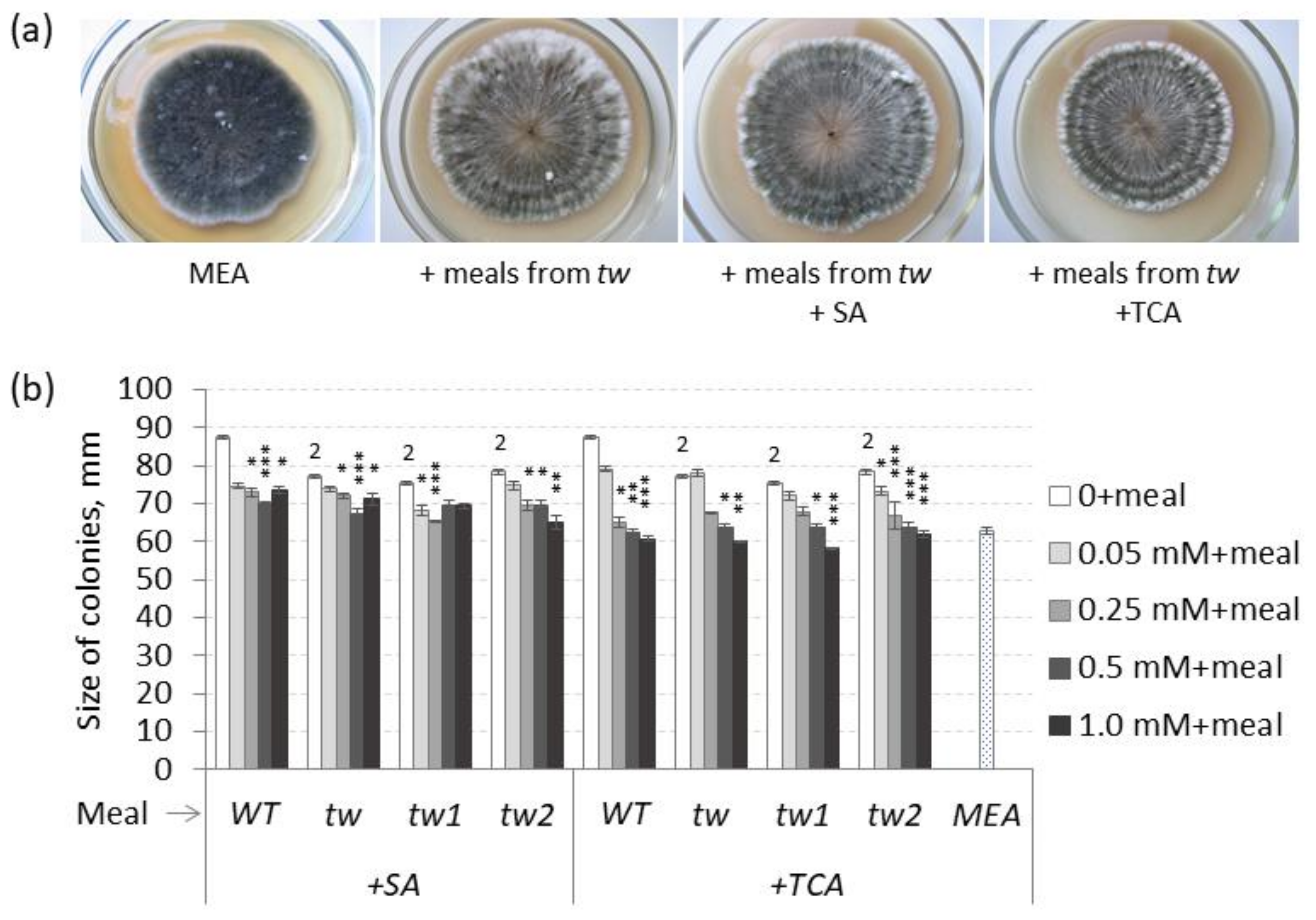
2.4. Investigation of Effects of Meal on B. sorokiniana Growth
2.5. Statistical Analysis
3. Results
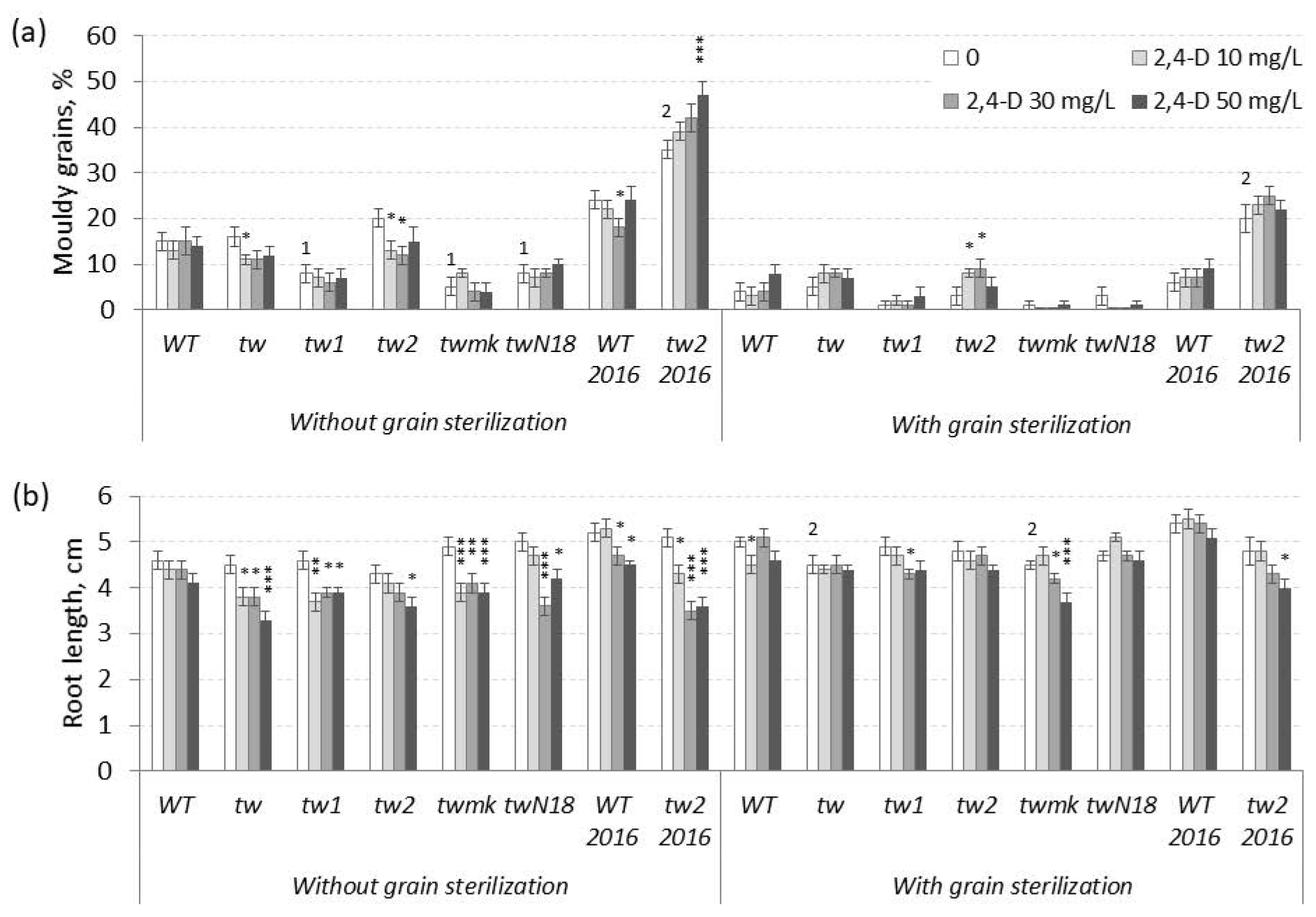
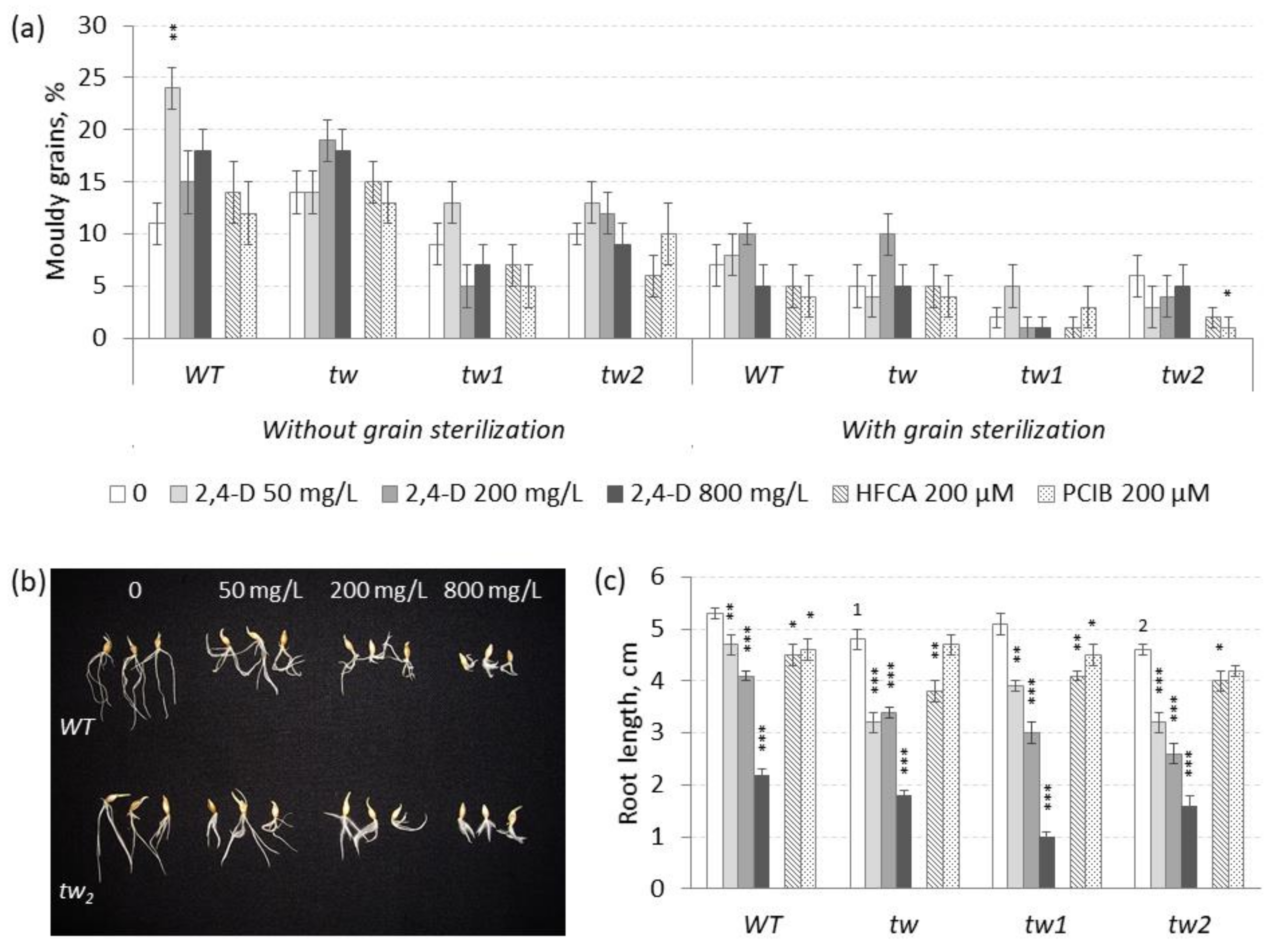
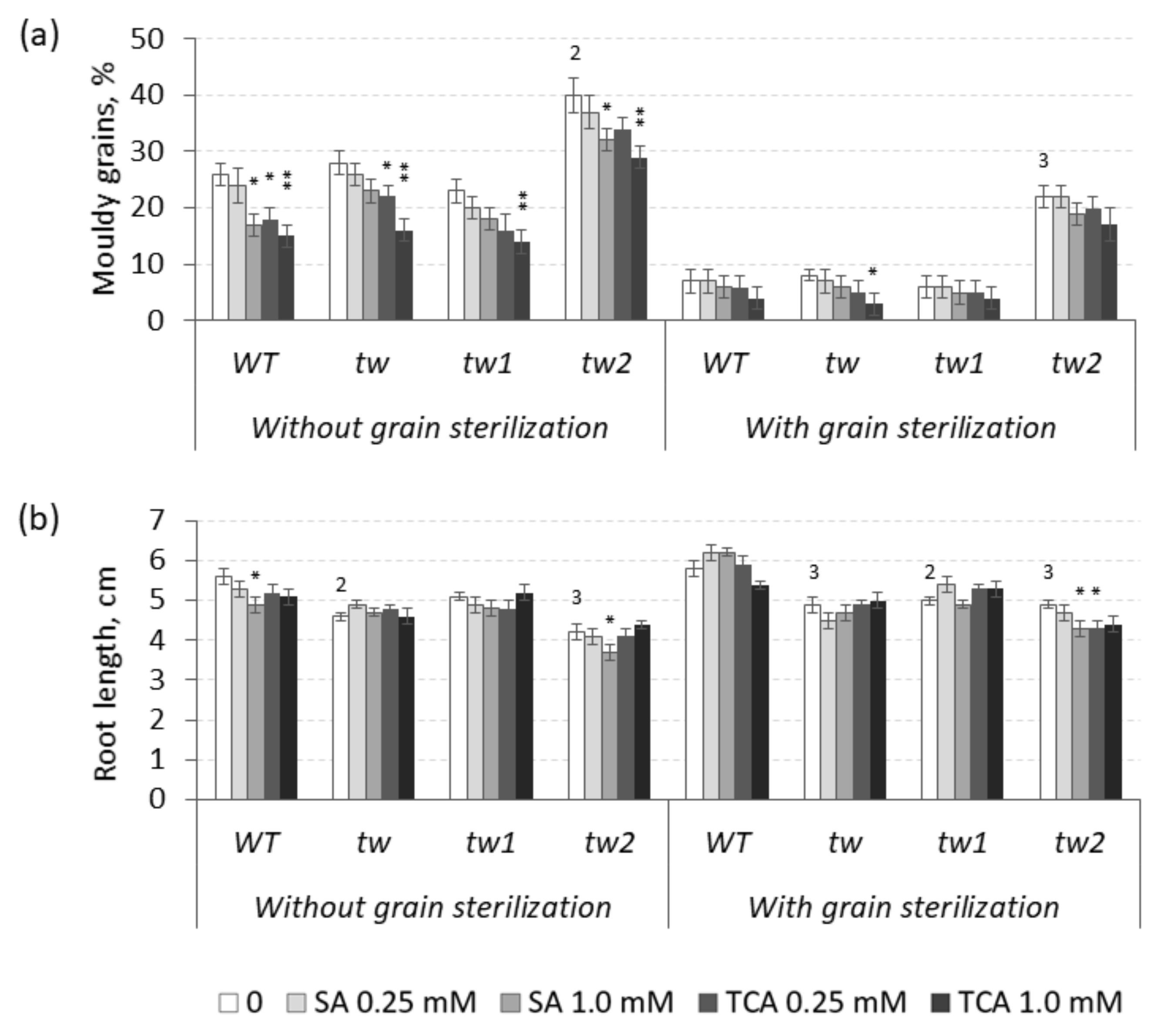
3.1. The Action of 2,4-D, SA and TCA on MGG and Root Growth of Allelic Tweaky Spike Mutants
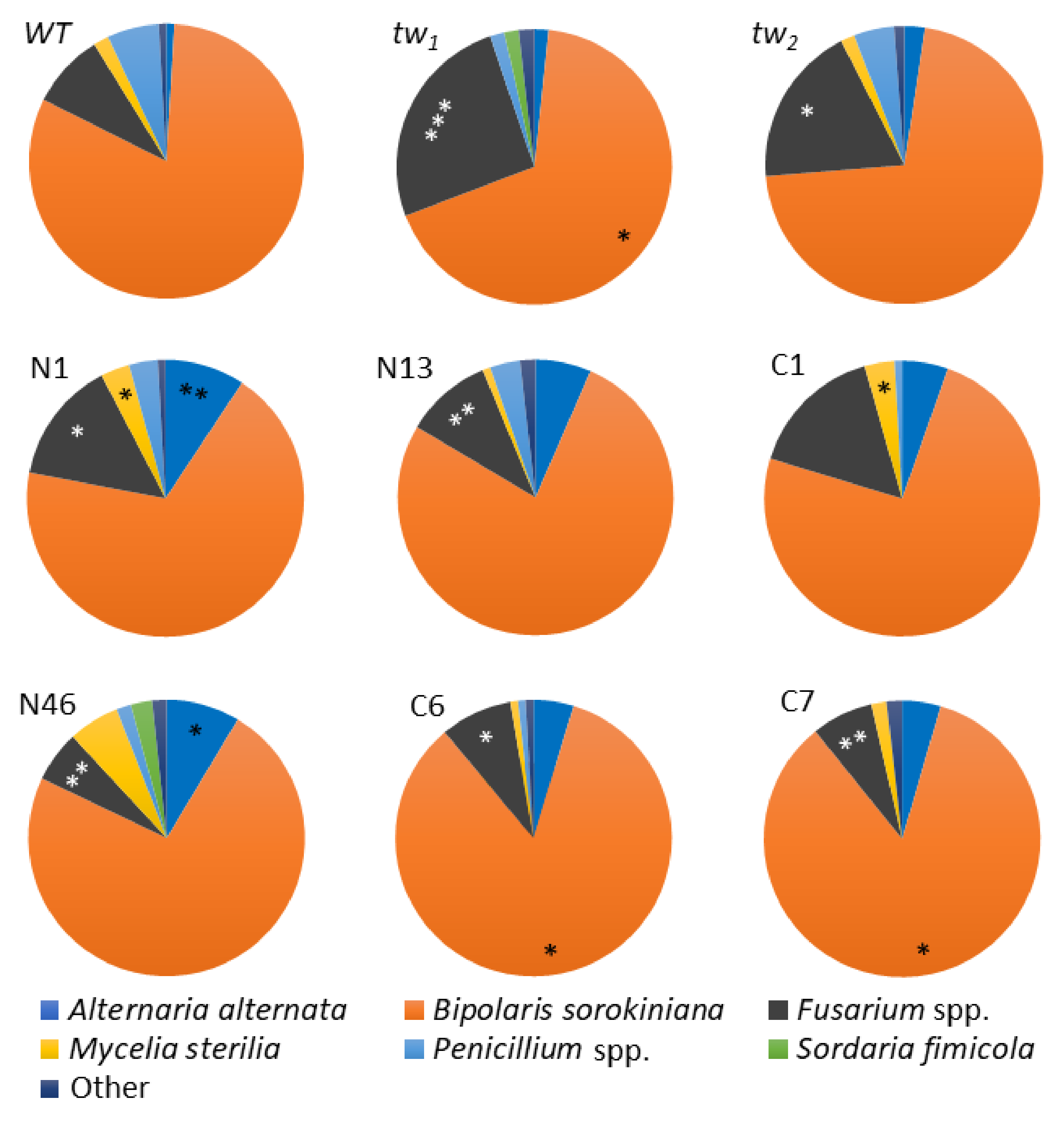
3.2. Fungi Spectrum in the Internal Grain Tissues of Barley tw-Type Mutants and Revertants
3.3. The Impact of Meals from Grains of tw-Type Mutants on the Colony Growth of Bipolaris sorokiniana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rančelis, V.; Žilinskaitė, S.; Vaitkūnienė, V.; Kačergius, A.; Kasparavičius, J. Immunodeficiency of barley allelic mutants ‘tweaky spike’.1. Susceptibility to Ustilago nuda (Jens.) Rostr. and Claviceps purpurea Tul. Biologija 1994, 4, 14–22. [Google Scholar]
- Mačkinaitė, R.; Kačergius, A.; Kasparavičius, J.; Balčiūnienė, L.; Vaitkūnienė, V.; Žilinskaitė, S.; Rančelis, V. Immunodeficiency of barley allelic mutants ‘tweaky spike’. II. Mildewed germinating grains and micromycetes. Biologija 1996, 4, 11–18. [Google Scholar]
- Assunção, R.; Silva, M.J.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef] [Green Version]
- Bengyella, L.; Iftikhar, S.; Nawaz, K.; Fonmboh, D.J.; Yekwa, E.L.; Jones, R.C.; Njanu, Y.M.T.; Roy, P. Biotechnological application of endophytic filamentous Bipolaris and Curvularia: A review on bioeconomy impact. World J. Microbiol. Biotechnol. 2019, 35, 69. [Google Scholar] [CrossRef] [PubMed]
- Janssen, E.M.; Liu, C.; Van der Fels-Klerx, H.J. Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J. 2018, 11, 33–46. [Google Scholar] [CrossRef]
- Šiukšta, R.; Vaitkūnienė, V.; Kaselytė, G.; Okockytė, V.; Žukauskaitė, J.; Žvingila, D.; Rančelis, V. Inherited phenotype instability of inflorescence and floral organ development in homeotic barley double mutants and its specific modification by auxin inhibitors and 2,4-D. Ann. Bot. 2015, 115, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šiukšta, R.; Vaitkūnienė, V.; Rančelis, V. Is auxin involved in the induction of genetic instability in barley homeotic double mutants? Planta 2018, 247, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Bacteria and fungi controlling plant growth by manipulating auxin: Balance between development and defense. J. Plant Physiol. 2015, 172, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Doohan, F.M.; Hodkinson, T.R. Persistent fungal root endophytes isolated from a wild barley species suppress seed-borne infections in a barley cultivar. Biocontrol 2015, 60, 281–292. [Google Scholar] [CrossRef]
- Qiang, X.; Ding, J.; Lin, W.; Li, Q.; Xu, C.; Zheng, Q.; Li, Y. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil 2019, 439, 373–391. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanclud, E.; Morel, J.B. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D. Pathological hormone imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Y.; Guo, Z. Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res. 2008, 18, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, B.N.; Kadoo, N.Y.; Dombrecht, B.; Tekeoglu, M.; Gardiner, D.M.; Thatcher, L.F.; Aitken, E.A.; Schenk, P.M.; Manners, J.M.; Kazan, K. Auxin signaling and transport promote susceptibility to the root-infecting fungal pathogen Fusarium oxysporum in Arabidopsis. Mol. Plant Microbe Interact. 2011, 24, 733–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheitz, K.; Lüthen, H.; Schenck, D. Rapid auxin-induced root growth inhibition requires the TIR and AFB auxin receptors. Planta 2013, 238, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Vaitkūnienė, V.; Čėsnienė, T.; Balčiūnienė, L.; Barysas, D.; Vaišnienė, V.; Rančelis, V. Immunodeficiency of barley allelic mutants tweaky spike. 4. Action of thiolic compounds and ascorbic acid on the frequency of mouldy grains. Biologija 1997, 2, 6–11. [Google Scholar]
- Vaitkūnienė, V.; Balčiūnienė, L.; Varnaitė, A.; Rančelis, V. Response of barley immunodeficient mutants tweaky spike to salicylic acid in field conditions. Biologija 2005, 2, 13–20. [Google Scholar]
- Vaitkūnienė, V.; Balčiūnienė, L.; Varnaitė, A.; Rančelis, V. Quality of seed material of barley tw type mutants according to susceptibility to micromycetes after treatment of previous generation with salicylic acid. Biologija 2005, 3, 41–45. [Google Scholar]
- Vaitkūnienė, V.; Varnaitė, A.; Balčiūnienė, L.; Rančelis, V.; Mačkinaitė, R.; Leistrumaitė, A. Two types of revertants from the same homeotic barley mutants tweaky spike. Biologija 2006, 2, 18–23. [Google Scholar]
- Vaitkūnienė, V.; Varnaitė, A.; Rančelis, V. Interaction of barley tweaky spike and laxatum mutations in F1 hybrids. Biologija 2004, 4, 10–15. [Google Scholar]
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi; Danish Government Institute of Seed Pathology for Developing Countries: Copenhagen, Denmark, 2003.
- Mačkinaitė, R. Internal mycobiota of wild and cultivated common caraway (Carum carvi L.) seeds. Zemdirbyste 2011, 98, 183–194. [Google Scholar]
- Aggarwal, R.; Gupta, S.; Banerjee, S.; Singh, V.B. Development of a SCAR marker for detection of Bipolaris sorokiniana causing spot blotch of wheat. Can J. Microbiol. 2011, 57, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Te Beest, D.E.; Paveley, N.D.; Shaw, M.W.; van den Bosch, F. Disease-weather relationships for powdery mildew and yellow rust on winter wheat. Phytopathology 2008, 98, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junk, J.; Kouadio, L.; Delfosse, P.; El Jarroudi, M. Effects of regional climate change on brown rust disease in winter wheat. Clim. Chang. 2016, 135, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The most important fungal diseases of cereals—Problems and possible solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Balčiūnienė, L.; Bieliūnienė, A.; Kazlauskaitė, D.; Vaitkūnienė, V.; Rančelis, V. Barley mutants with phenotypical gradient of spike development. Proc. Latv. Acad. Sci. B Nat. Exact Appl. Sci. 2001, 55, 207–211. [Google Scholar]
- Sauer, D.B.; Burroughs, R. Disinfection of seed surfaces with sodium hypochlorite. Phytopathology 1986, 76, 745–749. [Google Scholar] [CrossRef]
- Miché, L.; Balandreau, J. Effects of rice seed surface sterilization with hypochlorite on inoculated Burkholderia vietnamiensis. Appl. Environ. Microbiol. 2001, 67, 3046–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoudpour, Y.; Schmidt, M.; Calabrese, F.; Richnow, H.H.; Musat, N. High resolution microscopy to evaluate the efficiency of surface sterilization of Zea mays seeds. PLoS ONE 2020, 15, e0242247. [Google Scholar] [CrossRef]
- Stotz, H.U.; Jikumaru, Y.; Shimada, Y.; Sasaki, E.; Stingl, N.; Mueller, M.J.; Kamiya, Y. Jasmonate-dependent and COI1-independent defense responses against Sclerotinia sclerotiorum in Arabidopsis thaliana: Auxin is part of COI1-independent defense signaling. Plant Cell Physiol. 2011, 52, 1941–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorente, F.; Muskett, P.; Sánchez-Vallet, A.; López, G.; Ramos, B.; Sánchez-Rodríguez, C.; Jordá, L.; Parker, J.; Molina, A. Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol. Plant 2008, 1, 496–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meents, A.K.; Furch, A.; Almeida-Trapp, M.; Özyürek, S.; Scholz, S.S.; Kirbis, A.; Lenser, T.; Theißen, G.; Grabe, V.; Hansson, B.; et al. Beneficial and pathogenic Arabidopsis root-interacting fungi differently affect auxin levels and responsive genes during early infection. Front. Microbiol. 2019, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, X.; Fernando, W. Directing Trophic Divergence in Plant-Pathogen Interactions: Antagonistic Phytohormones with NO Doubt? Front. Plant Sci. 2020, 11, 600063. [Google Scholar] [CrossRef] [PubMed]
- Petti, C.; Reiber, K.; Ali, S.S.; Berney, M.; Doohan, F.M. Auxin as a player in the biocontrol of Fusarium head blight disease of barley and its potential as a disease control agent. BMC Plant Biol. 2012, 12, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, K.; Rocheleau, H.; Qi, P.F.; Zheng, Y.L.; Zhao, H.Y.; Ouellet, T. Indole-3-acetic acid in Fusarium graminearum: Identification of biosynthetic pathways and characterization of physiological effects. Fungal Biol. 2016, 120, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Schäfer, P.; Hückelhoven, R.; Langen, G.; Baltruschat, H.; Stein, E.; Nagarajan, S.; Kogel, K.H. Bipolaris sorokiniana, a cereal pathogen of global concern: Cytological and molecular approaches towards better control. Mol. Plant Pathol. 2002, 3, 185–195. [Google Scholar] [CrossRef]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant.Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, L. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P. Separate jasmonate-dependent and salicylate-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, D.; Li, B.; Zhang, B.; Miao, F.; Zhou, L. Bioactivity and structure-activity relationship of cinnamic acid esters and their derivatives as potential antifungal agents for plant protection. PLoS ONE 2017, 12, e0176189. [Google Scholar] [CrossRef]
- Steenackers, W.J.; Klíma, P.; Quareshy, M.; Cesarino, I.; Kumpf, R.P.; Corneillie, S.; Araújo, P.; Viaene, T.; Goeminne, G.; Nowack, M.K.; et al. Cis-cinnamic acid is a novel, natural auxin efflux inhibitor that promotes lateral root formation. Plant Physiol. 2016, 173, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Sooväli, P.; Koppel, M.; Kangor, T. Effectiveness of seed treatment against Fusarium spp. and Cochliobolus sativus of spring barley in different conditions. Agron. Res. 2018, 15, 280–287. [Google Scholar]
- Liatukas, Ž.; Supronienė, S.; Ruzgas, V.; Leistrumaitė, A. Effects of organic seed treatment methods on spring barley seed quality, crop, productivity and disease incidence. Zemdirbyste 2019, 106, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Chand, R.; Vasistha, N.K.; Pandey, S.P.; Kumar, U.; Mishra, V.K.; Joshi, A.K. Spot blotch disease of wheat: The current status of research on genetics and breeding. Plant. Pathol. 2018, 67, 508–531. [Google Scholar] [CrossRef]
- Lindsey, B.E.; Rivero, L.; Calhoun, C.S.; Grotewold, E.; Brkljacic, J. Standardized method for high-throughput sterilization of Arabidopsis seeds. J. Vis. Exp. 2017, 128, 56587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalewaja, J.D.; Woznica, Z.; Matysiak, R. 2,4-D amine antagonism by salts. Weed Technol. 1991, 5, 873–880. [Google Scholar] [CrossRef]
- Nalewaja, J.D.; Matysiak, R. 2,4-D and salt combinations affect glyphosate phytotoxicity. Weed Technol. 1992, 6, 322–327. [Google Scholar] [CrossRef]
| Year | Position in Spike | WT | tw | tw1 | tw2 | References |
|---|---|---|---|---|---|---|
| 1992 | Total u.s. | 25 ± 4 | 42 ± 2 3 | 40 ± 5 1 | 38 ± 5 1 | [4] |
| 1993 | Total u.s. | 19 ± 4 | 33 ± 2 2c | 21 ± 4 c | 45 ± 5 3c | |
| 1996 | Total u.s. | 11.0 ± 2.2 | - | 34.5 ± 3.4 3 | 28.5 ± 3.2 3 | [19] |
| 2002 | Total u.s. | 11.6 ± 2.3 | 18.0 ± 2.7 | 18.4 ± 2.8 | 19.2 ± 2.8 1 | [21] |
| 1999 | Total u.s. | 7.8 ± 1.0 | 18.7 ± 1.6 3 | 20.2 ± 2.9 2 | 17.0 ± 1.5 3 | [30] |
| 2012 | Total u.s. | 34.5 ± 2.9 | - | 32.0 ± 2.6 c | 48.0 ± 2.5 3c | Unpublished data |
| 2013 | Total u.s. | 11 ± 2 | 14 ± 2 | 9 ± 2 | 10 ± 1 | Present study |
| 2013 | Total st. | 7 ± 2 | 5 ± 2 | 2 ± 1 | 6 ± 2 | [30] |
| 2013 | Total u.s. | 15 ± 2 | 16 ± 2 | 8 ± 2 1a | 20 ± 2 b | Unpublished data |
| 2013 | Total st. | 4 ± 2 | 5 ± 2 | 1 ± 1 | 3 ± 2 | Present study |
| 2016 | Total u.s. | 24 ± 4 | - | - | 35 ± 2 2 | |
| 2016 | Total st. | 6 ± 2 | - | - | 20 ± 3 2 | |
| 2017 | Total u.s. | 6 ± 2 | 13 ± 2 1a | - | 19 ± 2 3a | |
| 2018 | Total u.s. | 8 ± 2 | - | 17 ± 2 2 | 22 ± 2 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šiukšta, R.; Vaitkūnienė, V.; Mačkinaitė, R.; Rančelis, V. Application of Barley Tweaky Spike Mutants for the Study of Effects of Plant Immunity-Related Substances. Agronomy 2021, 11, 2180. https://doi.org/10.3390/agronomy11112180
Šiukšta R, Vaitkūnienė V, Mačkinaitė R, Rančelis V. Application of Barley Tweaky Spike Mutants for the Study of Effects of Plant Immunity-Related Substances. Agronomy. 2021; 11(11):2180. https://doi.org/10.3390/agronomy11112180
Chicago/Turabian StyleŠiukšta, Raimondas, Virginija Vaitkūnienė, Rimutė Mačkinaitė, and Vytautas Rančelis. 2021. "Application of Barley Tweaky Spike Mutants for the Study of Effects of Plant Immunity-Related Substances" Agronomy 11, no. 11: 2180. https://doi.org/10.3390/agronomy11112180
APA StyleŠiukšta, R., Vaitkūnienė, V., Mačkinaitė, R., & Rančelis, V. (2021). Application of Barley Tweaky Spike Mutants for the Study of Effects of Plant Immunity-Related Substances. Agronomy, 11(11), 2180. https://doi.org/10.3390/agronomy11112180







