Abstract
Wild lowbush blueberry fields are characterized by high genetic diversity, with a large number of genotypes coexisting in every field. Yield also varies among genotypes, which could be related to the variation in physiological and structural traits, but this has not been rigorously tested. In this study, we aimed to quantify the inter-genotype variation in yield, as well as leaf and stem functional traits, and to establish the relationship between functional traits and yield-related traits in wild blueberries. To do so, we carried out a study during the 2019 harvest season measuring structural and functional traits including stem number, stem length, stem diameter, leaf chlorophyll concentration, leaf mass area, leaf area per stem, leaf number per stem, number of branches per stem, leaf temperature, soil temperature, and soil water content and yield data including yield, berry size (weight of 100 berries), number of berries per stem, and length of berry cluster from two wild blueberry farms. We found high variations in structural, functional, and yield-related traits among genotypes, but not between two fields. We also found negative associations of the leaf mass per unit area and midday leaf temperature with the yield, whereas the leaf chlorophyll concentration was positively associated with the yield. Additionally, we found a quadratic relationship between yield-related traits (weight of 100 berries, number of berries per stem, and length of berry cluster) and stem length, with the optimum stem length for yield at 25 cm. Our results suggest that several leaf and stem functional traits are related with yield-related traits; thereby, those traits can be used to predict wild blueberry yields. Our findings could help growers and breeders select better-yielding genotypes based on structural and functional traits.
1. Introduction
Wild blueberry (Vaccinium angustifolium Aiton) is an important crop of Maine, USA, Quebec, and maritime Canada. It mostly reproduces by obligate cross-pollination [1]. Each seed gives rise to a genetically unique plant, which expands horizontally in the field via the vegetative (clonal) growth of rhizomes and forms a patch of connected aboveground stems [2]. A patch of stems with identical genetic information is called a genotype or clone with a different genetic make-up than other wild blueberry genotypes [3]. Among genotypes, there are significant differences in morphology, structural and functional traits, physiological performance, and yield [4,5,6]. Crop yield is regulated by several genes named quantitative trait loci and is also influenced by external environmental factors [7]. Yield is also indirectly determined by several morphological and physiological traits [7]. An optimal plant architecture is beneficial for higher yields [8]. In wild blueberries, the high intergenotype variations in structural and physiological traits can indirectly impact the overall yield of wild blueberries, but the associations have not been established.
The yield of crops is strongly associated with and dependent on plant growth and development. Plant architecture, leaf structure, and vascular architecture are major developmental features of plants [9]. Plant functional traits can directly reflect the physiological performance of a plant and its response to environmental change and stress [10]. Many studies have shown that leaf features and plant architectural traits can predict plant growth and performance as the quantity of light interception, photosynthetic capacity, and the source energy of plants are determined by several functional traits [9,11]. Also, the mobilization of photosynthates from source to sink, which is related to vascular architecture, is crucial for the efficient partitioning of photo-assimilated carbon [9]. Thus, these features can be considered the parts of a developmental module that dictates crop performance and yield; the optimization of these developmental features is essential for the efficient performance of crop plants. Genotypes of wild blueberries with a greater vegetative biomass, which is represented by several structural traits, i.e., stem height and number of leaves, can produce more flowers, which ultimately results in higher fruit biomass [12]. Plant stem height and diameter are closely related to biomass production and are important morphological traits affecting yield performance [10]. Plants with a higher stem height and larger diameter stems normally have larger diameter vessels, leading to a higher hydraulic conductance [13]. A higher hydraulic conductance facilitates higher stomatal conductance, leading to more photosynthetic carbon gain [14]. Another critical factor in hydraulic conductance is leaf vein density [15]. High leaf vein density leads to higher photosynthetic and growth rates by providing a faster water supply [16]. However, with increasing height, the risk of xylem cavitation also increases, leading to more vulnerability to drought compared to shorter plants [17,18]. Wild blueberry plants with a higher stem height might also be more prone to winter wind damage and could result in reduced yield. That is why an optimal architecture is required to achieve a high yield [8], and an understanding of the optimal architecture for the wild blueberry crop could aid in the selection process of cultivar development.
Physiological traits of crops determine the health of plants and correlate with yield. Crop yield can be increased by (i) increased photosynthesis per unit land area, which can be achieved by effective agronomic practices such as irrigation, fertilization, and pollination and (ii) the increased partitioning of crop biomass to the harvested product, which can be achieved by plant breeding [19]. Leaf chlorophyll concentration (LCC) is a good indicator of leaf photosynthetic capacity and is positively correlated with yield [20]. In wheat, researchers have found positive correlations of yield with the rate of CO₂ fixation, LCC, and stomatal conductance and a negative correlation with the loss of chlorophyll [21]. Understanding the effects of LCC on wild blueberry yield can explain the soil nutrient availability in a field and the uptake of nutrients because LCC production in leaves depends on the nitrogen and moisture availability in the soil [22]. Understanding the relationships between LCC and yield can also help facilitate the selection of high-yielding genotypes. Plant canopy temperature is related to stomatal conductance and water stress [21]. A negative correlation between yield and canopy temperature was found in wheat [21]. Canopy temperature can be used to assess the health condition status of a plant and allow an assessment of the current on-farm water use efficiency and the development of mitigation strategies.
Plant breeding can increase crop yield by achieving a high-rate partitioning of crop biomass to the harvested product [19]. However, the breeding of wild blueberry is complicated due to the characteristics of heterozygosity, ploidy, complex heritability of QTLs, and long timeframe for establishing mature fruit-bearing progeny [4]. Hall (1983) noted improvements in wild blueberry cultivars in terms of size and yield resulting from a plant breeding program [23]. Researchers also found heritable genetic components linked to wild blueberry yield [24]. Griffing (1956) also demonstrated evidence of phenotypic selections that resulted in genetically advanced inbred lines [25]. The overall productivity of blueberry fields can be improved by increasing blueberry cover in between inter-genotype empty spaces in existing fields and replacing the less productive genotypes with high-yielding genotypes [26]. Understanding the direction and strength of the relationships between yield-related traits and functional traits is very important to improve the efficiency of genetic selection in plant breeding programs [27]. Studies have already quantified associations between yield-related traits and functional traits in different crops [20,27,28,29], but these associations have not been quantified in the wild blueberry.
To do so, the objectives of this research were to (1) quantify the variation of major leaf and stem functional traits and yield-related traits among selected genotypes and between fields of wild blueberries, and (2) establish relationships between plant functional traits and yield-related traits in wild blueberries. This study will help to clarify the magnitudes and directions of correlations among functional traits and yield-related traits in wild blueberries. This can also be helpful for both growers and breeders in selecting better yielding genotypes based on structural and functional traits.
2. Materials and Methods
2.1. Study Sites and Plant Materials
The study sites were located in Washington County in the Downeast coastal region of Maine, USA. One site was located at the University of Maine Blueberry Hill Farm (BBHF) in Jonesboro (Latitude: 67°38′53″ W, Longitude: 44°38′44″ N), Maine, USA, and another site was located at Jasper Wyman & Son (Wyman’s) blueberry farm (Latitude: 67°59′58″ W, Longitude: 44°44′07″ N) in Deblois, ME, USA. Soils at both sites were well-drained acidic sandy loam with 0–3% slope. The Downeast coastal region of Maine has a temperate climate with an average low of −10.6 °C and a high of 24.2 °C, and a monthly average precipitation low of 85.1 mm and high of 136.4 mm. Wild blueberries are managed on a two-year cycle; in prune years, plants are pruned and grow vegetatively, and in harvest years, the plants flower and produce a fruit crop. The study was conducted in 2019, which was a crop or harvest year for both fields. Wild blueberry fields contain numerous diverse genotypes [30]; 30 genotypes were selected from BBHF, and 15 genotypes were selected from Wyman’s based on their morphological and phenological differences to include a high variety of genotypes. Different genotypes could be easily identified visually based on morphological and phenological differences including leaf color, stem color, berry density, berry color (Figure 1), as well as phenology.

Figure 1.
High variations in leaf color, stem height, berry density, and berry color among genotypes in a wild blueberry field at the Wyman’s Farm, Deblois, ME, USA. Different genotypes form patches connecting each other in the field. The photo was taken during the fruit production stage by Dr. Xiaoxue Mo.
2.1.1. Leaf and Stem Structural Traits
Six wild blueberry shoots were arbitrarily selected from each randomly selected genotype of the BBHF and Wyman’s fields in August 2019 during the harvest season. Structural traits including stem length and diameter, leaf number, as well as total leaf area and dry leaf biomass in a stem were measured. A caliper was used to measure stem length (from the soil line) and diameter. Leaf area was determined using an LI-3000A area meter (Li-Cor, Lincoln, NE, USA), after which leaves were oven-dried at 70 °C to constant mass and weighed. Leaf mass per area (LMA) was determined as leaf dry mass divided by leaf area (g/m2).
2.1.2. Leaf Chlorophyll Concentrations
Six wild blueberry stems were arbitrarily selected from each genotype to measure leaf chlorophyll concentration (LCC). Chlorophyll concentration per unit leaf area was measured in the field with a SPAD Chlorophyll Meter (SPAD 502; Minolta Corp., Osaka, Japan) on samples from the Wyman’s Farm and with an atLEAF Digital Chlorophyll Meter (FT Green LLC, Wilmington, DE, USA) on samples from BBHF. Both the SPAD and atLeaf data were converted to values for LCC (µg/cm2) by the formula relating SPAD/atLEAF values and laboratory-measured chlorophyll values using chemical extraction methods [31].
2.1.3. Leaf Temperature, Soil Temperature, and Soil Water Content
Leaf temperature (LeafT) was measured in the field by using a Fluke 62 Max+ handheld infrared thermometer (Fluke Corporation, Everett, WA, USA) on four arbitrarily selected leaves from each genotype. Soil temperature (SoilT) and soil water content (SWC) were measured in the field by a Fieldscout TDR 150 Soil Moisture Meter (Spectrum Technologies Inc., Aurora, IL, USA) from four random places within the boundaries of each genotype. These measurements were taken during midday (12:00 to 14:00) on 4 August 2019, a sunny day, and the leaves were exposed to direct sunlight. The measurements were carried out under similar conditions for all genotypes. These measurements were only taken at Wyman’s Farm due to limited resources and the time taken to measure each genotype.
2.1.4. Yield and Yield-Related Traits
Yield and yield-related traits were quantified by establishing a 0.3 by 0.3-m quadrat within each genotype (Table 1). A hand rake was used to collect the fruit sample within each quadrat and then weighed on a portable balance in the field to obtain the total fruit yield (g) per quadrat. The weight (g) of 100 berries (WT) from each quadrat was also quantified as a measure of berry size. The length of berry cluster (LBC) in stem (cm) and the number of berries per stem (NBS) were quantified in the field by selecting six stems in the quadrat. All measurements were conducted at both research sites.

Table 1.
List of yield-related, morphological, and functional traits used in this study along with their abbreviations and units.
2.2. Calculation for Estimating Optimal Sample Size of Genotypes to Estimate Yield
Using estimates of variance from the genotype yield data collected in the two fields and two levels of precision (SE/mean ratios of 0.10 and 0.25), the optimal sample size of genotypes for estimating yield was calculated using the formula developed by Cochran (1977) [32]:
where N = optimal sample size for a given level of precision; t(0.05) is 1.96 or the t-value for = 0.05 when n approaches ∞; S2 = estimated variance of yield/genotype; m = mean yield/genotype; and P = level of precision defined as the proportion of the standard error/mean.
N = (t(0.05) × S2/(m2 × P2)
The use of the t-value in the formula estimates the optimal sample size for a 95% likelihood of obtaining the desired precision in yield for the calculated number of genotypes required.
2.3. Data Analyses
Statistical analyses were conducted using SPSS v23 (IBM Corp., Armonk, NY, USA), JMP v15 (SAS Institute Inc., Cary, NC, USA), and RStudio software (RStudio, PBC, Vienna, Austria). The truncated gaussian kernel density estimation was performed using the ggplot package in RStudio. To determine trait differences between the two fields and genotypes within fields, we used a hierarchical nested mixed model with the field as the fixed effect and the genotype nested within the field as the random effect (α = 0.05). Harrison et al. [33] suggested this approach for split-plot designs (genotypes within fields). The statistical software JMP version 15 was used to fit the model with the restricted maximum likelihood. The Pearson correlation analysis was conducted using the corrplot package in RStudio at an alpha (α) level of 0.05 and a 95% confidence interval. The structural and functional traits were averaged within genotypes for a regression analysis using SPSS. We analyzed the relationship between functional traits using linear (in the form of a + bx) or quadratic (in the form a + bx + cx2) regressions according to which best approximated the structure of the relationship. We determined the statistical significance of the relationship using the coefficient of determination and its significance (α) at p < 0.05. With the mean trait values of each genotype, a Principal Component Analysis (PCA) was employed to characterize the variance of structural and functional traits for the two examined fields in RStudio. Multiple linear regression analysis was conducted in RStudio to test the overall contribution of several functional traits (StemL, LMA, and LCC) on the yield for studied fields. We used a generalized linear model with the Gaussian or Normal distribution and the identity linkage function. The model was fit using maximum likelihood.
3. Results
3.1. Estimation of Optimal Sample Size of Genotypes to Estimate Yield
Based upon the average yields in the two fields, we estimated that 31 genotypes should be sampled if it is desired to estimate the yield of a field with a standard error\mean ratio precision of 0.25. If higher precisions are desired, the necessary sample sizes increase geometrically: 196 genotypes for a precision of 0.10 and 784 genotypes for a precision of 0.05. However, if one is willing to sacrifice 95% confidence in estimating yield with a given precision, then 16, 100, and 400 genotypes would need to be sampled to estimate yield with precisions of 0.25, 0.10, and 0.05, respectively.
3.2. Variation in Yield, Structural and Functional Traits between Wyman’s and BBHF Blueberry Fields and among the Genotypes
We found that both Wyman’s and BBHF fields had yield distributions that were heavily skewed toward the yield range 2200–2500 (g/m2) (Figure 2). Though the average yield in the BBHF field appeared higher compared to the Wyman’s field yield (Figure 2), the difference was not statistically significant (Table 2).
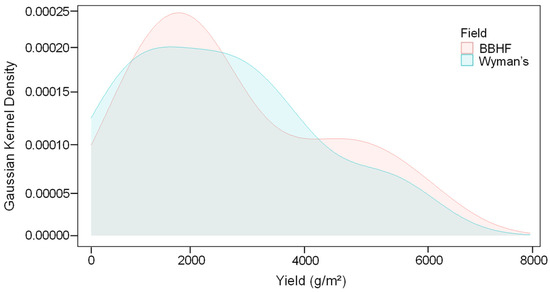
Figure 2.
Gaussian kernel density estimates of the wild blueberry yield (g/m2) for the two studied wild blueberry fields, Wyman’s and BBHF.

Table 2.
Comparison between Jasper Wyman & Son Blueberry Farm (Wyman’s) and from Blueberry Hill Farm (BBHF) blueberry fields in yield-related, morphological, and functional traits in the minimum (Min), maximum (Max), mean, and standard deviation (SD) of Yield, WT, NBS, LBC, StemN, StemL, StemD, LCC, LMA, LAPS, LNPS, NBPS, LeafT, SoilT, and SWC. To determine trait differences between the two fields and genotypes within fields, we used a hierarchical nested mixed model with field as the fixed effect and genotype nested with-in field as the random effect. LeafT, SoilT, and SWC data were only available for the Wyman’s field. For definitions of trait abbreviations, please see Table 1.
We found a high variation in the observed yields, structural and functional traits among genotypes, and within and between fields (Table 2). The yield varied by 46-fold, ranging from 122.22 to 5577.7 g/m2 in the Wyman’s field. The yield varied by 36-fold, ranging from 177.77 to 6355.5 g/m2 in the BBHF field. The weight of 100 berries varied by 1.7-fold, ranging from 40 to 69 g in the Wyman’s field, and varied by 4-fold, ranging from 22 to 88 g in the BBHF field. The number of berries on a stem varied by 24.7-fold, ranging from 0.8 to 20.5 in the Wyman’s field, and varied by 14.3-fold, ranging from 1.9 to 26.7 in the BBHF field. The length of a berry cluster varied by 12.8-fold, ranging from 0.5 to 5.8 cm in the Wyman’s field, and varied by 6.8-fold, ranging from 0.6 to 4.0 cm in the BBHF field. Stem length varied by 1.9-fold, ranging from 14.7 to 28.3 cm in the Wyman’s field, and varied by 2.3-fold, ranging from 10.7 to 24.3 cm in the BBHF field. Leaf mass area varied by 1.3-fold, ranging from 64.5 to 82.9 g/m2 in the Wyman’s field, and varied by 1.3-fold, ranging from 60.9 to 81.0 g/m2 in the BBHF field. Leaf chlorophyll concentration (LCC) varied by 3.4-fold, ranging from 0.1 to 0.3 μg/cm2 in the Wyman’s field, and varied by 3.4-fold, ranging from 0.1 to 0.3 μg/cm2 in the BBHF field. Leaf canopy temperature varied by 1.3-fold, ranging from 25.4 to 32.3 °C in the Wyman’s field.
When comparing between the fields, we did not find any significant differences between Wyman’s and BBHF in yield-related traits: yield (g/m2), weight of 100 berries (WT), number of berries on a stem (NBS), and the length of a berry cluster (LBC) (Table 2). We found significant differences between the two fields for stem structural traits including the stem number per plot (StemN) and stem length (StemL) but not for the stem diameter (StemD). Both total StemN and mean StemL were higher in the Wyman’s field compared to the BBHF field (Table 2). In terms of leaf structural and functional traits, a significant difference was only found for leaf number per stem (LNPS), and mean LNPS was higher in the Wyman’s field.
When comparing among genotypes of each field, significant differences in yield-related traits; NBS and LBC were found among the genotypes of the Wyman’s field as well as the BBHF (Table 2). We found significant differences among genotypes for stem structural traits including stem length (StemL), and stem diameter (StemD). In terms of leaf structural and functional traits, we found significant differences among genotypes LCC and leaf area per stem (LAPS) but not between the Wyman’s and BBHF fields. The same pattern was found for the number of branches/stem (NBPS). The LNPS was found significantly different between fields as well as among the genotypes in those fields. We did not find any significant difference in leaf mass per area (LMA) between the fields or among the genotypes in those fields. Significant differences among genotypes in Wyman’s field were also found with water condition, leaf temperature (LeafT), soil temperature (SoilT), and soil water content (SWC).
3.3. Structural and Functional Traits in Relation to Yield and Yield Related Traits of Wild Blueberry Fields
We used Pearson correlation as well as bivariate linear and quadratic regression relationships to determine if different structural and functional traits can be used to predict yield and yield-related traits WT, NBS, and LBC for the wild blueberries. We found relationships of yield with the LMA, LCC, and LeafT, but the relationships were not always consistent within and across fields (Figure 3 and Figure 4). We found significant negative linear relationships between the yield and LMA for the Wyman’s field (Figure 4a: R2 = 0.41, p < 0.05) as well as combining the data of the two fields (Figure 4c: R2 = 0.18, p < 0.05), but not for the BBHF field. We also found significant quadratic relationships for Wyman’s field (Figure 4a: R2 = 0.37, p < 0.05) as well as for the combined field data (Figure 4c: R2 = 0.10, p < 0.05), whereas the quadratic relationships were not significant for the BBHF field (Figure 4b: R2 = 0.07, p = 0.16). However, coefficients of determination (R2) were higher for the linear relationships compared to quadratic relationships between yield and LMA. When we analyzed the relationship between LCC and yield, we did not find any significant linear or quadratic relationships for Wyman’s field or the BBHF field. When the data for the two fields were combined, we found a borderline significant quadratic relationship between LCC and yield (Figure 4f: R2 = 0.11, p = 0.08). We also found significant linear (Figure 4g: R2 = 0.42, p < 0.01) and quadratic relationships (Figure 4g: R2 = 0.42, p < 0.05) when we analyzed the relationship between LeafT and Yield (g/m2).
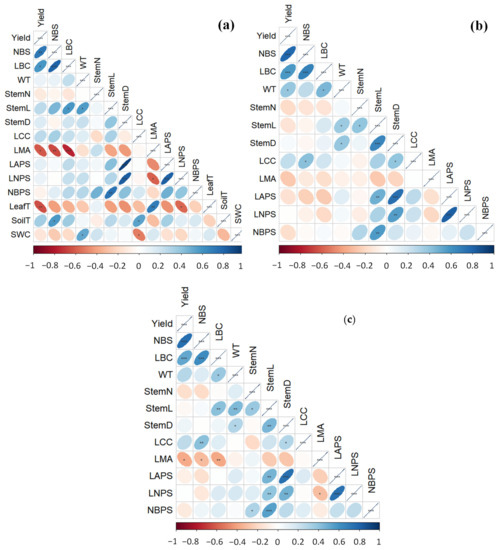
Figure 3.
Pearson correlation heat map of the structural, functional, and yield-related traits of wild blueberries for (a) Wyman’s field, (b) BBHF field, and (c) the combined data from both fields. The horizontal axis represents the degree and direction of Pearson correlation r. The sign of the significance for each correlation is shown as ***, p < 0.001; **, p < 0.01; *, p < 0.05. For definitions of trait abbreviations, please see Table 1. LeafT, SoilT, and SWC data were only available for the Wyman’s field.
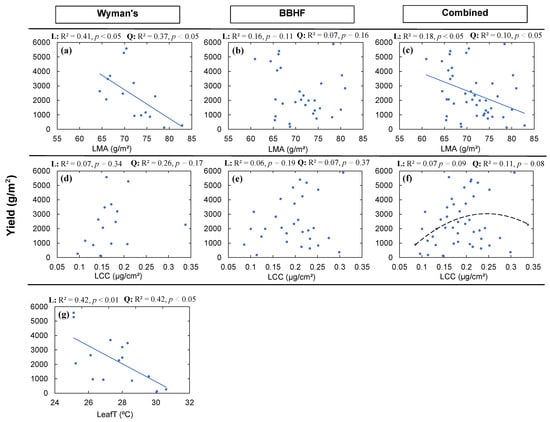
Figure 4.
Yield (g/m2) of wild blueberry fields in relation to leaf mass per area (LMA) (a–c); leaf chlorophyll concentration(LCC) (d–f) and leaf temperature (LeafT) (g). The solid lines indicate significant (p < 0.05) linear relationships, and black lines indicate significant (p < 0.05) quadratic relationships. Dashed lines indicate marginally significant (p < 0.10) relationships. Here among all the structural and functional traits, only significant or marginally significant relationships to yield were plotted. The strongest relationship was chosen by the high level of significance (p-value) and higher coefficient of determination (R2). For definitions of trait abbreviations, please see Table 1.
We found relationships of the average number of berries (NBS) with StemL, LCC, LMA, LAPS, and SoilT, but they were not consistent within and across fields (Figure 3 and Figure 5). We found a marginally significant linear (Figure 5a: R2 = 0.21, p = 0.08) and quadratic (Figure 5a: R2 = 0.33, p = 0.08) relationship between NBS and StemL among the genotypes in the Wyman’s field. Significant relationships were absent for the BBHF field and when the two fields were combined. We did not find any significant linear or quadratic relationships between LCC and NBS for Wyman’s wild blueberry field (Figure 5d) but found significant positive linear relationships for the BBHF field (Figure 5e: R2 = 0.17, p < 0.05) and combined data (Figure 5f: R2 = 0.17, p < 0.05). For the combined data, we also found a significant quadratic relationship (Figure 5f: R2 = 0.21, p < 0.01), but for the BBHF field, the quadratic relationship was borderline significant (Figure 5e: R2 = 0.18, p = 0.07). We found significant negative linear relationships between NBS and LMA for Wyman’s field (Figure 5g: R2 = 0.43, p < 0.01) and the combining data for the two fields (Figure 5i: R2 = 0.09, p < 0.05), but no significant relationship for the BBHF field. While comparing the leaf structural trait average leaf area per stem (LAPS) with NBS, only a significant quadratic relationship was found for the BBHF field (Figure 5k: R2 = 0.20, p < 0.05). We also found significant linear (Figure 5m: R2 = 0.35, p < 0.05) and quadratic relationships (Figure 5m: R2 = 0.33, p < 0.05) between soil temperature (SoilT) and NBS; however, we found a higher coefficient of determination (R2) associated with the linear relationship.
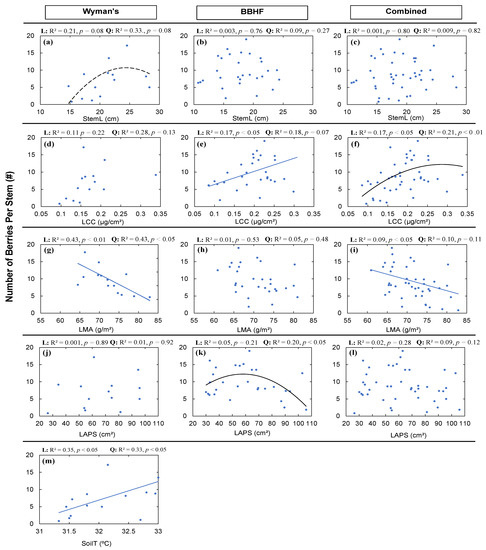
Figure 5.
Average number of berries per stem (NBS) in wild blueberry fields in relation to average stem length (StemL) (a–c); average leaf chlorophyll concentration (LCC) (d–f); average leaf mass per area (LMA) (g–i); average leaf area per stem (LAPS) (j–l); and average soil temperature (SoilT) (m). The solid blue lines indicate significant (p < 0.05) linear relationships, and black lines indicate significant (p < 0.05) quadratic relationships. Dashed lines indicate marginally significant (p < 0.10) relationships. Here, among all the structural and functional traits, only significant or marginally significant relationships to NBS were plotted. The strongest relationship was selected from the high level of significance (p-value) and higher coefficient of determination (R2).
The length of a berry cluster in stem (LBC) was significantly related to StemL, LMA, and LeafT. However, relationships within and across fields were not consistent (Figure 3 and Figure 6). This was also the case for yield (Figure 3) and NBS (Figure 5). We found a significant positive linear (Figure 6a: R2 = 0.34, p < 0.05) and marginally significant quadratic (Figure 6a: R2 = 0.35, p = 0.07) relationship of LBC with the StemL among the genotypes in the Wyman’s field, whereas significant relationships were absent for the BBHF field. However, we found significant linear and quadratic relationships (Figure 6c) for the combined data from the two fields. The relationships of LMA with the LBC were similar to what we found for NBS (Figure 6d–f). We found significant negative linear and quadratic relationships between LBC and LMA for the Wyman’s field (Figure 6d: R2 = 0.58, p < 0.001) and for the combined data of the two fields (Figure 6g: R2 = 0.15, p < 0.05). No significant relationship was found for the BBHF field. A quadratic relationship was found between LeafT and LBC (Figure 6g: R2 = 0.38, p < 0.05).
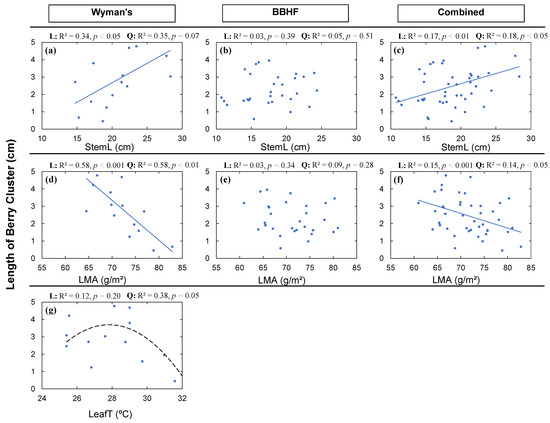
Figure 6.
Average length of berry cluster (LBC) in wild blueberry fields in relation to average stem length (StemL) (a–c); average leaf mass per area (LMA) (d–f); and average leaf temperature (LeafT) (g). The solid blue lines indicate significant (p < 0.05) linear relationships, and black lines indicate significant (p < 0.05) quadratic relationships. Dashed lines indicate marginally significant (p < 0.10) relationships. Here, among all the structural and functional traits, only significant or marginally significant relationships to LBC were plotted. The strongest relationship was selected from the high level of significance (p-value) and higher coefficient of determination (R2).
Berry size, indicated by the weight of 100 berries (WT), was found to be related to StemL, StemD, and SWC. These relationships were consistent among fields for StemL and WT (Figure 7a–c), but not for StemD (Figure 7d–f). We found a significant positive linear (Figure 7a: R2 = 0.32, p < 0.05) and marginally significant quadratic (Figure 7a: R2 = 0.32, p = 0.10) relationship for WT with StemL among genotypes in the Wyman’s field, whereas for the BBHF field, we found no significant relationships for the BBHF field (Figure 7b) or for the combined data (Figure 7c). We found significant positive linear and quadratic relationships (Figure 7e) for the BBHF field as well as for the combined data of two fields between StemD and WT, but no relationship was found for the Wyman’s field. We also found a significant increase in WT with an increase in SWC (Figure 7g: R2 = 0.27, p < 0.05).
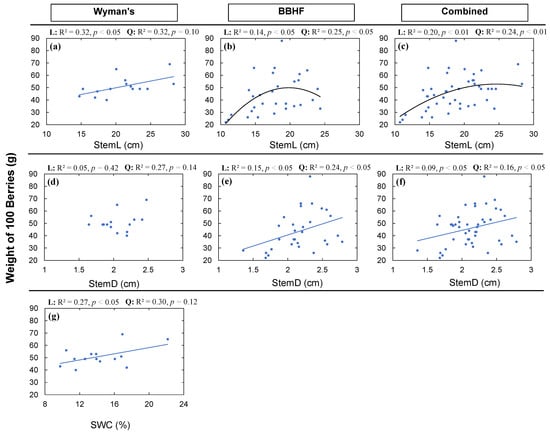
Figure 7.
Berry size (weight of 100 berries) (WT) in relation to average stem length (StemL) (a–c); average stem diameter (StemD) (d–f); and average soil water content (SWC) (g). The solid blue lines indicate significant (p < 0.05) linear relationships, and black lines indicate significant (p < 0.05) quadratic relationships. Dashed lines indicate marginally significant (p < 0.10) relationships. Here, among all the structural and functional traits, only significant or marginally significant relationships to WT were plotted. The strongest relationship was selected from the high level of significance (p-value) and higher coefficient of determination (R2).
Combining the data from both fields, we found that the multiple linear regression model tested was statistically significant (R2 = 0.345, p = 0.01), and the fitted regression model was Sqrt yield = −0.93 × StemL + 73.50 × LCC − 1.17 × LMA − ((LCC − 0.19) × (StemL − 18.76) × (− 30.07)). LMA (β = −1.17, p < 0.05) and LCC (β = 73.50, p < 0.05) significantly predicted yield (Table 3). The effect of LMA was negative on the yield, whereas the effect of LCC was found to be positive. It was also found that StemL did not significantly predict yield (β = −0.93, p = 0.057). When the parameters are centered and scaled, the relative importance of the predictors determining a unit of yield are: −25.3, 28.2, −41.4, and −42.1 for the parameters StemL, LCC, LMA, and StemL × LCC, respectively. The R2 of 0.345 of this multiple linear regression was higher than those of single traits (Figure 4).

Table 3.
Multiple linear regression analysis to predict yield (g/m2) using several functional traits stem length (StemL), leaf mass area (LMA), and leaf chlorophyll concentration (LCC) for the two studied fields combined. The best model, chosen after Lasso variable selection (least absolute shrinkage and selection operator) had an AICc of 382.1 and a coefficient of determination of R2 = 0.345. None of the predictors were correlated (p > 0.05), and the predictor distributions were not significantly different than a Normal distribution.
In the PCA analysis of the observed common structural and functional traits of the Wyman’s and BBHF field, principal component axis 1 (PC1) explained 28.4% while principal component axis 2 (PC2) explained 21.2% of the total variance (Figure 8). The PC1 was positively associated with stem structural traits StemL, StemD, and leaf size-related traits (LeafN and LAPS) and negatively with LMA. The PC2 was positively associated with leaf size-related traits (LeafN and LAPS), and negatively associated with yield and the yield-related traits NBS, LBC, and WT. The first principal component axis (PC1) represented a trade-off between investment in leaf toughness and endurance vs. investment in structural features, while the second principal component (PC2) represented a trade-off between productivity and investment in leaf structure.
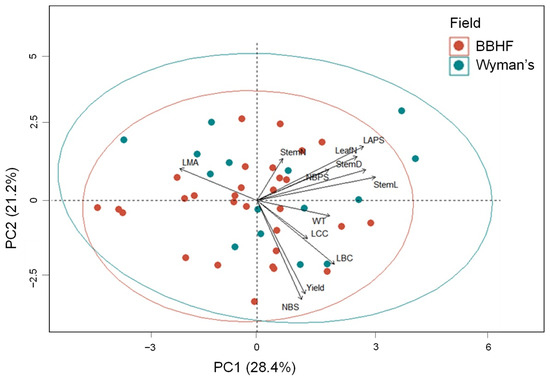
Figure 8.
Principal Component Analysis (PCA) of mean values of studied common structural and functional traits. The red circle represents BBHF’s field, and the blue circle represents Wyman’s field mean trait values. For definitions of trait abbreviations, please see Table 1.
4. Discussion
We found high variations in structural, functional, and yield-related traits among genotypes in both wild lowbush blueberry fields. Particularly, the yield varied by 46-fold among genotypes in the Wyman’s field and 36-fold in the BBHF field, which are surprisingly high and suggest the potential for breeding programs and precise management to increase yield. The high variations within fields could be explained by the fact that wild blueberry farms are semi-natural ecosystems with plants naturally growing in the field. Interestingly, no significant differences were found between the two studied fields in traits except StemN, StemL, and LNPS, despite different management practices and environmental conditions. To guide further studies, we found that the optimal sample size to estimate the yield of an entire field is 31 genotypes with a standard error\mean ratio precision of 0.25. Additionally, our results suggest the possible use of leaf mass per area, leaf chlorophyll concentration, stem length, and midday leaf temperature in predicting yield for wild blueberries. An R2 of 0.345 of the multiple linear regression model suggests important contributions to yield by other factors such as pollination and diseases. Nevertheless, the significant relationships revealed here suggest important biological causal factors in determining yield, which could be used to direct the design of controlled experiments in wild blueberries and other berry crops.
Wild blueberries are known to exhibit a high degree of intra-species diversity in terms of structural and functional traits and yield [34,35]. This is confirmed in our study. Additionally, we did not find any significant differences in any traits except StemN, StemL, and LNPS between the two studied fields (Table 2). This pattern agrees with the genetic variation across genotypes and fields, with more than 75 to 92% of the total genetic variation explained by inter-genotype variance within fields and only 8 to 25% explained by between-field variance [24,30]. Our results suggest overlaps in yield and many functional traits at the two farms despite different management practices. This could be explained by the fact that wild blueberry fields are semi-natural systems with blueberry plants naturally growing in the field and competing with each other. Thus, different genotypes with diverse functional performance are filling different niches of the ecosystem, resulting in a high range of functional traits in different farms. The absence of significant differences between these two farms might also be partially explained by seed dispersal by mammals and birds. Genetic differentiation among blueberry fields does not occur until between-field distances reach 12.5 km [30], suggesting that the effective genetic neighborhood is quite large, and gene flow through both pollen and seed dispersal operates at km distances. Therefore, fields close to one another will have genotypes that are genetically similar. Structural and functional features of plants also can be influenced by micro-climatic conditions [36]. We found a high variation in soil water content in the Wyman’s field (Table 2), which suggests the heterogeneity of environmental factors within the field. High inter-genotype diversity along with the spatial heterogeneity of the micro-climatic conditions might together shape the significant variation of structural and functional traits in wild blueberries [37].
We found that with an increase of LMA, the yield-related traits indicated by Yield, NBS, and LBC decreased. A leaf with a high leaf mass per area (LMA) has more fiber content and mass density resulted from the high N accumulation in the cell wall [38,39]. This type of investment in leaf structure increases plant endurance and resistance to environmental stress [40]. However, high N concentrations in the cell wall reduce N concentrations in the photosynthetic machinery, reducing the overall photosynthetic performance [38,39]. Moreover, thicker cell walls increase leaf endurance but increase CO2 diffusion resistance to chloroplasts, resulting in reduced photosynthetic capacity [41,42]. A higher LMA is linked to better survival but slower growth and yield performance; thus, it reduces overall yield [43], whereas a lower LMA results in better resource acquisition and usage efficiency, producing faster growth and higher yield [44]. Our finding in the regression analysis is also supported by our PCA analysis, where PC1 was shown to be positively correlated with stem structural features and leaf size-related features and negatively correlated with LMA. The first axis (PC1) indicated a trade-off in investment between leaf toughness and durability vs. structural features related to resource acquisition for photosynthesis, whereas the second axis (PC2) represented a trade-off between productivity and leaf structure. This investment in leaves vs. reproduction tradeoff is also supported by the finding that the removal of flowers of wild blueberry plants enhanced earlier leaf production and higher leaf production rates [45].
A positive link of yield with CO2 fixation rate, LCC, and stomatal conductance and a negative correlation with LCC has been found in wheat [21]. In general, LCC correlates positively with yield performance of crops as it reflects photosynthetic capacity [20]. We also found a positive impact of LCC on yield (Figure 4f: borderline significance) and yield-related traits like NBS (Figure 5e,f). Chlorophyll production in leaves is dependent on soil nitrogen availability as well as the effectiveness of plants to uptake nitrogen from soil [22]. The significant variation of LCC among wild blueberry genotypes and between fields might be related to the variability of soil nitrogen availability as well as variability in the effectiveness to uptake nitrogen from soils due to distinct genotypic features or a combination of both.
We found quadratic relationships between yield-related traits and StemL, suggesting that there is an optimum height for maximum wild blueberry yields. Plants with a higher stem length and larger diameter normally have bigger vessels, which can lead to higher hydraulic conductivity [13], facilitating higher stomatal conductance and photosynthetic carbon gain [14]. Higher hydraulic conductivity and photosynthesis are also related to higher plant growth rates [16], ultimately impacting yield performance. This explains positive associations between yield-related traits and stem structural traits, which need further physiological studies to confirm. It was previously observed in wild blueberries that greater stem length is related to higher fruit biomass [12]. Additionally, genotypes with a higher StemL might be benefitted in terms of successful pollination by bees, which is one of the major yield determining factors of wild blueberries [46]. However, as length increases, the danger of xylem cavitation increases, making plants more susceptible to drought [17,18]. Additionally, taller wild blueberry plants might be more prone to winter wind damage, which could result in reduced yield.
We found a negative association between the LeafT with Yield (Figure 4g) and a quadratic association with the yield-related trait LBC (Figure 6g). LeafT is a good indicator of leaf transpiration and water status [47]. There is a direct relationship between leaf temperature and plant water status. When water is limited, the plant reduces its transpiration rate, resulting in higher leaf temperatures than non-stressed well-watered plants [47]. We did not find any significant relationship between yield and soil water content, but we found a significant positive association between WT and soil water content (Figure 7g). A reason for the positive association might be that the weight of berries mostly consists of water; thus, a higher soil water content might be a driver of berry weight.
Between the two fields, we found that plants in Wyman’s field showed significantly higher investment in vegetative features compared to the genotypes in BBHF, as we can see significantly higher numbers of stems per quadrat, stem length, and leaf number per stem. However, the difference in yield-related traits between the two studied fields was not statistically significant. Notably, winter damages were found in the fields in 2019, and Wyman’s field with taller stems (higher stem length) may experience higher winter damages and more reduction in yield. Overall, although the difference in management practices and environmental conditions between these two farms resulted in differences in vegetative features, it seems they had limited effects on yield, at least in 2019.
5. Conclusions
Our study confirms the presence of high levels of variation among wild blueberry genotypes in structural, functional, and yield-related traits. The negative association of leaf mass per area (LMA) with yield-related traits suggests that there is a tradeoff between maintenance traits and yield performance traits, which is to be expected based on the cost/benefit balance theory. This interesting tradeoff could exist in other berry crops as well, which deserves further investigation. Meanwhile, although genotypes with a high LMA showed lower yield, they may be more drought resistant and produce a higher yield in drought years. As drought is frequent in wild blueberry fields [48], and future warming will decrease the relative humidity and enhance drought effects [49,50], the functional diversity in this semi-natural system could be important in maintaining the stability of wild blueberry production under increasing climate variability. Overall, our findings imply that a number of leaf and stem functional traits are linked to yield-related traits, and thus these traits can be used to predict wild blueberry productivity to facilitate precise management. We also identified that 25 cm was the optimal stem length for maximizing yield. Our findings are useful for growers and breeders in selecting superior yielding genotypes based on structural and functional features. Increased high-yielding blueberry genotypes planted in between inter-genotype unoccupied spaces in existing fields, as well as replacing less productive genotypes with high-yielding genotypes, would enhance crop production.
Author Contributions
Conceptualization, Y.-J.Z.; methodology, Y.-J.Z.; formal analysis, K.B. and F.D.; investigation, L.C., H.V., F.D., Y.-J.Z. and M.W.; resources, M.W. and B.H.; data curation, K.B. and Y.-J.Z.; software, K.B.; writing—original draft preparation, K.B.; writing—review and editing, L.C., H.V., F.D., B.H. and Y.-J.Z.; visualization, K.B.; supervision, Y.-J.Z.; project administration, Y.-J.Z.; funding acquisition, Y.-J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the USDA National Institute of Food and Agriculture, Hatch Project Numbers ME0-21832 and ME0-22021, through the Maine Agricultural and Forest Experiment Station. This research was also supported by the Wild Blueberry Commission of Maine, the Maine Department of Agriculture, Conservation and Forestry (SCBGP), and the UMaine RRF Fund and Faculty Summer Research Award. Maine Agricultural and Forest Experiment Station Publication Number 3885.
Data Availability Statement
Any data and codes used in this study are available upon request from the corresponding author (yongjiang.zhang@maine.edu).
Acknowledgments
We would like to acknowledge Bryan Peterson from the School of Food and Agriculture at the University of Maine, for his suggestions to prepare the writings of the draft manuscript. We would also like to acknowledge Yu-Ying Arin Chen, Anthony Ayers, Xiaoxue Mo, Zhiquan Mo, Wenlan Liu, and Huanrui Henry Zhang for helping with the data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drummond, F. Reproductive Biology of Wild Blueberry (Vaccinium angustifolium Aiton). Agriculture 2019, 9, 69. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Drummond, F. Simulation-Based Modeling of Wild Blueberry Pollination. Comput. Electron. Agric. 2018, 144, 94–101. [Google Scholar] [CrossRef]
- Bell, D.J.; Rowland, L.J.; Zhang, D.; Drummond, F.A. Spatial Genetic Structure of Lowbush Blueberry, Vaccinium angustifolium, in Four Fields in Maine. Botany 2009, 87, 932–946. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.J.; Rowland, L.J.; Smagula, J.; Drummond, F.A. Recent Advances in the Biology and Genetics of Lowbush Blueberry. Tech. Bull. 2009, 203, 1–28. [Google Scholar]
- Tasnim, R.; Zhang, Y.-J. Are Wild Blueberries a Crop with Low Photosynthetic Capacity? Chamber-Size Effects in Measuring Photosynthesis. Agronomy 2021, 11, 1572. [Google Scholar] [CrossRef]
- Drummond, F.A. Wild Blueberry Fruit Drop: A Consequence of Seed Set? Agronomy 2020, 10, 939. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of Grain Size, Shape and Quality by OsSPL16 in Rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural Variation in Ghd7 Is an Important Regulator of Heading Date and Yield Potential in Rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef]
- Mathan, J.; Bhattacharya, J.; Ranjan, A. Enhancing Crop Yield by Optimizing Plant Developmental Features. Development 2016, 143, 3283–3294. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.-M.; Fan, Z.-X.; Fu, P.-L.; Chen, H.; Lin, L.-X. Size Dependent Associations between Tree Diameter Growth Rates and Functional Traits in an Asian Tropical Seasonal Rainforest. Funct. Plant Biol. 2021, 48, 231–240. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf Size and Angle Vary Widely across Species: What Consequences for Light Interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef] [Green Version]
- Fournier, M.-P.; Paré, M.C.; Buttò, V.; Delagrange, S.; Lafond, J.; Deslauriers, A. How Plant Allometry Influences Bud Phenology and Fruit Yield in Two Vaccinium Species. Ann. Bot. 2020, 126, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and Function in Conifer Tracheids and Angiosperm Vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.S.; Goldstein, G.; Meinzer, F.C.; Fisher, J.B.; Machado, K.; Woodruff, D.; Jones, T. Leaf Photosynthetic Traits Scale with Hydraulic Conductivity and Wood Density in Panamanian Forest Canopy Trees. Oecologia 2004, 140, 543–550. [Google Scholar] [CrossRef]
- Sack, L.; Frole, K. Leaf Structural Diversity Is Related to Hydraulic Capacity in Tropical Rain Forest Trees. Ecology 2006, 87, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf Maximum Photosynthetic Rate and Venation Are Linked by Hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.G.; Phillips, N.; Lunch, C.; Bond, B.J.; Ryan, M.G. An Investigation of Hydraulic Limitation and Compensation in Large, Old Douglas-Fir Trees. Tree Physiol. 2002, 22, 763–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, O.L.; van der Heijden, G.; Lewis, S.L.; López-González, G.; Aragão, L.E.O.C.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; Almeida, S.; Dávila, E.A.; et al. Drought–Mortality Relationships for Tropical Forests. New Phytol. 2010, 187, 631–646. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.A. Selectable Traits to Increase Crop Photosynthesis and Yield of Grain Crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Ghimire, B.; Timsina, D.; Nepal, J. Analysis of Chlorophyll Content and Its Correlation with Yield Attributing Traits on Early Varieties of Maize (Zea mays L.). J. Maize Res. Dev. 2015, 1, 134–145. [Google Scholar] [CrossRef]
- Silva-Pérez, V.; De Faveri, J.; Molero, G.; Deery, D.M.; Condon, A.G.; Reynolds, M.P.; Evans, J.R.; Furbank, R.T. Genetic Variation for Photosynthetic Capacity and Efficiency in Spring Wheat. J. Exp. Bot. 2020, 71, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Percival, D.; Sanderson, K. Main and Interactive Effects of Vegetative-Year Applications of Nitrogen, Phosphorus, and Potassium Fertilizers on the Wild Blueberry. Small Fruits Rev. 2004, 3, 105–121. [Google Scholar] [CrossRef]
- Hall, I.V. Genetic Improvement of the Lowbush Blueberry, Vaccinium angustifolium. Can. J. Plant Sci. 1983, 63, 1091–1092. [Google Scholar] [CrossRef]
- Bell, D.J. Spatial and Genetic Factors Influencing Yield in Lowbush Blueberry (Vaccinium angustifolium Ait.) in Maine. Ph.D. Thesis, The University of Maine, Orono, ME, USA, 2009. [Google Scholar]
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Hepler, P.R.; Yarborough, D.E. Natural Variability in Yield of Lowbush Blueberries. HortScience 1991, 26, 245–246. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, M.; Ashraf, U.; Liu, S.; Zhang, J. Exploring the Relationships between Yield and Yield-Related Traits for Rice Varieties Released in China from 1978 to 2017. Front. Plant Sci. 2019, 10, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.S. Chapter 20—Breeding for Abiotic Stress Resistance in Sorghum. In Breeding Sorghum for Diverse End Uses; Aruna, C., Visarada, K.B.R.S., Bhat, B.V., Tonapi, V.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2019; pp. 325–340. ISBN 9780081018798. [Google Scholar]
- Reynolds, M.P.; Balota, M.; Delgado, M.I.B.; Amani, I.; Fischer, R.A. Physiological and Morphological Traits Associated With Spring Wheat Yield Under Hot, Irrigated Conditions. Funct. Plant Biol. 1994, 21, 717–730. [Google Scholar] [CrossRef]
- Beers, L.; Rowland, L.J.; Drummond, F. Genetic Diversity of Lowbush Blueberry throughout the United States in Managed and Non-Managed Populations. Agriculture 2019, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Tremblay, N.; Liang, Y. Comparing SPAD and AtLEAF Values for Chlorophyll Assessment in Crop Species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Sampling Techniques, 3rd Edition. Wiley. Available online: https://www.wiley.com/en-us/Sampling+Techniques%2C+3rd+Edition-p-9780471162407 (accessed on 11 January 2022).
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [Green Version]
- Kloet, S.P.V. The Taxonomic Status of Vaccinium pallidum, the Hillside Blueberries Including Vaccinium vacillans. Can. J. Bot. 1978, 56, 1559–1574. [Google Scholar] [CrossRef]
- Smagula, J.M.; Litten, W.; Chen, Y.; Dunham, S. Variation of fruit set and fruit characteristics of wild lowbush blueberries (Vaccinium angustifolium ait.) in a managed field. Acta Hortic. 1997, 109–118. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific Functional Variability: Extent, Structure and Sources of Variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A Multi-Trait Approach Reveals the Structure and the Relative Importance of Intra- vs. Interspecific Variability in Plant Traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.F.; Clissold, F.J.; Cornelissen, J.H.C.; Díaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global Patterns of Leaf Mechanical Properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, E.T.F.; Lamont, B.B. Leaf Specific Mass Confounds Leaf Density and Thickness. Oecologia 1991, 88, 486–493. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Sack, L.; Cao, K.-F.; Wei, X.-M.; Li, N. Speed versus Endurance Tradeoff in Plants: Leaves with Higher Photosynthetic Rates Show Stronger Seasonal Declines. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, U. Components of Leaf Dry Mass per Area—Thickness and Density—Alter Photosynthetic Capacity in Reverse Directions in Woody Plants. New Phytol. 1999, 144, 35–47. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From Tropics to Tundra: Global Convergence in Plant Functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [Green Version]
- Rüger, N.; Wirth, C.; Wright, S.J.; Condit, R. Functional Traits Explain Light and Size Response of Growth Rates in Tropical Tree Species. Ecology 2012, 93, 2626–2636. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent Variation in Growth Rate between Higher Plants: A Search for Physiological Causes and Ecological Consequences. Adv. Ecol. Res. 2004, 34, 283–362. [Google Scholar]
- Bajcz, A.W.; Drummond, F.A. Bearing Fruit: Flower Removal Reveals the Trade-Offs Associated with High Reproductive Effort for Lowbush Blueberry. Oecologia 2017, 185, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Yarborough, D.E. Factors Contributing to the Increase in Productivity in the Wild Blueberry Industry. Small Fruits Rev. 2004, 3, 33–43. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.J.; Fereres, E. Applicability and Limitations of Using the Crop Water Stress Index as an Indicator of Water Deficits in Citrus Orchards. Agric. For. Meteorol. 2014, 198–199, 94–104. [Google Scholar] [CrossRef]
- Barai, K.; Tasnim, R.; Hall, B.; Rahimzadeh-Bajgiran, P.; Zhang, Y.-J. Is Drought Increasing in Maine and Hurting Wild Blueberry Production? Climate 2021, 9, 178. [Google Scholar] [CrossRef]
- Tasnim, R.; Drummond, F.; Zhang, Y.-J. Climate Change Patterns of Wild Blueberry Fields in Downeast, Maine over the Past 40 Years. Water 2021, 13, 594. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Pahadi, P.; Calderwood, L.; Annis, S.; Drummond, F.; Zhang, Y.-J. Will Climate Warming Alter Biotic Stresses in Wild Lowbush Blueberries? Agronomy 2022, 12, 371. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).