Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments and Crop Husbandry
2.3. Growth Traits
2.4. Physiological Traits
2.5. Chlorophyll and Carotenoid Contents
2.6. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Contents
2.7. Total Soluble Proteins and Free Amino Acids
2.8. Anti-Oxidant Activities
2.9. Determination of Yield Traits
2.10. Determination of Cadmium Concentrations in Root, Shoot and Seed
2.11. Statistical Analysis
3. Results
3.1. Growth Traits
3.2. Photosynthetic Pigments
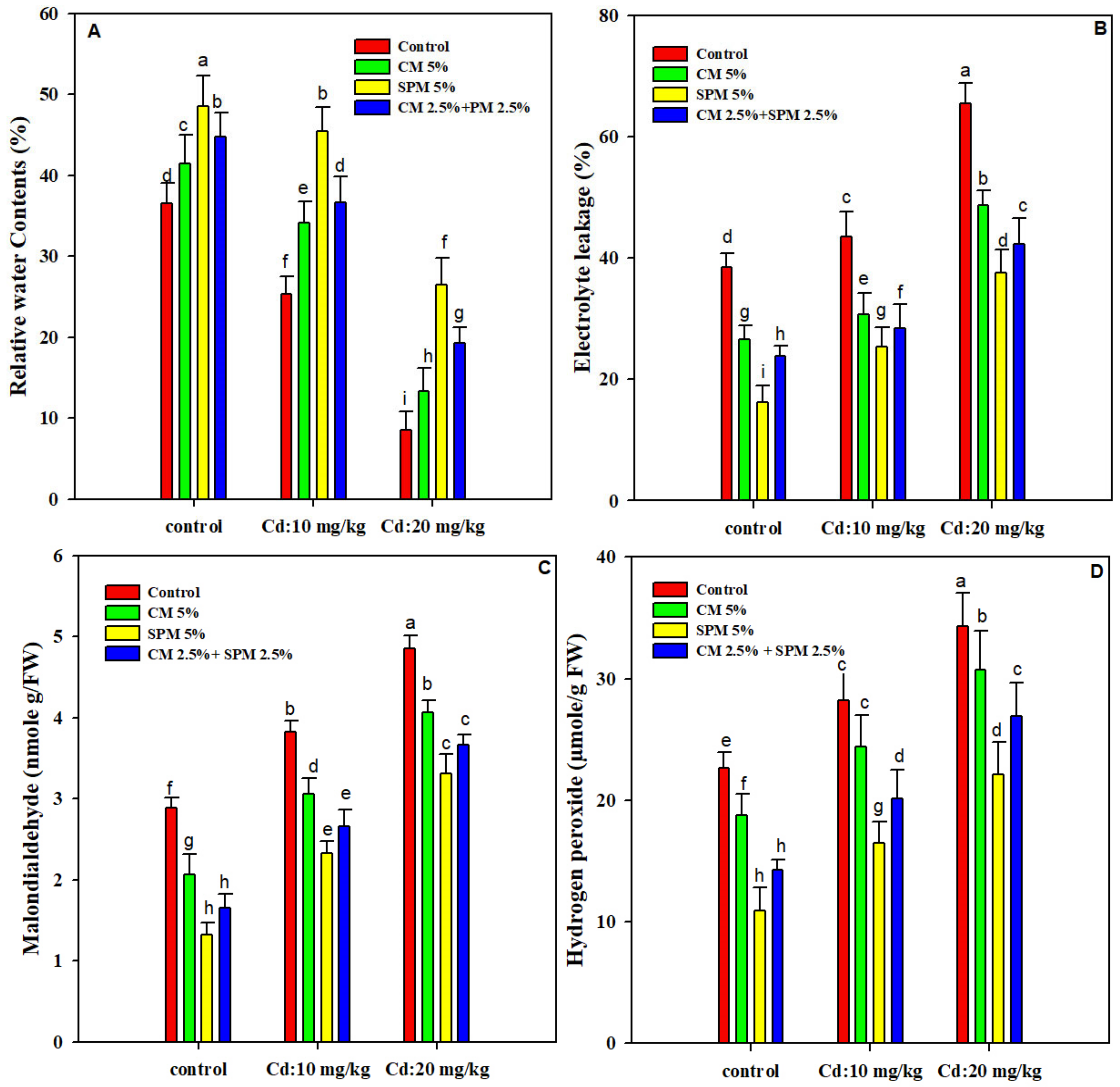
3.3. Relative Water Contents and Electrolyte Leakage
3.4. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Contents
3.5. Anti-Oxidant Activities
3.6. Total Soluble Proteins and Free Amino Acids
3.7. Yield Traits
3.8. Cadmium Concentration Different Plant Parts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Poll. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka India. J. Saud. Soc. Agric. Sci. 2017, 16, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Chaoua, S.; Boussaa, S.; Gharmali, A.E.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saud. Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Khan, I.; Seleiman, M.F.; Rasheed, A.; Nawaz, M.; Rehman, A.; Talha, A.M.; et al. Sugarcane distillery spent wash (DSW) as a bio-nutrient supplement: A win-win option for sustainable crop production. Agronomy 2021, 11, 183. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, P.; Singh, R.P.; Araujo, A.S.F.D.; Singh, S. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci. 2019, 5, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Aamer, M.; Muhammad, U.H.; Li, Z.; Abid, A.; Su, Q.; Liu, Y.; Adnan, R.; Muhammad, A.U.K.; Tahir, A.K.; Huang, G. Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of anti-oxidant system. Appl. Ecol. Environ. Res. 2018, 16, 7575–7583. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Ghafoor, A.; Naeem, A.; Ali, S.; Sabir, M.; Qayyum, M.F. Effect of inorganic amendments for in situ stabilization of cadmium in contaminated soils and its phytoavailability to wheat and rice under rotation. Environ. Sci. Pollut. Res. 2015, 22, 16897–16906. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Höckner, M. Cadmium protection strategies—A hidden trade-off? Int. J. Mol. Sci. 2016, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2014. [Google Scholar]
- Mombo, S.; Foucault, Y.; Deola, F.; Gaillard, I.; Goix, S.; Shahid, M.; Schreck, E.; Pierart, A.; Dumat, C. Management of human health risk in the context of kitchen gardens polluted by lead and cadmium near a lead recycling company. J. Soils Sediment. 2016, 16, 1214–1224. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, T.; Zhang, W.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol. Environ. Saf. 2018, 147, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Imran, K.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.; Izzet, W.; Alhammad, B.A.; Rana, R.; Hassan, M.U. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Hort. Agrobot. 2021, 49, 12303. [Google Scholar]
- Gallego, S.M.; Pena, L.B.; Barcia, C.E.; Azpilicueta, M.F.; Iannone, E.P.; Rosales, M.S.; Zawoznik, M.D.; Groppa, A.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [Green Version]
- Nas, F.S.; Ali, M. The effect of lead on plants in terms of growing and biochemical parameters: A review. MOJ Ecol. Environ. Sci. 2018, 3, 265–268. [Google Scholar]
- Shi, G.; Liu, C.; Cai, Q.; Liu, Q.; Hou, C. Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidative enzymes. Bull. Environ. Contam. Toxicol. 2010, 85, 256–263. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Choi, Y.J.; Kim, J.G. In situ stabilization of cadmium, lead, and zinc-contaminated soil using various amendments. Chemosphere 2009, 77, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; We, Y.M.; Qium, Q.Y.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Niemsdorff, P.V.F.U.; Heb, J. Effect of different organic wastes on soil properties and plant growth and yield: A review. Sci. Agric. Bohem. 2017, 48, 224–237. [Google Scholar]
- Hussain, M.; Farooq, M.; Nawaz, A.M.; Al-Sadi, Z.M.; Solaiman, S.S.; Alghamdi, U.; Ammara, U.; Ok, Y.S.; Siddique, K.M. Biochar for crop production: Potential benefits and risks. J. Soils Sed. 2017, 17, 685–716. [Google Scholar] [CrossRef]
- Sharma, S.S.; Tiwari, A.; Hasan, V.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. Biotechnology 2018, 8, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Murtaza, G.; Saifullah, M.; Farooq, M. Comparable effect of commercial composts on chemical properties of sandy clay loam soil and accumulation of trace elements in soil-plant system. Int. J. Agric. Biol. 2018, 20, 85–92. [Google Scholar]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, Y.; Sun, Y.; Gao, H.; Yang, H.; Xu, H. Effects of amendments on heavy metal immobilization and uptake by Rhizoma chuanxiong on copper and cadmium contaminated soil. Roy. Soc. Open Sci. 2018, 5, 181–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.; Zawar, H.; Anwarzeb, K.; Muhammad, A.K.; Abdur, R.; Muhammad, A.; Muhammad, A.S.; Asim, M. The effects of organic amendments on heavy metals bioavailability in mine impacted soil and associated human health risk. Sci. Hort. 2020, 262, 109067. [Google Scholar] [CrossRef]
- Swati, A.; Hait, S. Fate and bioavailability of heavy metals during vermicomposting of various organic wastes—A review. Process Saf. Environ. Prot. 2017, 109, 30–45. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Barbanti, L.; Chattha, M.B.; Khan, I.; Usman, M.; Ali, A.; Nawaz, M. Composted sugarcane by-product press mud cake supports wheat growth and improves soil properties. Int. J. Plant Prod. 2019, 13, 241–249. [Google Scholar] [CrossRef]
- Nawaz, M.; Chattha, M.U.; Chattha, M.B.; Ahmad, R.; Munir, H.; Usman, M.; Hassan, M.U.; Khan, S.; Kharal, M. Assessment of compost as nutrient supplement for spring planted sugarcane (Saccharum officinarum L.). J. Anim. Plant Sci. 2017, 27, 283–293. [Google Scholar]
- Nawaz, M.; Khan, S.; Ali, H.; Ijaz, M.; Chattha, M.U.; Hassan, M.U.; Irshad, S.; Hussain, S.; Khan, S. Assessment of environment-friendly usage of spent wash and its nutritional potential for sugarcane production. Comm. Soil Sci. Plant Anal. 2019, 50, 1239–1249. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ali, R.; Javid, H.N.; Asghar, I.; Ahmad, M.Z.; Iqbal, Z.; Khaliq, A. Organic and inorganic amendments immobilized cadmium and improved maize growth and yield in Cd-contaminated soil. Int. J. Agric. Biol. 2019, 22, 1497–1506. [Google Scholar]
- Mahmood, T. Phytoextraction of heavy metals-the process and scope for remediation of contaminated soils. Soil Environ. 2010, 29, 91–109. [Google Scholar]
- Bashir, S.; Bashir, S.; Gulshan, A.B.; Khan, M.J.; Iqbal, J.; Sherani, J.; Husain, A.; Ahmed, N.; Shah, A.N.; Bukhari, M.A.; et al. The role of different organic amendments to improve maize growth in wastewater irrigated soil. J. King Saud Uni. Sci. 2021, 7, 101583. [Google Scholar] [CrossRef]
- Saleem, M.; Asghar, H.N.; Khan, M.Y.; Zahir, Z.A. Gibberellic acid in combination with pressmud enhances the growth of sunflower and stabilizes chromium (VI)-contaminated soil. Environ. Sci. Poll. Res. 2015, 22, 10610–10617. [Google Scholar] [CrossRef]
- Azhar, M.; Rehman, M.Z.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; Haq, M.A.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef]
- Senthilvalavan, P.V.; VInothkumar, R.; Singaravel, R.; Parthasarthi, R. Mobilization of lead and cadmium influenced by soil amendments. Pollut. Res. 2020, 39, 692–701. [Google Scholar]
- Li, S.; Liu, R.; Wang, M.; Wang, X.; Shen, H.; Wang, H. Phytoavailability of cadmium to cherry-red radish in soils applied composed chicken or pig manure. Geoderma 2006, 136, 260–271. [Google Scholar] [CrossRef]
- Park, J.; Cho, K.H.; Ligaray, M.; Choi, M.J. Organic matter composition of manure and its potential impact on plant growth. Sustainability 2019, 11, 2346. [Google Scholar] [CrossRef] [Green Version]
- Kiran, Y.K.; Barkat, A.; Cui, X.; Ying, F.; Pan, F.S.; Lin, T.; Yang, X.E. Cow manure and cow manure-derived biochar application as a soil amendment for reducing cadmium availability and accumulation by Brassica chinensis L. in acidic red soil. J. Integr. Agric. 2017, 16, 725–734. [Google Scholar] [CrossRef]
- Chiu, K.K.; Ye, Z.H.; Wong, M.H. Growth of Vetiveria zizanioides and Phragmities australis on Pb/Zn and Cu mine tailing amended with manure compost and sewage sludge: A greenhouse study. Bioresour. Tech. 2006, 97, 158–170. [Google Scholar] [CrossRef]
- Putwattana, N.; Kruatrachue, M.; Pokethitiyook, P.; Chaiyarat, R. Immobilization of cadmium in soil by cow manure and silicate fertilizer, and reduced accumulation of cadmium in sweet basil (Ocimum basilicum). Sci. Asia 2010, 36, 349–354. [Google Scholar] [CrossRef]
- Majeed, A.; Niaz, A.; Rizwan, M.; Imran, M.; Alsahli, A.A.; Alyemeni, M.N.; Ali, S. Effects of biochar, farm manure, and pressmud on mineral nutrients and cadmium availability to wheat (Triticum aestivum L.) in Cd-contaminated soil. Physiol. Plant. 2021, 73, 191–200. [Google Scholar]
- Pichtel, J.; Bradway, D. Conventional crops and organic amendments for Pb, Cd and Zn treatment at a severely contaminated site. Bioresour. Tech. 2008, 99, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Shamsuzzaman, S.M.; Islam, M.R.; Samsuri, A.W.; Uddin, M.K. Cadmium availability and uptake by rice from lime, cow-dung and poultry manure amended Ca-contaminated paddy soil. Bangladesh J. Bot. 2017, 46, 291–296. [Google Scholar]
- Li, B.; Yang, L.; Wang, C.Q.; Zheng, S.Q.; Xiao, R.; Guo, Y. Effects of organic-inorganic amendments on the cadmium fraction in soil and its accumulation in rice (Oryza sativa L.). Environ. Sci. Poll. Res. 2019, 26, 13762–13772. [Google Scholar] [CrossRef] [PubMed]
- Boysan, C.S.; Bozkurt, M.A.; Kipcak, S. The effects of organic amendments on cadmium uptake of spinach (Spinacia oleracea L.) and plant growth under cadmium toxicity. Fresenius Environ. Bull. 2018, 27, 3174–3179. [Google Scholar]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, I.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.; Iqbal, J.; Barbanti, L.; Sher, A.; Shabbir, G.; Rabbani, G.; Rafiq, M.K.; Tareen, M.N.; Tareen, M.J.; Amin, B.A.Z. Mash Bean [Vigna mungo (L.) Hepper] Germplasm evaluation at different ecological conditions of Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6643–6654. [Google Scholar] [CrossRef]
- Sharma, P.; Sekhon, H.S.; Bains, T.S. Performance and growth analysis in Mash bean genotypes. World J. Agric. Sci. 2012, 8, 303–308. [Google Scholar]
- Karrou, M.; Maranville, J. Response of wheat cultivars to different soil nitrogen and moisture regimes: III. Leaf water content, conductance, and photosynthesis. J. Plant Nutr. 1995, 18, 777–791. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981, 21, 43–47. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembrane. Methods Enzymol. 1987, 148, 350–352. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Buege, J.A.; Steven, D.A. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Vanslyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidase. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Asada, K.; Takahashi, M. Production and Scavenging of Active Oxygen in Chloroplasts. In Photoinhibition; Elsevier: Amsterdam, The Neherlands, 1987; pp. 227–287. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiolog. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. Soil Testing Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics, A Biometrical Approach, 3rd ed.; McGraw Hill, Inc. Book Co.: New York, NY, USA, 1997; pp. 352–358. [Google Scholar]
- Saidi, I.; Ayouni, M.; Dhieb, A.; Chtourou, Y.; Chaïbi, W.; Djebali, W. Oxidative damages induced by short-term exposure to cadmium in bean plants: Protective role of salicylic acid. S. Afr. J. Bot. 2013, 85, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Carpenter-Boggs, L.; Hoque, M.A.; Ahammed, G.J. Effect of soil amendments on antioxidant activity and photosynthetic pigments in pea crops grown in arsenic contaminated soil. Heliyon 2020, 6, e05475. [Google Scholar]
- WU, F.Z.; Yang, W.Q.; Zhang, J.; Zhou, L.Q. Effects of cadmium stress on growth and nutrient accumulation, distribution and utilization in Osmanthus fragrans var. thunbergii. Chin. J. Plant Ecol. 2010, 34, 1220. [Google Scholar]
- Shakirova, F.M. Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. In Salicylic Acid: A Plant Hormone; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–89. [Google Scholar]
- Li, M.; Ren, L.; Zhang, J.; Luo, L.; Qin, P.; Zhou, Y.; Huang, C.; Tang, J.; Huang, H.; Chen, A. Population characteristics and influential factors of nitrogen cycling functional genes in heavy metal contaminated soil remediated by biochar and compost. Sci. Total Environ. 2019, 651, 2166–2174. [Google Scholar]
- Bashir, S.; Bakhsh, G.A.; Iqbal, J.; Husain, A.; Alwahibi, M.S.; Alkahtani, J.; Dwiningsih, Y.; Bakhsh, A.; Ahmed, N.; Jamal, M.; et al. Comparative role of animal manure and vegetable waste induced compost for polluted soil restoration and maize growth. Saudi J. Biol. Sci. 2021, 28, 2534–2539. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Chen, X.; Pu, G.; Huang, Y.; Mo, L. Effects of thallium and cadmium stress on growth and photosynthetic characteristics of Arundo donax. Guangxi Zhiwu Guihaia 2019, 39, 743–751. [Google Scholar]
- Song, X.; Yue, X.; Chen, W.; Jiang, H.; Han, Y.; Li, X. Detection of cadmium risk to the photosynthetic performance of hybrid Pennisetum. Front. Plant Sci. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Vijendra, P.D.; Huchappa, K.M.; Lingappa, R.; Basappa, G.; Jayanna, G.S.; Kumar, V. Physiological and biochemical changes in moth bean (Vigna aconitifolia L.) under cadmium stress. J. Bot. 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Abbas, Q.; Wang, R.; Imtiaz, M.; Zia-ur-Rehman, M. Investigating the uptake and acquisition of potentially toxic elements in plants and health risks associated with the addition of fresh biowaste amendments to industrially contaminated soil. Land Degrad. Dev. 2017, 28, 2596–2607. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. SpringerPlus 2016, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef]
- Palma, J.M.; Sandalio, L.M.; Corpas, F.J.; Romero-Puertas, M.C.; McCarthy, I.; Luis, A. Plant proteases, protein degradation, and oxidative stress: Role of peroxisomes. Plant Physiol. Biochem. 2002, 40, 521–530. [Google Scholar]
- Zemanová, V.; Pavlík, M.; Pavlíková, D.; Tlustoš, P. The significance of methionine, histidine and tryptophan in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014, 60, 426–432. [Google Scholar] [CrossRef]
- Borchard, N.; Spokas, K.; Prost, K.; Siemens, J. Greenhouse gas production in mixtures of soil with composted and non-composted biochars is governed by char associated organic compounds. J. Environ. Qual. 2014, 43, 971–979. [Google Scholar] [CrossRef]
- Karwal, M.; Kaushik, A. Co-composting and vermicomposting of coal fly-ash with press mud: Changes in nutrients, micro-nutrients and enzyme activities. Environ. Technol. Inn. 2020, 18, 100708. [Google Scholar] [CrossRef]
- Bashir, S.; Salam, A.; Chhajro, M.A.; Fu, Q.; Khan, M.J.; Zhu, J.; Shaaban, M.; Kubar, K.A.; Ali, U.; Hu, H. Comparative efficiency of rice husk-derived biochar (RHB) and steel slag (SS) on cadmium (Cd) mobility and its uptake by Chinese cabbage in highly contaminated soil. Int. J. Phytoremed. 2018, 20, 1221–1228. [Google Scholar] [CrossRef]
- Bashir, S.; Qayyum, M.A.; Husain, A.; Bakhsh, A.; Ahmed, N.; Hussain, M.B.; Elshikh, M.S.; Alwahibi, M.S.; Almunqedhi, B.M.A.; Hussain, R.; et al. Efficiency of different types of biochars to mitigate Cd stress and growth of sunflower (Helianthus; L.) in wastewater irrigated agricultural soil. Saudi J. Biol. Sci. 2021, 28, 2453–2459. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Bashir, S.; Ali, U.; Shaaban, M.; Gulshan, A.B.; Iqbal, J.; Khan, S.; Husain, A.; Ahmed, N.; Mehmood, S.; Kamran, M.; et al. Role of sepiolite for cadmium (Cd) polluted soil restoration and spinach growth in wastewater irrigated agricultural soil. J. Environ. Manag. 2020, 258, 110020. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim Rehman, M.Z.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Azeem, M.; Naveed, M.; Latif, A.; Bashir, S.; Ali, A.; Bilal, M.; Ali, L. Synergistic use of biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int. J. Phytorem. 2020, 22, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Noureen, S.; Shakoor, M.B.; Haroon, M.Y.; Rizwan, M.; Jilani, A.; Arif, M.S.; Khalil, U. Comparative evaluation of wheat straw and press mud biochars for Cr(VI) elimination from contaminated aqueous solution. Environ. Technol. Innov. 2020, 19, 101017. [Google Scholar] [CrossRef]
- Huang, X.; Jia, Z.; Guo, J.; Li, T.; Sun, D.; Meng, H.; Yu, G.; He, X.; Ran, W.; Zhang, S. Ten-year long-term organic fertilization enhances carbon sequestration and calcium-mediated stabilization of aggregate-associated organic carbon in a reclaimed Cambisol. Geoderma 2019, 355, 11388. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; El Sabagh, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants andphytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]





| Cadmium Stress | OA | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Carotenoid (mg/g FW) |
|---|---|---|---|---|
| (Control) | Control | 2.13c ± 0.01 | 1.40e ± 0.03 | 1.60e ± 0.03 |
| CM 5% | 2.27b ± 0.009 | 1.67d ± 0.05 | 1.88c ± 0.06 | |
| SPM 5% | 2.40a ± 0.02 | 2.80a ± 0.08 | 2.40a ± 0.07 | |
| CM 2.5% + SMP 2.5% | 2.33ab ± 0.02 | 2.12c ± 0.12 | 2.04b ± 0.02 | |
| 10 mg/kg | Control | 1.48e ± 0.02 | 1.15f ± 0.03 | 1.13h ± 0.06 |
| CM 5% | 1.52e ± 0.009 | 1.32e ± 0.09 | 1.54e ± 0.05 | |
| SPM 5% | 1.66d ± 0.02 | 2.37b ± 0.09 | 2.10b ± 0.07 | |
| CM 2.5% + SMP 2.5% | 1.58de ± 0.006 | 2.14c ± 0.05 | 1.76d ± 0.07 | |
| 20 mg/kg | Control | 0.90h ± 0.09 | 0.77g ± 0.6 | 0.73i ± 0.08 |
| CM 5% | 1.13g ± 0.06 | 1.08f ± 0.02 | 1.25g ± 0.03 | |
| SPM 5% | 1.34f ± 0.06 | 1.72d ± 0.11 | 1.88c ± 0.04 | |
| CM 2.5% +SMP 2.5% | 1.20g ± 0.005 | 1.37e ± 0.08 | 1.44f ± 0.06 |
| Cadmium Stress | OA | Pod Length (cm) | Grains/Pod | 100-Grain Weight (g) | Grain Yield/Pot | Biological Yield/Pot |
|---|---|---|---|---|---|---|
| (Control) | Control | 11.38b ± 0.07 | 10.67bcd ± 0.33 | 6.31b ± 0.06 | 40.00bc ± 1.73 | 71.67b ± 1.67 |
| CM 5% | 11.71b ± 0.10 | 11.00bc ± 0.58 | 6.40b ± 0.11 | 43.00b ± 1.00 | 72.33b ± 1.45 | |
| SPM 5% | 12.63a ± 0.15 | 13.33a ± 0.39 | 6.90a ± 0.05 | 48.00a ± 1.53 | 80.67a ± 2.19 | |
| CM 2.5% + SMP 2.5% | 12.38a ± 0.12 | 11.67b ± 0.29 | 6.72a ± 0.07 | 46.67a ± 0.33 | 78.00a ± 1.53 | |
| 10 mg/kg | Control | 9.57d ± 0.15 | 9.00fgh ± 0.34 | 5.11e ± 0.14 | 31.00ef ± 0.58 | 59.00d ± 2.08 |
| CM 5% | 10.00d ± 0.44 | 9.33efg ± 0.26 | 5.43d ± 0.07 | 33.33de ± 0.88 | 64.33c ± 1.20 | |
| SPM 5% | 10.87c ± 0.12 | 10.33cde ± 0.27 | 5.96c ± 0.09 | 36.67cd ± 2.03 | 67.67bc ± 1.76 | |
| CM 2.5% + SMP 2.5% | 10.57c ± 0.04 | 9.67def ± 0.41 | 5.67d ± 0.08 | 35.67d ± 1.20 | 66.67c ± 1.20 | |
| 20 mg/kg | Control | 7.92f ± 0.06 | 8.00i ± 0.49 | 4.14h ± 0.04 | 22.00i ± 1.00 | 45.67f ± 0.67 |
| CM 5% | 8.10ef ± 0.15 | 7.67hi ± 0.35 | 4.37gh ± 0.07 | 24.33hi ± 0.67 | 47.33f ± 1.33 | |
| SPM 5% | 8.80e ± 0.21 | 8.33ghi ± 0.32 | 4.67f ± 0.011 | 29.33fg ± 1.45 | 54.00e ± 1.00 | |
| CM 2.5% + SMP 2.5% | 8.40f ± 0.25 | 8.00hi ± 0.24 | 4.45fh ± 0.07 | 26.00gh ± 0.58 | 50.00ef ± 2.89 |
| Cadmium Stress | OA | Root Cd (µg g−1 DW) | Stem Cd (µg g−1 DW) | Leaf (µg g−1 DW) | Grain Cd (µg g−1 DW) |
|---|---|---|---|---|---|
| (Control) | Control | 2.24g ± 1.44 | 1.60f ± 0.087 | 1.04h ± 0.054 | 0.89f |
| CM 5% | 2.10g ± 1.46 | 1.48f ± 0.060 | 1.04h ± 0.042 | 0.83f | |
| SPM 5% | 1.70g ± 1.92 | 1.33f ± 0.044 | 0.95h ± 0.021 | 0.72f | |
| CM 2.5% + SMP 2.5% | 1.95g ± 1.82 | 1.45f ± 0.021 | 1.01h ± 0.086 | 0.78f | |
| 10 mg/kg | Control | 17.73d ± 3.26 | 11.17b ± 0.601 | 6.83e ± 0.203 | 3.73d |
| CM 5% | 14.87e ± 1.95 | 9.96cd ± 0.125 | 6.65e ± 0.118 | 3.64d | |
| SPM 5% | 11.70f ± 1.71 | 8.57e ± 0.470 | 6.21g ± 0.067 | 3.10e | |
| CM 2.5% + SMP 2.5% | 14.00e ± 8.25 | 9.29de ± 0.162 | 6.44fg ± 0.157 | 3.22e | |
| 20 mg/kg | Control | 26.13a ± 3.88 | 12.17a ± 0.441 | 9.82a ± 0.055 | 4.73a |
| CM 5% | 24.43b ± 2.37 | 11.53ab ± 0.437 | 9.06b ± 0.142 | 4.43b | |
| SPM 5% | 22.10bc ± 3.85 | 10.73bc ± 0.393 | 8.31d ± 0.055 | 4.10c | |
| CM 2.5% + SMP 2.5% | 23.17c ± 2.87 | 10.86bc ± 0.197 | 8.69c ± 0.142 | 4.15c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umer Chattha, M.; Arif, W.; Khan, I.; Soufan, W.; Bilal Chattha, M.; Hassan, M.U.; Ullah, N.; Sabagh, A.E.; Qari, S.H. Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]. Agronomy 2021, 11, 2152. https://doi.org/10.3390/agronomy11112152
Umer Chattha M, Arif W, Khan I, Soufan W, Bilal Chattha M, Hassan MU, Ullah N, Sabagh AE, Qari SH. Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]. Agronomy. 2021; 11(11):2152. https://doi.org/10.3390/agronomy11112152
Chicago/Turabian StyleUmer Chattha, Muhammad, Warda Arif, Imran Khan, Walid Soufan, Muhammad Bilal Chattha, Muhammad Umair Hassan, Najeeb Ullah, Ayman El Sabagh, and Sameer H. Qari. 2021. "Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]" Agronomy 11, no. 11: 2152. https://doi.org/10.3390/agronomy11112152
APA StyleUmer Chattha, M., Arif, W., Khan, I., Soufan, W., Bilal Chattha, M., Hassan, M. U., Ullah, N., Sabagh, A. E., & Qari, S. H. (2021). Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]. Agronomy, 11(11), 2152. https://doi.org/10.3390/agronomy11112152











