Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Sample Preparation and Analysis
2.3. Data Analysis
3. Results
3.1. Metal Concentrations in Soil
3.2. Metal Concentrations in Plants
4. Discussion
4.1. Considerations on the Analytical Methodology
4.2. Differences among Soil–Plant Relations
4.3. Differences among Plant Species
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 15 June 2021).
- Mok, H.F.; Williamson, V.G.; Grove, J.R.; Burry, K.; Barker, S.F.; Hamilton, A.J. Strawberry fields forever? Urban agriculture in developed countries: A review. Agron. Sustain. Dev. 2014, 34, 21–43. [Google Scholar] [CrossRef] [Green Version]
- FAO HABITAT III. Revised Zero Draft of the New Urban Agenda. In Proceedings of the United Nations Conference on Housing and Sustainable Urban Development, Quito, Ecuador, 17–20 October 2016. [Google Scholar]
- Golden, S. Urban Agriculture Impacts: Social, Health, and Economic: A Literature Review; University of California Agriculture and Natural Resources: Davis, CA, USA, 2013. [Google Scholar]
- Pfeiffer, A.; Silva, E.; Colquhoun, J. Innovation in urban agricultural practices: Responding to diverse production environments. Renew. Agric. Food Syst. 2015, 30, 79–91. [Google Scholar] [CrossRef]
- Johnson, M.S.; Lathuillière, M.J.; Tooke, T.R.; Coops, N.C. Attenuation of urban agricultural production potential and crop water footprint due to shading from buildings and trees. Environ. Res. Lett. 2015, 10, 64007. [Google Scholar] [CrossRef]
- Wielemaker, R.; Oenema, O.; Zeeman, G.; Weijma, J. Fertile cities: Nutrient management practices in urban agriculture. Sci. Total Environ. 2019, 668, 1277–1288. [Google Scholar] [CrossRef]
- Watson, D.L.B.; Moore, H.J. Community gardening and obesity. Perspect. Public Health 2011, 131, 163–164. [Google Scholar] [CrossRef] [Green Version]
- Gaspéri, J.; Ayrault, S.; Moreau-Guigon, E.; Alliot, F.; Labadie, P.; Budzinski, H.; Blanchard, M.; Muresan, B.; Caupos, E.; Cladière, M.; et al. Contamination of soils by metals and organic micropollutants: Case study of the Parisian conurbation. Environ. Sci. Pollut. Res. 2016, 25, 23559–23573. [Google Scholar] [CrossRef] [Green Version]
- Meharg, A.A. Perspective: City farming needs monitoring. Nature 2016, 531, S60. [Google Scholar] [CrossRef]
- Schlecht, M.T.; Säumel, I. Wild growing mushrooms for the Edible City? Cadmium and lead content in edible mushrooms harvested within the urban agglomeration of Berlin, Germany. Environ. Pollut. 2015, 204, 298–305. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Taylor, M.P.; Isley, C.F.; Fry, K.L.; Liu, X.; Gillings, M.M.; Rouillon, M.; Soltani, N.S.; Gore, D.B.; Filippelli, G.M. A citizen science approach to identifying trace metal contamination risks in urban gardens. Environ. Int. 2021, 155, 106582. [Google Scholar] [CrossRef]
- Konwuruk, N.; Borquaye, L.S.; Darko, G.; Dodd, M. Distribution, bioaccessibility and human health risks of toxic metals in peri-urban topsoils of the Kumasi Metropolis. Sci. Afr. 2021, 11, e00701. [Google Scholar] [CrossRef]
- López, R.; Hallat, J.; Castro, A.; Miras, A.; Burgos, P. Heavy metal pollution in soils and urban-grown organic vegetables in the province of Sevilla, Spain. Biol. Agric. Hortic. 2019, 35, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.M.; Mawodza, T.; Sarkar, B.; Menon, M. Assessment of potentially toxic trace element contamination in urban allotment soils and their uptake by onions: A preliminary case study from Sheffield, England. Ecotoxicol. Environ. Saf. 2019, 170, 156–165. [Google Scholar] [CrossRef]
- Paltseva, A.; Cheng, Z.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640–641, 273–283. [Google Scholar] [CrossRef]
- Alfaro, M.R.; do Nascimento, C.W.A.; Ugarte, O.M.; Álvarez, A.M.; de Aguiar Accioly, A.M.; Martín, B.C.; Jiménez, T.L.; Aguilar, M.G. First national-wide survey of trace elements in Cuban urban agriculture. Agron. Sustain. Dev. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Schmeltz, M.T.; Grassman, J.A.; Cheng, Z. Assessing Soil Lead Exposure for Gardeners in New York City—A Pilot Study; Springer: Cham, Switzerland, 2020; pp. 4–11. [Google Scholar] [CrossRef]
- Entwistle, J.A.; Amaibi, P.M.; Dean, J.R.; Deary, M.E.; Medock, D.; Morton, J.; Rodushkin, I.; Bramwell, L. An apple a day? Assessing gardeners’ lead exposure in urban agriculture sites to improve the derivation of soil assessment criteria. Environ. Int. 2019, 122, 130–141. [Google Scholar] [CrossRef]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in urban soils: A real or perceived concern for urban agriculture? J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.; Waterlot, C.; Sahmer, K.; Marot, F.; Douay, F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables—A review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar] [CrossRef]
- Paltseva, A.A.; Cheng, Z.; Egendorf, S.P.; Groffman, P.M. Remediation of an urban garden with elevated levels of soil contamination. Sci. Total Environ. 2020, 722, 137965. [Google Scholar] [CrossRef]
- Cooper, A.M.; Felix, D.; Alcantara, F.; Zaslavsky, I.; Work, A.; Watson, P.L.; Pezzoli, K.; Yu, Q.; Zhu, D.; Scavo, A.J.; et al. Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: A case study of an urban community garden. Plant Direct 2020, 4, e00198. [Google Scholar] [CrossRef] [Green Version]
- Egendorf, S.P.; Cheng, Z.; Deeb, M.; Flores, V.; Paltseva, A.; Walsh, D.; Groffman, P.; Mielke, H.W. Constructed soils for mitigating lead (Pb) exposure and promoting urban community gardening: The New York City Clean Soil Bank pilot study. Landsc. Urban Plan. 2018, 175, 184–194. [Google Scholar] [CrossRef]
- Arrobas, M.; Lopes, H.; Rodrigues, M.Â. Urban agriculture in Bragança, Northeast Portugal: Assessing the nutrient dynamic in the soil and plants, and their contamination with trace metals. Biol. Agric. Hortic. 2016, 33, 1–13. [Google Scholar] [CrossRef]
- Darko, G.; Adjei, S.; Nkansah, M.A.; Borquaye, L.S.; Boakye, K.O.; Dodd, M. Accumulation and bioaccessibility of toxic metals in root tubers and soils from gold mining and farming communities in the Ashanti region of Ghana. Int. J. Environ. Health Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Alloway, B.J. Contamination of soils in domestic gardens and allotments: A brief overview. L. Contam. Reclam. 2004, 12, 179–187. [Google Scholar] [CrossRef]
- Christodoulou, A.; Christidis, P. Evaluating congestion in urban areas: The case of Seville. Res. Transp. Bus. Manag. 2021, 39, 100577. [Google Scholar] [CrossRef]
- de Galdeano, C.S.; Vera, J.A. Stratigraphic record and palaeogeographical context of the Neogene basins in the Betic Cordillera, Spain. Basin Res. 1992, 4, 21–36. [Google Scholar] [CrossRef]
- Amils, R.; González-Toril, E.; Fernández-Remolar, D.; Gómez, F.; Aguilera, Á.; Rodríguez, N.; Malki, M.; García-Moyano, A.; Fairén, A.G.; de la Fuente, V.; et al. Extreme environments as Mars terrestrial analogs: The Rio Tinto case. Planet. Space Sci. 2007, 55, 370–381. [Google Scholar] [CrossRef]
- Galán, E.; Fernández-Caliani, J.C.; González, I.; Aparicio, P.; Romero, A. Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. J. Geochem. Explor. 2008, 98, 89–106. [Google Scholar] [CrossRef]
- EPA Method 6200: Field Portable X-Ray Fluorescence Spectrometry for the Determination of Elemental Concentrations in Soil and Sediment: Rev 0. February 2007. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/6200.pdf (accessed on 12 February 2019).
- López-Núñez, R.; Ajmal-Poley, F.; González-Pérez, J.A.; Bello-López, M.A.; Burgos-Doménech, P. Quick Analysis of Organic Amendments via Portable X-ray Fluorescence Spectrometry. Int. J. Environ. Res. Public Health 2019, 16, 4317. [Google Scholar] [CrossRef] [Green Version]
- Analizer XRF NitonTM XL3t GOLDD+. Available online: https://www.thermofisher.com/order/catalog/product/XL3TGOLDDPLUS#/XL3TGOLDDPLUS (accessed on 2 July 2021).
- Standard Reference Material® 2709a San Joaquin Soil. Available online: https://www-s.nist.gov/m-srmors/certificates/2709a.pdf (accessed on 1 September 2021).
- Liu, W.; Zhao, J.; Ouyang, Z.; Söderlund, L.; Liu, G. Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environ. Int. 2005, 31, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Ruíz, J.; Galán-Huertos, E.; Gómez-Ariza, J. Estudio de Elementos Traza en Suelos de Andalucía. Junta de Andalucía: Seville, Spain.
- Bu-Olayan, A.H.; Bivin, V.T. Translocation and Bioaccumulation of Trace Metals in Desert Plants of Kuwait Governorates. Res. J. Environ. Sci. 2009, 3, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for environmental assessment of soils: Not just a point and shoot method. Environ. Int. 2020, 134, 105250. [Google Scholar] [CrossRef]
- Ravansari, R.; Lemke, L.D. Portable X-ray fluorescence trace metal measurement in organic rich soils: pXRF response as a function of organic matter fraction. Geoderma 2018, 319, 175–184. [Google Scholar] [CrossRef]
- Samsøe-Petersen, L.; Larsen, E.H.; Larsen, P.B.; Bruun, P. Uptake of Trace Elements and PAHs by Fruit and Vegetables from Contaminated Soils. Environ. Sci. Technol. 2002, 36, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Sipter, E.; Rózsa, E.; Gruiz, K.; Tátrai, E.; Morvai, V. Site-specific risk assessment in contaminated vegetable gardens. Chemosphere 2008, 71, 1301–1307. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Abreu, M.M.; Santos, E.S.; Leidi, E.O. Soil–plant system and potential human health risk of Chinese cabbage and oregano growing in soils from Mn- and Fe-abandoned mines: Microcosm assay. Environ. Geochem. Health. 2020, 42, 4073–4086. [Google Scholar] [CrossRef]
- Rossini_Oliva, S.; Valdés, B.; Mingorance, M.D. Evaluation of some pollutant levels in bitter orange trees: Implications for human health. Food Chem. Toxicol. 2008, 46, 65–72. [Google Scholar] [CrossRef]
- McBride, M.B.; Shayler, H.A.; Russell-Anelli, J.M.; Spliethoff, H.M.; Marquez-Bravo, L.G. Arsenic and Lead Uptake by Vegetable Crops Grown on an Old Orchard Site Amended with Compost. Water Air Soil Pollut. 2015, 226, 265. [Google Scholar] [CrossRef] [Green Version]
- McBride, M.B.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Monaci, F.; Moni, F.; Lanciotti, E.; Grechi, D.; Bargagli, R. Biomonitoring of airborne metals in urban environments: New tracers of vehicle emission, in place of lead. Environ. Pollut. 2000, 107, 321–327. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Espinosa, A.J.F. Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem. J. 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Salminen, R.; Batista, M.J.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W. FOREGS Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; Geological Survey of Finland: Espoo, Finland, 2005. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils ad Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Monaci, F.; Leidi, E.O.; Mingorance, M.D.; Valdés, B.; Oliva, S.R.; Bargagli, R. Selective uptake of major and trace elements in Erica andevalensis, an endemic species to extreme habitats in the Iberian Pyrite Belt. J. Environ. Sci. 2011, 23, 444–452. [Google Scholar] [CrossRef]
- Codex Alimentarius Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTF. 2016. Available online: https://www.fao.org/3/i3243e/i3243e.pdf (accessed on 19 September 2019).
- Junta_de_Andalucía. Decree 18/2015, of January 27, Approving the Regulation about Contaminated Soils; Boletín Oficial de la Junta de Andalucía: Sevilla, Spain, 2015; pp. 28–64. [Google Scholar]
- Alexander, P.D.; Alloway, B.J.; Dourado, A.M. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef]
- Ge, Y.; Murray, P.; Hendershot, W.H. Trace metal speciation and bioavailability in urban soils. Environ. Pollut. 2000, 107, 137–144. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Alegría, A.; Barberá, R.; Boluda, R.; Errecalde, F.; Farré, R.; Lagarda, J.M. Environmental cadmium, lead and nickel contamination: Possible relationship between soil and vegetable content. Fresenius J. Anal. Chem. 1991, 339, 654–657. [Google Scholar] [CrossRef]

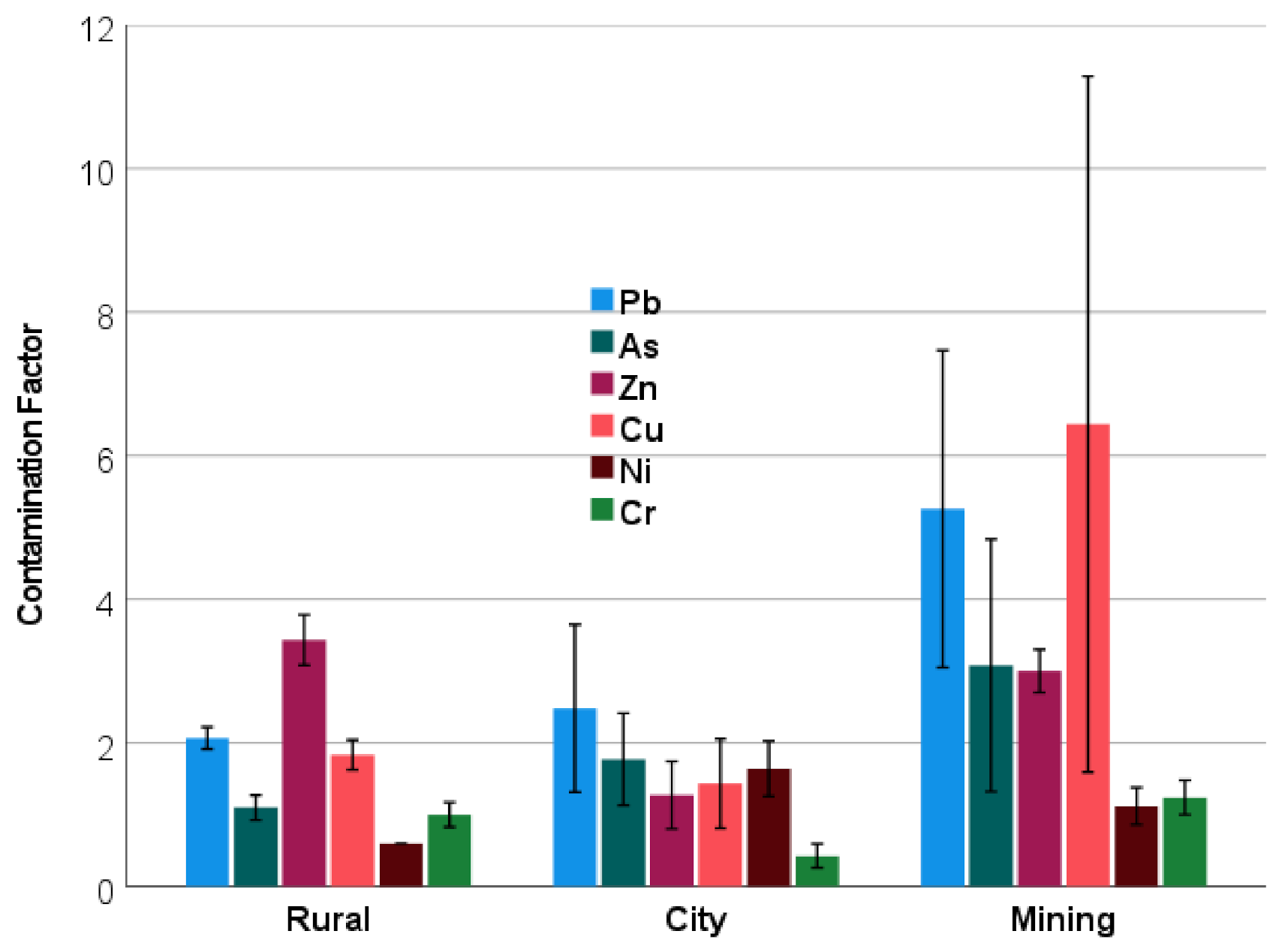
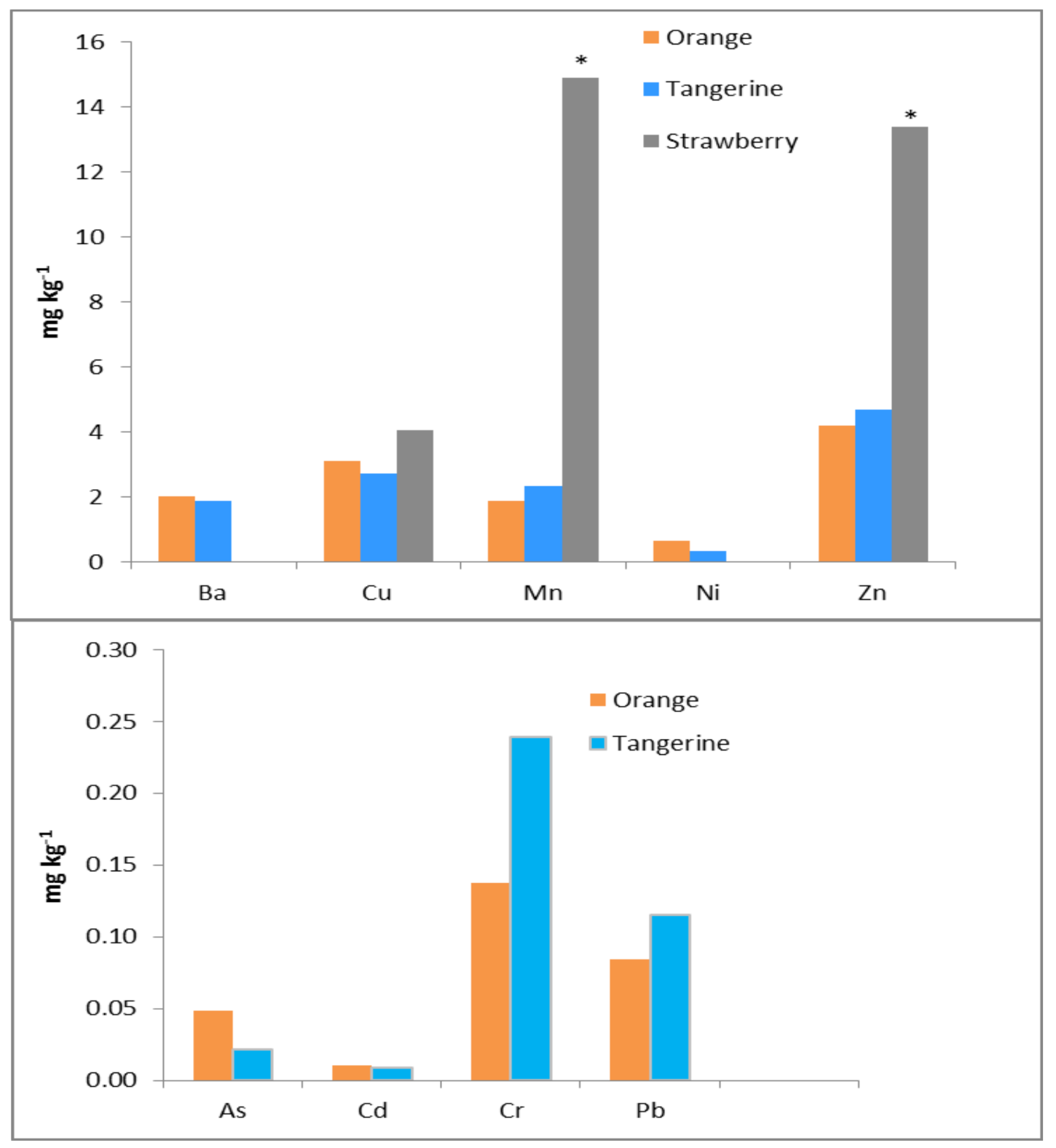
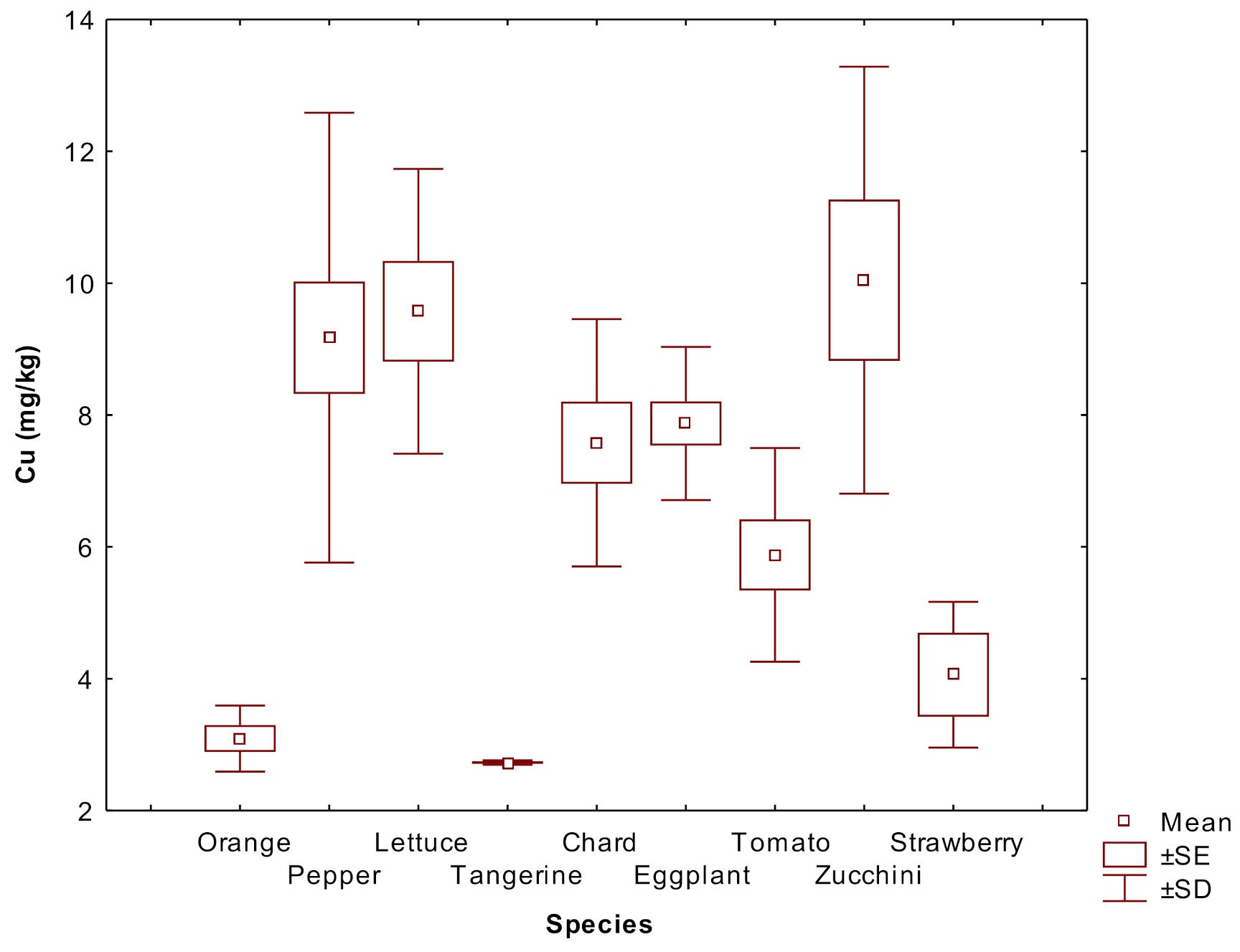
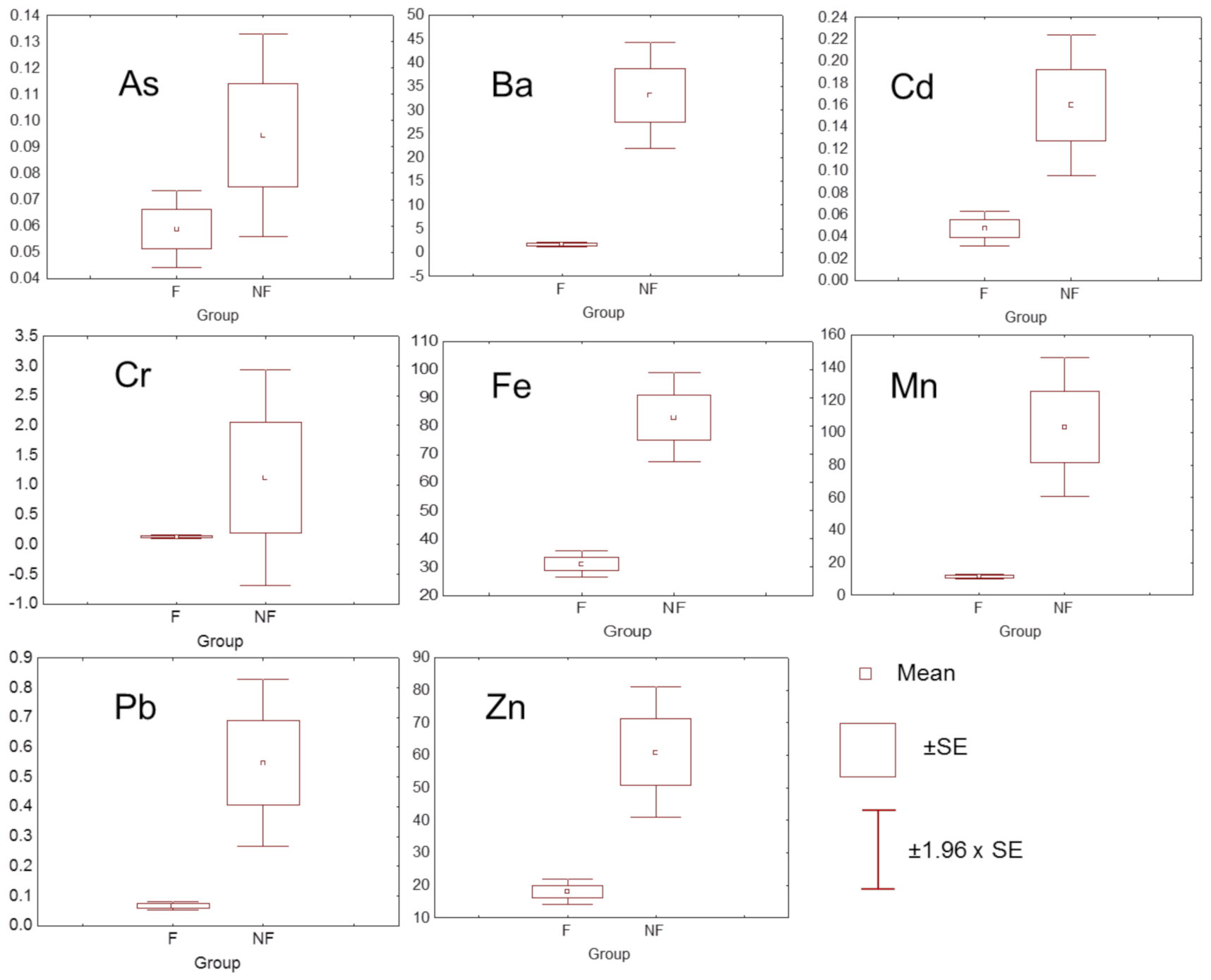
| Metal | City n = 16 | Rural n = 3 | Mining n = 5 | NIST 2709a |
|---|---|---|---|---|
| As | 10.4 a ± 3.8 | 21.0 a ± 3.4 | 77.0 b ± 44.1 | 10.5 |
| Ba | 370 b ± 50 | 264 a ± 129 | 332 ab ± 59 | 979 |
| Cr | 28.4 a ± 9.8 | 81.2 ab ± 13.4 | 117 b ± 22.0 | 130 |
| Cu | 34.5 a ±14.8 | 51.9 a ± 5.8 | 206 b ± 155 | 33.9 |
| Mn | 458 a ± 95 | 590 b ± 24 | 1331 c ± 128 | 529 |
| Ni | 48.8 ± 11.1 | BDL 1 | 39.5 ± 10.0 | 85 |
| Pb | 40.7 a ± 20.4 | 65.5 ab ± 4.7 | 199 b ± 84 | 17.3 |
| Sn | 11.5 a ± 2.8 | 7.1 a ± 0.0 | 22.5 b ± 9.4 | -- |
| Sr | 168 ± 105 | 59.6 ± 25.8 | 164 ± 42 | 239 |
| Zn | 68.8 a ± 27.5 | 272 c ± 28 | 228 b ± 22 | 103 |
| As | Ba | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pepper | City (n = 7) | --- | --- | BDL | BDL | 10.1 ± 3.18 | 30.6 ± 5.45 | 9.01 ± 2.10 | 0.65 ± 1.73 | BDL | 10.4 ± 3.29 |
| Mining (n = 6) | 0.05 ± 0.02 | 0.94 ± 0.53 | 0.05 ± 0.01 | 0.10 ± 0.05 | 7.30 ± 3.95 | 35.5 ± 15.1 | 8.81 ± 0.88 | 0.55 ± 0.46 | 0.07 ± 0.03 | 14.9 ± 2.66 | |
| Rural (n = 3) | 0.02 ± 0.00 | 1.42 ± 0.21 | 0.04 ± 0.00 | 0.10 ± 0.00 | 10.8 ± 0.61 | 43.0 ± 0.58 | 21.3 ± 1.08 | 0.33 ± 0.02 | 0.07 ± 0.00 | 17.5 ± 0.28 | |
| Significance | ns | ns | ns | ns | ns | ns | * | ns | ns | ns | |
| Eggplant | City (n = 3) | --- | --- | BDL | BDL | 8.41 ± 2.00 | 22.2 ± 4.02 | 12.9 ± 2.75 | BDL | 0.57 ± 0.98 | 18.3 ± 0.36 |
| Mining (n = 6) | 0.10 ± 0.00 | 1.27 ± 0.67 | 0.09 ± 0.03 | 0.09 ± 0.01 | 7.69 ± 0.85 | 30.1 ± 3.51 | 14.0 ± 3.05 | 0.30 ± 0.12 | 0.06 ± 0.01 | 18.4 ± 1.07 | |
| Rural (n = 3) | 0.02 ± 0.00 | 6.41 ± 1.32 | 0.03 ± 0.00 | 0.09 ± 0.02 | 7.68 ± 0.99 | 25.4 ± 2.34 | 17.0 ± 0.65 | 0.06 ± 0.01 | 0.06 ± 0.01 | 17.1 ± 1.13 | |
| Significance | * | * | * | ns | ns | * | ns | * | ns | ns | |
| Chard | City (n = 5) | --- | --- | BDL | BDL | 7.45 ± 2.31 | 107.6 ± 36.8 | 168 ± 59.7 | BDL | BDL | 25.0 ± 2.63 |
| Mining (n = 3) | 0.13 ± 0.00 | 19.2 ± 0.19 | 0.21 ± 0.00 | 0.29 ± 0.05 | 9.94 ± 1.41 | 101.8 ± 10.0 | 54.2 ± 4.10 | 0.28 ± 0.00 | 0.39 ± 0.22 | 26.3 ± 0.22 | |
| Rural (n = 3) | 0.09 ± 0.01 | 42.4 ± 1.00 | 0.17 ± 0.00 | 2.05 ± 3.19 | 6.16 ± 0.25 | 65.4 ± 4.17 | 253 ± 18.2 | 0.25 ± 0.02 | 0.83 ± 0.07 | 102 ± 4.47 | |
| Significance | * | * | * | ns | ns | ns | * | ns | * | * |
| Cu | Fe | Mn | Zn | ||
|---|---|---|---|---|---|
| Tomato | City (n = 6) | 5.62 ± 1.95 | 18.5 ± 4.37 | 7.33 ± 1.78 | 14.0 ± 3.66 |
| Rural (n = 3) | 6.40 ± 0.89 | 60.8 ± 12.9 | 17.0 ± 2.41 | 20.6 ± 2.07 | |
| Significance | ns | * | * | * | |
| Zucchini | City (n = 4) | 11.5 ± 3.8 | 49.9 ±22.8 | 15.9 ± 5.4 | 44.9 ±22.5 |
| Rural (n = 3) | 8.12 ± 0.06 | 56.2 ± 5.6 | 21.7 ± 1.4 | 57.1 ± 3.2 | |
| Significance | ns | ns | ns | ns | |
| Lettuce | City (n = 5) | 9.03 ± 2.67 | 92.8 ± 41.7 | 33.6 ± 11.0 | 41.0 ± 21.4 |
| Rural (n = 3) | 10.5 ± 0.38 | 46.5 ± 1.40 | 55.1 ± 1.92 | 123 ± 3.38 | |
| Significance | ns | ns | * | * |
| As | Ba | Cr | Cu | Mn | Ni | Pb | Zn | ||
|---|---|---|---|---|---|---|---|---|---|
| Tomato | City | -- | -- | -- | 0.13 | 0.01 | -- | -- | 0.19 |
| Rural | -- | -- | -- | 0.10 | 0.02 | -- | 0.001 | 0.07 | |
| Zucchini | City | -- | -- | -- | 0.27 | 0.03 | -- | -- | 0.57 |
| Control | -- | -- | -- | 0.15 | 0.03 | -- | -- | 0.21 | |
| Pepper | City | -- | -- | -- | 0.28 | 0.01 | 0.01 | -- | 0.13 |
| Mining | 0.0008 | 0.004 | 0.0008 | 0.05 | 0.006 | 0.01 | 0.0003 | 0.06 | |
| Rural | 0.0008 | 0.003 | 0.001 | 0.23 | 0.03 | -- | 0.0009 | 0.08 | |
| Lettuce | City | -- | -- | -- | 0.26 | 0.07 | -- | -- | 0.63 |
| Rural | -- | -- | -- | 0.18 | 0.08 | -- | 0.007 | 0.49 | |
| Eggplant | City | -- | -- | -- | 0.21 | 0.02 | -- | -- | 0.11 |
| Mining | 0.001 | 0.003 | 0.0009 | 0.05 | 0.01 | 0.007 | 0.0003 | 0.08 | |
| Rural | 0.0009 | 0.02 | 0.001 | 0.09 | 0.02 | -- | 0.0009 | 0.06 | |
| Chard | City | -- | -- | -- | 0.18 | 0.30 | -- | -- | 0.33 |
| Mining | 0.002 | 0.06 | 0.002 | 0.07 | 0.04 | 0.006 | 0.002 | 0.11 | |
| Rural | 0.004 | 0.16 | 0.02 | 0.09 | 0.42 | -- | 0.01 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossini-Oliva, S.; López-Núñez, R. Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions. Agronomy 2021, 11, 2151. https://doi.org/10.3390/agronomy11112151
Rossini-Oliva S, López-Núñez R. Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions. Agronomy. 2021; 11(11):2151. https://doi.org/10.3390/agronomy11112151
Chicago/Turabian StyleRossini-Oliva, Sabina, and Rafael López-Núñez. 2021. "Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions" Agronomy 11, no. 11: 2151. https://doi.org/10.3390/agronomy11112151
APA StyleRossini-Oliva, S., & López-Núñez, R. (2021). Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions. Agronomy, 11(11), 2151. https://doi.org/10.3390/agronomy11112151







