Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Plant Material
2.2. Growing Conditions and Experimental Design
2.3. 13CO2 Labeling of Plants
2.4. Harvest and Analyses
2.5. C Rhizodeposition Determination
2.6. Enzyme Activities and Stoichiometry
2.7. Statistical Analysis
3. Results
3.1. Soil Characteristics
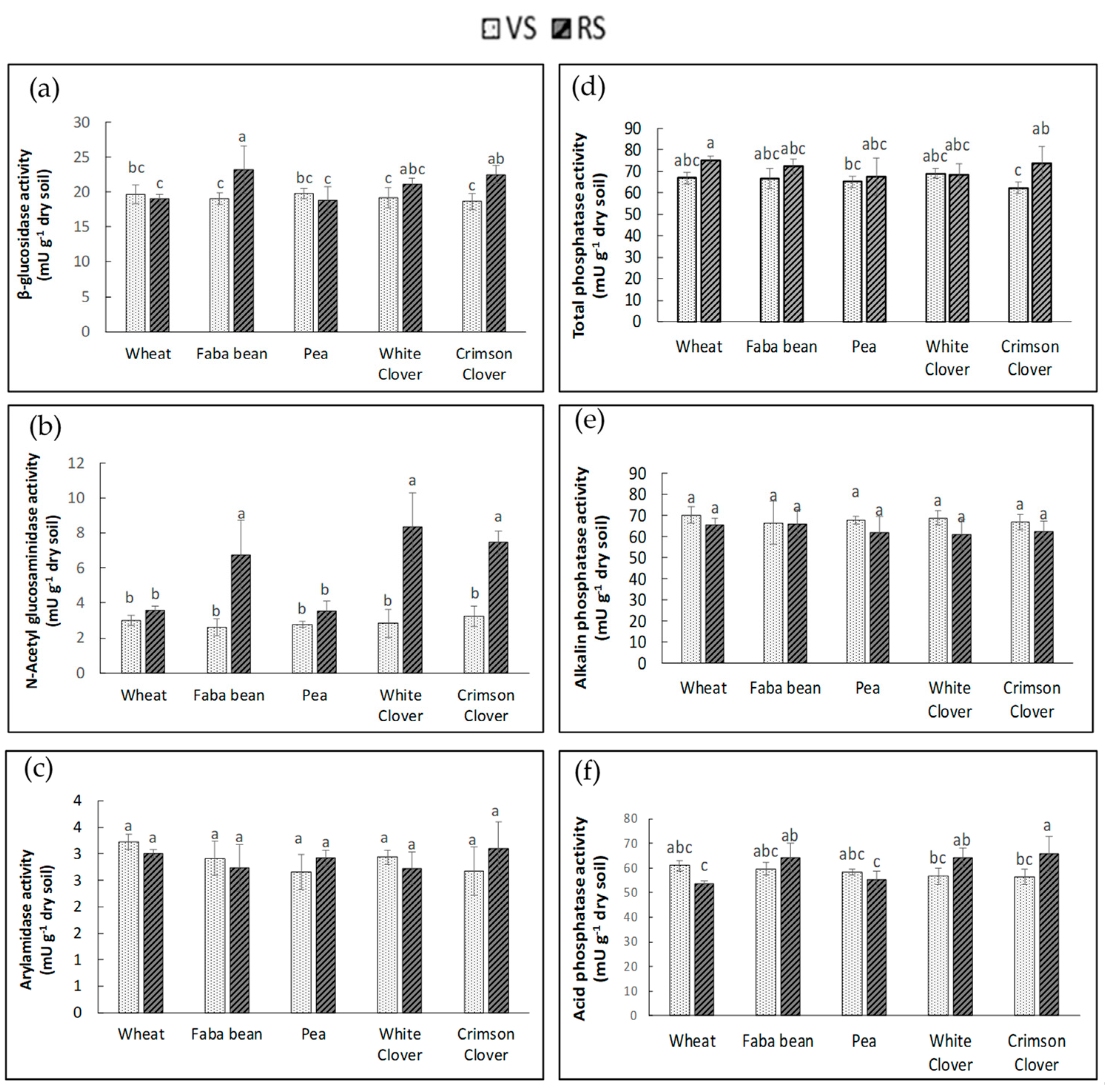
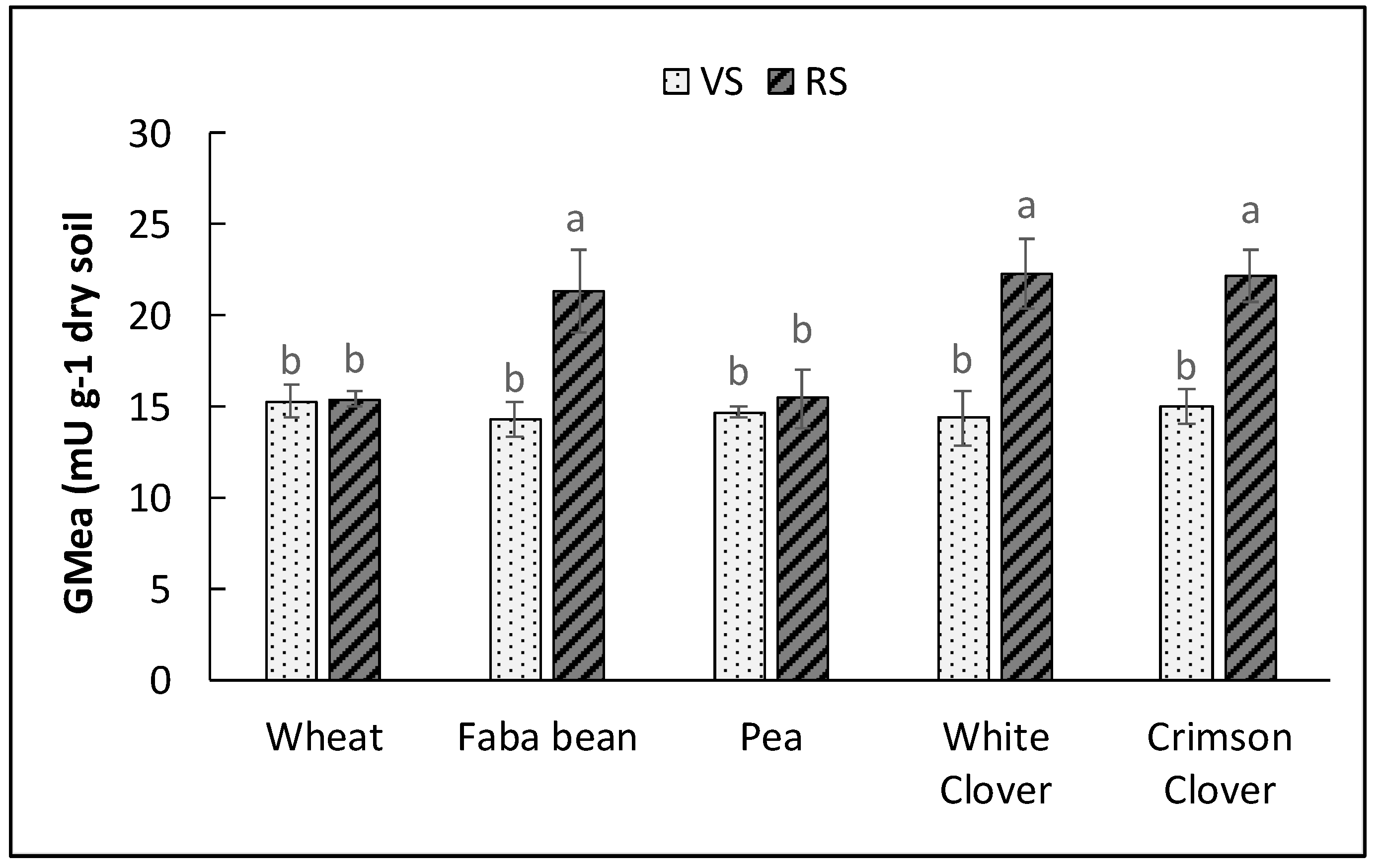
3.2. Enzymatic Activities
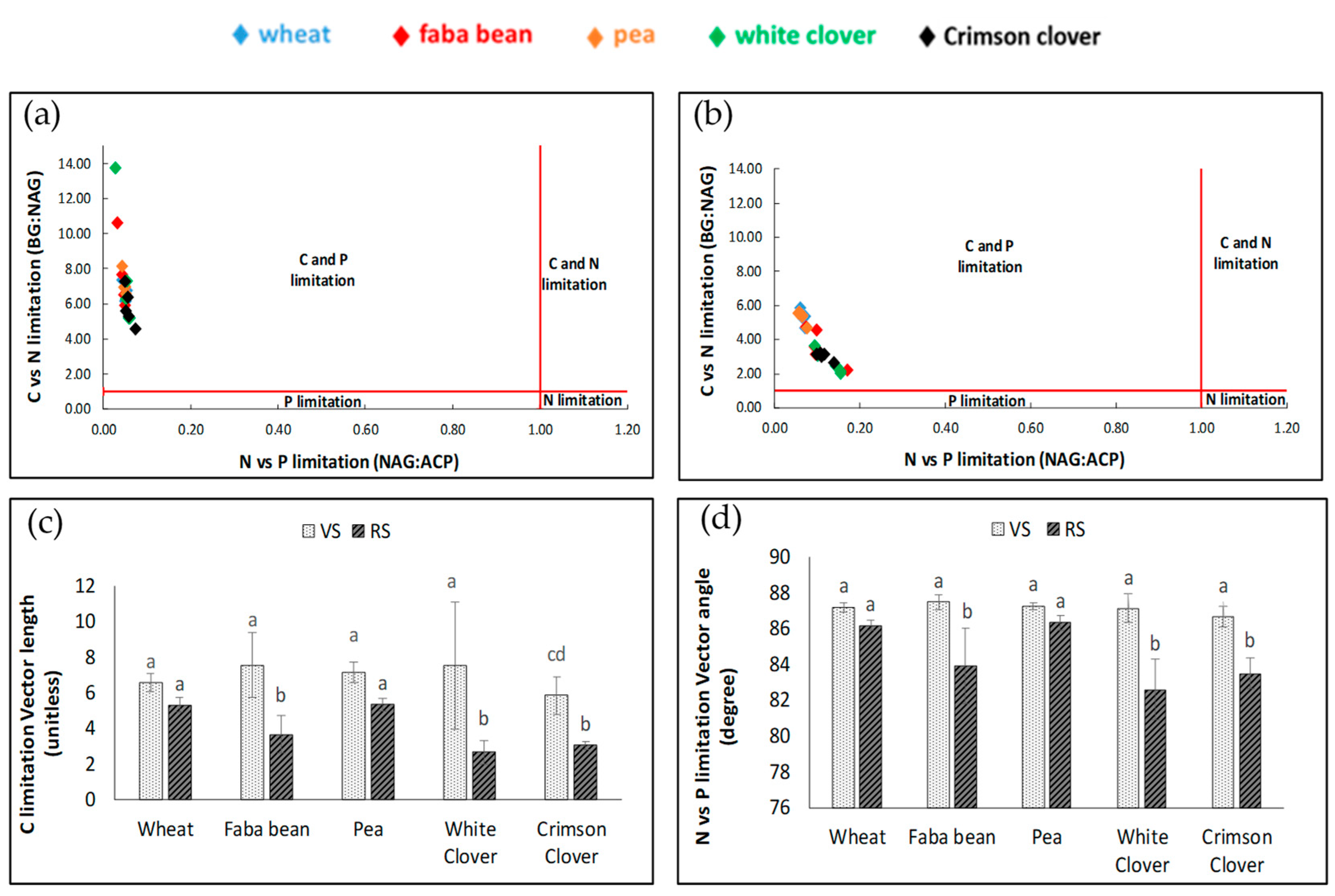
3.3. Enzyme’s Stoichiometry
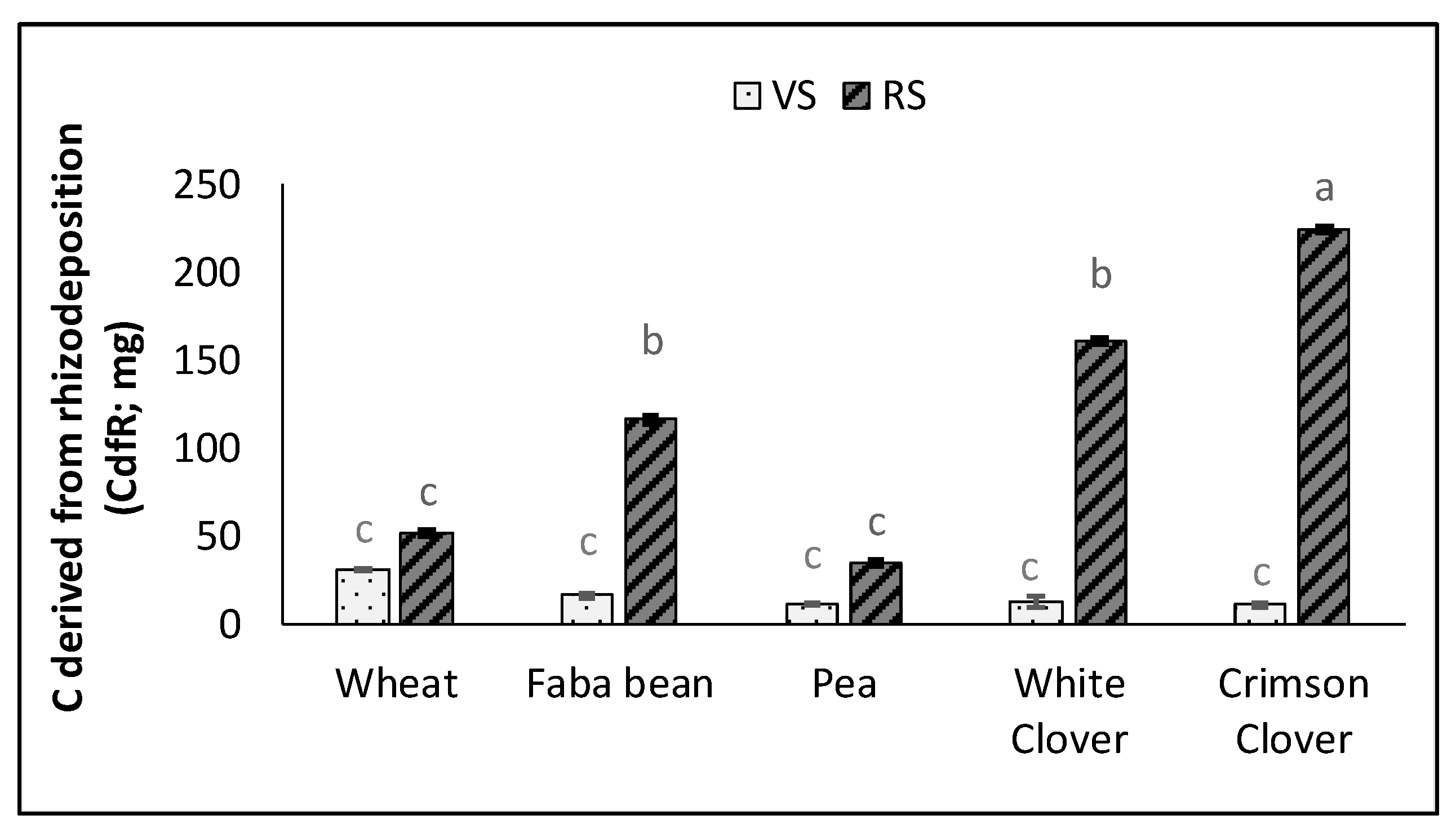
3.4. Plant Traits and C Rhizodeposition
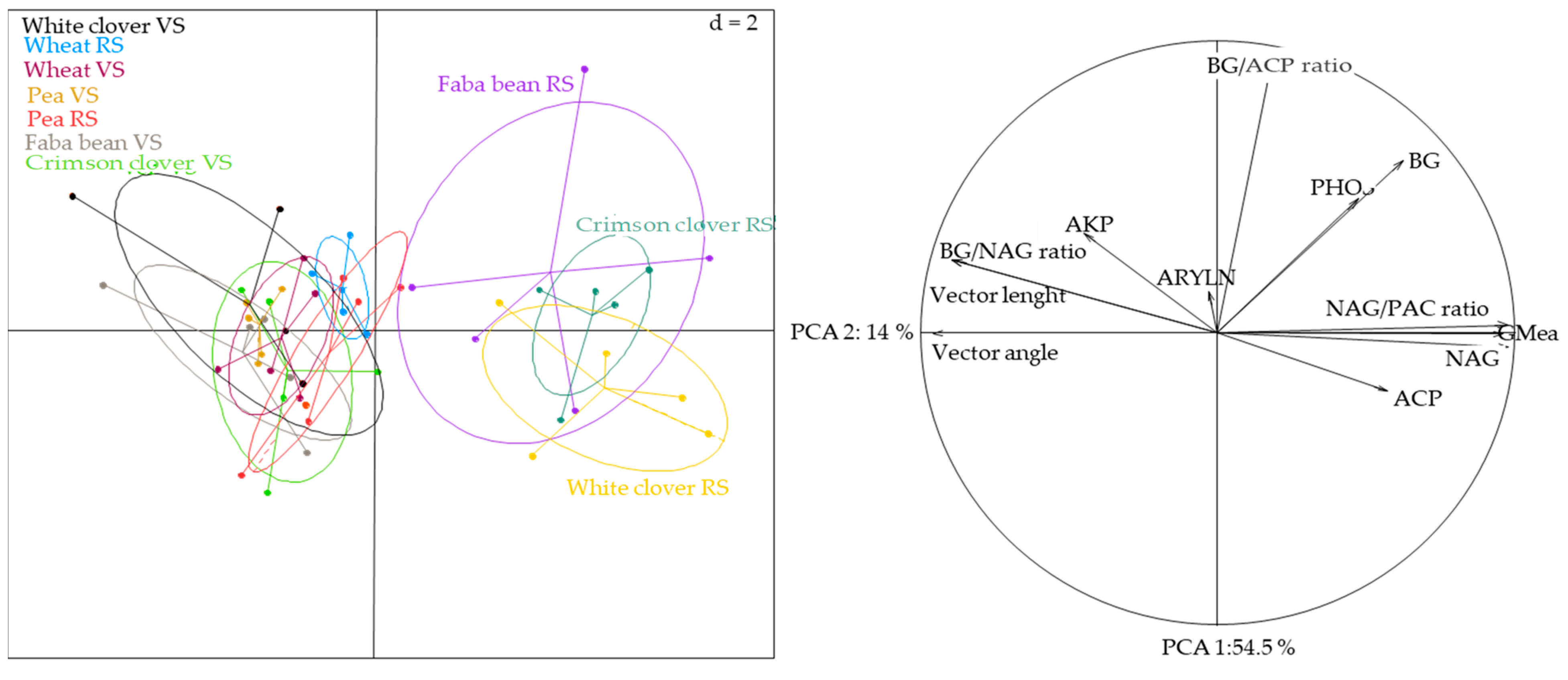
3.5. Linking Plant, Soil and Microbial Components
4. Discussion
4.1. Variability of Enzymatic Activities According to Plant Cover Crops
4.2. Microbial Resource Requirement under the Different Crops
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howden, S.M.; Soussana, J.-F.; Tubiello, F.N.; Chhetri, N.; Dunlop, M.; Meinke, H. Adapting Agriculture to Climate Change. Proc. Natl. Acad. Sci. USA 2007, 104, 19691–19696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burney, J.A.; Davis, S.J.; Lobell, D.B. Greenhouse Gas Mitigation by Agricultural Intensification. Proc. Natl. Acad. Sci. USA 2010, 107, 12052–12057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerson, M.; Morales, M.B.; Oñate, J.J.; Batáry, P.; Berendse, F.; Liira, J.; Aavik, T.; Guerrero, I.; Bommarco, R.; Eggers, S.; et al. Chapter Two—How Agricultural Intensification Affects Biodiversity and Ecosystem Services. In Advances in Ecological Research; Dumbrell, A.J., Kordas, R.L., Woodward, G., Eds.; Large-Scale Ecology: Model Systems to Global Perspectives; Academic Press: Cambridge, MA, USA, 2016; Volume 55, pp. 43–97. [Google Scholar]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What Is Sustainable Agriculture? A Systematic Review. Sustainability 2015, 7, 7833–7865. [Google Scholar] [CrossRef] [Green Version]
- DeLonge, M.S.; Miles, A.; Carlisle, L. Investing in the Transition to Sustainable Agriculture. Environ. Sci. Policy 2016, 55, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Wezel, A.; Jauneau, J.-C. Agroecology—Interpretations, Approaches and Their Links to Nature Conservation, Rural Development and Ecotourism. In Integrating Agriculture, Conservation and Ecotourism: Examples from the Field; Campbell, W.B., Lopez Ortiz, S., Eds.; Issues in Agroecology—Present Status and Future Prospectus; Springer: Dordrecht, The Netherlands, 2011; pp. 1–25. ISBN 978-94-007-1309-3. [Google Scholar]
- Kremen, C.; Miles, A. Comparing Biologically Diversified with Conventional Farming Systems: What Is Known about Environmental Benefits, Externalities and Tradeoffs among Crop Productivity and Ecosystem Services? Ecol. Soc. 2012, 17, 40. [Google Scholar] [CrossRef]
- Griffon, M. L’agroécologie, Un Nouvel Horizon Pour l’agriculture. Études 2014, 12, 31–39. [Google Scholar]
- Tittonell, P. Ecological Intensification of Agriculture—Sustainable by Nature. Curr. Opin. Environ. Sustain. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- Wezel, A.; Brives, H.; Casagrande, M.; Clément, C.; Dufour, A.; Vandenbroucke, P. Agroecology Territories: Places for Sustainable Agricultural and Food Systems and Biodiversity Conservation. Agroecol. Sustain. Food Syst. 2016, 40, 132–144. [Google Scholar] [CrossRef]
- Schneider, A.; Huyghe, C. Les Légumineuses Pour Des Systèmes Agricoles et Alimentaires Durables; éditions Quae: Versailles, France, 2015. [Google Scholar]
- Mylona, P.; Pawlowski, K.; Bisseling, T. Symbiotic Nitrogen Fixation. Plant Cell 1995, 7, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Das, A.; Yadav, G.S.; Lal, R. Legumes for Soil Health and Sustainable Management; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Altobelli, F.; Benedetti, A.; Calles, T.; Caon, L.; Charrondiere, R.; Gri Shiv, P.; Grande, F.; Muthuraman, R.P.; Pisante, M.; Pramar, B.; et al. Soils and Pulses: Symbiosis for Life—World 2016. Available online: https://reliefweb.int/report/world/soils-and-pulses-symbiosis-life (accessed on 1 October 2021).
- Adeboye, M.K.A.; Iwuafor, E.N.O.; Agbenin, J.O. Rotation Effects of Grain and Herbaceous Legumes on Maize Yield and Chemical Properties of an Alfisol in the Northern Guinea Savanna, Nigeria. Niger. J. Soil Environ. Res. 2005, 6, 22–31. [Google Scholar] [CrossRef]
- Vertès, F.; Jeuffroy, M.-H.; Louarn, G.; Voisin, A.-S.; Justes, E. Légumineuses et prairies temporaires: Des fournitures d’azote pour les rotations. Fourrages 2015, 223, 221–232. [Google Scholar]
- Rezgui, C.; Riah-Anglet, W.; Benoit, M.; Bernard, P.Y.; Laval, K.; Trinsoutrot-Gattin, I. Impacts of the Winter Pea Crop (Instead of Rapeseed) on Soil Microbial Communities, Nitrogen Balance and Wheat Yield. Agriculture 2020, 10, 548. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.-B. Nitrogen Rhizodeposition of Legumes. A Review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Wichern, F.; Eberhardt, E.; Mayer, J.; Joergensen, R.G.; Müller, T. Nitrogen Rhizodeposition in Agricultural Crops: Methods, Estimates and Future Prospects. Soil Biol. Biochem. 2008, 40, 30–48. [Google Scholar] [CrossRef]
- Chalk, P.M.; Peoples, M.B.; McNeill, A.M.; Boddey, R.M.; Unkovich, M.J.; Gardener, M.J.; Silva, C.F.; Chen, D. Methodologies for Estimating Nitrogen Transfer between Legumes and Companion Species in Agro-Ecosystems: A Review of 15N-Enriched Techniques. Soil Biol. Biochem. 2014, 73, 10–21. [Google Scholar] [CrossRef]
- Uren, N.C. Types, Amounts, and Possible Functions of Compounds Released into the Rhizosphere by Soil-Grown Plants. In The Rhizosphere; CRC Press: Boca Raton, FL, USA, 2001; pp. 35–56. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichern, F.; Mayer, J.; Joergensen, R.; Müller, T. Evaluation of the Wick Method for in Situ 13C and 15N Labelling of Annual Plants Using Sugar-Urea Mixtures. Plant Soil 2010, 329, 105–115. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Kennedy, A.C. Grain Legumes in Northern Great Plains: Impacts on Selected Biological Soil Processes. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of Organic C by Plants: Mechanisms and Controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon Input by Roots into the Soil: Quantification of Rhizodeposition from Root to Ecosystem Scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- El Haichar, F.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant Host Habitat and Root Exudates Shape Soil Bacterial Community Structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition Shapes Rhizosphere Microbial Community Structure in Organic Soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, A.; Palojärvi, A.; Schloter, M. Chapter 3.4—Soil Microbial Communities and Related Functions. In Perspectives for Agroecosystem Management; Schröder, P., Pfadenhauer, J., Munch, J.C., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 279–292. ISBN 978-0-444-51905-4. [Google Scholar]
- Ravi, R.K.; Anusuya, S.; Balachandar, M.; Muthukumar, T. Microbial interactions in soil formation and nutrient cycling. In Mycorrhizosphere and Pedogenesis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 363–382. [Google Scholar]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The role of microbial communities in the formation and decomposition of soil organic matter. In Soil Microbiology and Sustainable Crop Production; Springer: Berlin/Heidelberg, Germany, 2010; pp. 81–118. [Google Scholar]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Microorganisms and Their Roles in Fundamental Biogeochemical Cycles. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 775–833. [Google Scholar]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil Enzyme Activities as Biological Indicators of Soil Health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil Enzyme Activities, Microbial Communities, and Carbon and Nitrogen Availability in Organic Agroecosystems across an Intensively-Managed Agricultural Landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Chapter 33—Role of Soil Enzymes in Sustainable Crop Production. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 569–589. ISBN 978-0-12-813280-7. [Google Scholar]
- Rao, M.A.; Scelza, R.; Gianfreda, L. Soil Enzymes. In Enzymes in Agricultural Sciences; OMICS Group: Foster City, FL, USA, 2014; pp. 10–24. [Google Scholar]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Potential Use of Soil Enzymes as Soil Quality Indicators in Agriculture. In Frontiers in Soil and Environmental Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 57–64. [Google Scholar]
- Harvey, P.J.; Xiang, M.; Palmer, J.M. Extracellular Enzymes in the Rhizosphere. Biotechnol. Bioeng 2002, 44, 1132–1139. [Google Scholar]
- Chroma, L.; Mackova, M.; Kucerova, P.; In Der Wiesche, C.; Burkhard, J.; Macek, T. Enzymes in Plant Metabolism of PCBs and PAHs. Acta Biotechnol. 2002, 22, 35–41. [Google Scholar] [CrossRef]
- Gramss, G.; Voigt, K.-D.; Kirsche, B. Oxidoreductase Enzymes Liberated by Plant Roots and Their Effects on Soil Humic Material. Chemosphere 1999, 38, 1481–1494. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.V.N.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The Endophytic Microbiome as a Hotspot of Synergistic Interactions, with Prospects of Plant Growth Promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef]
- Dick, R.P. Soil Enzyme Activities as Indicators of Soil Quality. Defin. Soil Qual. A Sustain. Environ. 1994, 35, 107–124. [Google Scholar]
- Dick, R.P. Soil Enzyme Activities as Integrative Indicators of Soil Health. In Biological Indicators of Soil Health; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Eds.; USA7 CAB Internat: Wallingford, UK, 1997; pp. 121–156. [Google Scholar]
- Gianfreda, L. Enzymes of Importance to Rhizosphere Processes. J. Soil Sci. Plant Nutr. 2015, 15, 283–306. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Enzyme Activities in the Rhizosphere of Plants. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Soil Biology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2010; Volume 22, pp. 149–166. ISBN 978-3-642-14224-6. [Google Scholar]
- Yin, R.; Deng, H.; Wang, H.; Zhang, B. Vegetation Type Affects Soil Enzyme Activities and Microbial Functional Diversity Following Re-Vegetation of a Severely Eroded Red Soil in Sub-Tropical China. CATENA 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn-Szewczyk, B.; Antonkiewicz, J. Enzymatic Activity of Loess Soil in Organic and Conventional Farming Systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Mndzebele, B.; Ncube, B.; Fessehazion, M.; Mabhaudhi, T.; Amoo, S.; du Plooy, C.; Venter, S.; Modi, A. Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response. Agronomy 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Harasim, E.; Antonkiewicz, J.; Kwiatkowski, C.A. The Effects of Catch Crops and Tillage Systems on Selected Physical Properties and Enzymatic Activity of Loess Soil in a Spring Wheat Monoculture. Agronomy 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mi, G.; Chen, F.; Zhang, J.; Zhang, F. Rhizosphere Effect and Root Growth of Two Maize (Zea Mays L.) Genotypes with Contrasting P Efficiency at Low P Availability. Plant Sci. 2004, 167, 217–223. [Google Scholar] [CrossRef]
- Maseko, S.T.; Dakora, F.D. Plant Enzymes, Root Exudates, Cluster Roots and Mycorrhizal Symbiosis Are the Drivers of P Nutrition in Native Legumes Growing in P Deficient Soil of the Cape Fynbos in South Africa. J. Agric. Sci. Technol. A 2013, 3, 331. [Google Scholar]
- Maltais-Landry, G. Legumes Have a Greater Effect on Rhizosphere Properties (PH, Organic Acids and Enzyme Activity) but a Smaller Impact on Soil P Compared to Other Cover Crops. Plant Soil 2015, 394, 139–154. [Google Scholar] [CrossRef]
- Aschi, A.; Aubert, M.; Riah-Anglet, W.; Nélieu, S.; Dubois, C.; Akpa-Vinceslas, M.; Trinsoutrot-Gattin, I. Introduction of Faba Bean in Crop Rotation: Impacts on Soil Chemical and Biological Characteristics. Appl. Soil Ecol. 2017, 120, 219–228. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Kalembasa, S.; Kalembasa, D. Soil Microbial Activity of Faba Bean (Vicia Faba L.) and Wheat (Triticum Aestivum L.) Rhizosphere during Growing Season. Appl. Soil Ecol. 2018, 130, 34–39. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Chen, G.; Guo, J.; Li, Y. Enzyme Stoichiometry Indicates the Variation of Microbial Nutrient Requirements at Different Soil Depths in Subtropical Forests. PLoS ONE 2020, 15, e0220599. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Dippold, M.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular Enzyme Activity and Stoichiometry: The Effect of Soil Microbial Element Limitation during Leaf Litter Decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Li, Q.; Xue, S. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation during Secondary Succession of Natural Grassland on the Loess Plateau, China. Soil Tillage Res. 2020, 200, 104605. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F.; Findlay, S.G.; Kuehn, K.A.; Moorhead, D.L. Scaling Microbial Biomass, Metabolism and Resource Supply. Biogeochemistry 2015, 122, 175–190. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The Application of Ecological Stoichiometry to Plant–Microbial–Soil Organic Matter Transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 978-0-691-07491-7. [Google Scholar]
- Elser, J.J.; Urabe, J. The Stoichiometry of Consumer-Driven Nutrient Recycling: Theory, Observations, and Consequences. Ecology 1999, 80, 735–751. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular Enzyme Stoichiometry Reveals the Carbon and Phosphorus Limitations of Microbial Metabolisms in the Rhizosphere and Bulk Soils in Alpine Ecosystems. Plant Soil 2021, 458, 7–20. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic Stoichiometry of Microbial Nutrient Acquisition in Tropical Soils. Biogeochemistry 2014, 117, 101–113. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of Soil Enzyme Activity at Global Scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of Soil Extracellular Enzyme Activity along a Climatic Transect in Temperate Grasslands of Northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial Enzyme Stoichiometry and Nutrient Limitation in US Streams and Rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J.; Hill, B.H.; Elonen, C.M. Ecoenzymatic Stoichiometry of Stream Sediments with Comparison to Terrestrial Soils. Biogeochemistry 2012, 111, 455–467. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Xiao, K.; Wang, K. Soil Microbial Processes and Resource Limitation in Karst and Non-Karst Forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Lehto, L.L.P.; Sebestyen, S.D.; Seifert-Monson, L.R. Ecoenzymatic Stoichiometry and Microbial Processing of Organic Matter in Northern Bogs and Fens Reveals a Common P-Limitation between Peatland Types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Fanin, N.; Moorhead, D.; Bertrand, I. Eco-Enzymatic Stoichiometry and Enzymatic Vectors Reveal Differential C, N, P Dynamics in Decaying Litter along a Land-Use Gradient. Biogeochemistry 2016, 129, 21–36. [Google Scholar] [CrossRef]
- Pei, Z.; Leppert, K.N.; Eichenberg, D.; Bruelheide, H.; Niaus, P.A.; Buscot, F.; Gutknecht, J.L.M. Leaf Litter Diversity Alters Microbial Activity, Microbial Abundances, and Nutrient Cycling in a Subtropical Forest Ecosystem. Biogeochemistry 2017, 134, 163–181. [Google Scholar] [CrossRef]
- Ghosh, A.; Singh, A.B.; Kumar, R.V.; Manna, M.C.; Bhattacharyya, R.; Rahman, M.M.; Sharma, P.; Rajput, P.S.; Misra, S. Soil Enzymes and Microbial Elemental Stoichiometry as Bio-Indicators of Soil Quality in Diverse Cropping Systems and Nutrient Management Practices of Indian Vertisols. Appl. Soil Ecol. 2020, 145, 103304. [Google Scholar] [CrossRef]
- Cliquet, J.-B.; Deléens, E.; Bousser, A.; Martin, M.; Lescure, J.-C.; Prioul, J.-L.; Mariotti, A.; Morot-Gaudry, J.-F. Estimation of Carbon and Nitrogen Allocation during Stalk Elongation by 13C and 15N Tracing in Zea Mays L. Plant Physiol. 1990, 92, 79–87. [Google Scholar] [CrossRef] [Green Version]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of Enzyme Activities for the Monitoring of Soil Quality Improvement in Organic Agricultural Systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic Relationships between Microbial Biomass, Respiration, Inorganic Nutrients and Enzyme Activities: Informing Enzyme-Based Decomposition Models. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil Quality: Enzymatic Activity of Soil β-Glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–150. [Google Scholar]
- Dodor, D.E.; Tabatabai, M.A. Arylamidase Activity as an Index of Nitrogen Mineralization in Soils. Commun. Soil Sci. Plant Anal. 2007, 38, 2197–2207. [Google Scholar] [CrossRef]
- Ekenler, M.; Tabatabai, M.A. Arylamidase and Amidohydrolases in Soils as Affected by Liming and Tillage Systems. Soil Tillage Res. 2004, 77, 157–168. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.I.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil Extracellular Enzyme Activities, Soil Carbon and Nitrogen Storage under Nitrogen Fertilization: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Siczek, A.; Lipiec, J. Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season. Int. J. Mol. Sci. 2016, 17, 784. [Google Scholar] [CrossRef] [Green Version]
- Skujins, J. History of Abiontic Soil Enzyme Research; Soil Enzyme; Academic Press: New York, NY, USA, 1978; pp. 1–49. [Google Scholar]
- Zhou, Y.; Qin, Y.; Liu, X.; Feng, Z.; Zhu, H.; Yao, Q. Soil Bacterial Function Associated With Stylo (Legume) and Bahiagrass (Grass) Is Affected More Strongly by Soil Chemical Property Than by Bacterial Community Composition. Front Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- García-Palacios, P.; Maestre, F.T.; Gallardo, A. Soil Nutrient Heterogeneity Modulates Ecosystem Responses to Changes in the Identity and Richness of Plant Functional Groups. J. Ecol. 2011, 99, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of Carboxylates and Acid Phosphatase and Depletion of Different Phosphorus Fractions in the Rhizosphere of a Cereal and Three Grain Legumes. Plant Soil 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Faucon, M.-P.; Houben, D.; Lambers, H. Plant Functional Traits: Soil and Ecosystem Services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Navas, M.-L.; Grigulis, K. Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties; Oxford University Press: Oxford, UK, 2016; ISBN 978-0-19-875736-8. [Google Scholar]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in Microbial Biomass and Phosphorus Availability in the Rhizosphere of Intercropped Cereal and Legumes under Field Conditions. Soil Biol. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Pei, K.; Zhou, J.; Peixoto, L.; Gunina, A.; Zeng, Z.; Zang, H.; Rasmussen, J.; Kuzyakov, Y. Nitrogen Rhizodeposition by Legumes and Its Fate in Agroecosystems: A Field Study and Literature Review. Land Degrad. Dev. 2020, 32, 410–419. [Google Scholar] [CrossRef]
- Paynel, F.; Lesuffleur, F.; Bigot, J.; Diquélou, S.; Cliquet, J.-B. A Study of 15N Transfer between Legumes and Grasses. Agron. Sustain. Dev. 2008, 28, 281–290. [Google Scholar] [CrossRef]
- Treseder, K.K.; Vitousek, P.M. Effects of Soil Nutrient Availability on Investment in Acquisition of N and P in Hawaiian Rain Forests. Ecology 2001, 82, 946–954. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Wirén, N. von Root Nutrient Foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angers, D.A.; Caron, J. Plant-Induced Changes in Soil Structure: Processes and Feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Roumet, C.; Birouste, M.; Picon-Cochard, C.; Ghestem, M.; Osman, N.; Vrignon-Brenas, S.; Cao, K.; Stokes, A. Root Structure–Function Relationships in 74 Species: Evidence of a Root Economics Spectrum Related to Carbon Economy. New Phytol. 2016, 210, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, J.P.; Cantarel, A.A.M.; Simon, L.; el Zahar Haichar, F. Root Exudation Rate as Functional Trait Involved in Plant Nutrient-Use Strategy Classification. Ecol. Evol. 2018, 8, 8573–8581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Yang, S.; Dou, P.; Wang, H.; Wang, F.; Qian, S.; Yang, G.; Zhao, L.; Yang, Y.; Fanin, N. A Plant Economics Spectrum of Litter Decomposition among Coexisting Fern Species in a Subtropical Forest. Ann. Bot. 2019, 125. [Google Scholar] [CrossRef]
- Birouste, M.; Kazakou, E.; Blanchard, A.; Roumet, C. Plant Traits and Decomposition: Are the Relationships for Roots Comparable to Those for Leaves? Ann. Bot. 2012, 109, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardgett, R.D. Plant Trait-Based Approaches for Interrogating Belowground Function. Biol. Environ. Proc. R. Ir. Acad. 2017, 117B, 1–13. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant Rhizodeposition—An Important Source for Carbon Turnover in Soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.; Valori, F. Microbial Diversity and Microbial Activity in the Rhizosphere. Cienc. Suelo 2007, 25, 89–97. [Google Scholar]
- Weintraub, M.N.; Scott-Denton, L.E.; Schmidt, S.K.; Monson, R.K. The Effects of Tree Rhizodeposition on Soil Exoenzyme Activity, Dissolved Organic Carbon, and Nutrient Availability in a Subalpine Forest Ecosystem. Oecologia 2007, 154, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W. Rhizosphere Priming Effect: Its Functional Relationships with Microbial Turnover, Evapotranspiration, and C–N Budgets. Soil Biol. Biochem. 2009, 41, 1795–1801. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root Carbon Inputs to the Rhizosphere Stimulate Extracellular Enzyme Activity and Increase Nitrogen Availability in Temperate Forest Soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial Pattern of Enzyme Activities Depends on Root Exudate Composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Steinauer, K.; Chatzinotas, A.; Eisenhauer, N. Root Exudate Cocktails: The Link between Plant Diversity and Soil Microorganisms? Ecol. Evol. 2016, 6, 7387–7396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Waal, D.B.; Elser, J.J.; Martiny, A.C.; Sterner, R.W.; Cotner, J.B. Progress in Ecological Stoichiometry; Frontiers Research Topics; Frontiers Media SA: Lausanne, Switzerland, 2018; ISBN 978-2-88945-621-5. [Google Scholar]
- Gao, Y.; Zhou, P.; Mao, L.; Zhi, Y.; Zhang, C.; Shi, W. Effects of Plant Species Coexistence on Soil Enzyme Activities and Soil Microbial Community Structure under Cd and Pb Combined Pollution. J. Environ. Sci. 2010, 22, 1040–1048. [Google Scholar] [CrossRef]
- Sanaullah, M.; Blagodatskaya, E.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Drought Effects on Microbial Biomass and Enzyme Activities in the Rhizosphere of Grasses Depend on Plant Community Composition. Appl. Soil Ecol. 2011, 48, 38–44. [Google Scholar] [CrossRef]
- Elfstrand, S.; Hedlund, K.; Mårtensson, A. Soil Enzyme Activities, Microbial Community Composition and Function after 47 Years of Continuous Green Manuring. Appl. Soil Ecol. 2007, 35, 610–621. [Google Scholar] [CrossRef]
- Bell, C.; Carrillo, Y.; Boot, C.M.; Rocca, J.D.; Pendall, E.; Wallenstein, M.D. Rhizosphere Stoichiometry: Are C:N:P Ratios of Plants, Soils, and Enzymes Conserved at the Plant Species-Level? New Phytol. 2014, 201, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Bragazza, L.; Fontana, M.; Guillaume, T.; Scow, K.M.; Sinaj, S. Nutrient Stoichiometry of a Plant-Microbe-Soil System in Response to Cover Crop Species and Soil Type. Plant Soil 2021. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Allison, S.D. Cheaters, Diffusion and Nutrients Constrain Decomposition by Microbial Enzymes in Spatially Structured Environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of Microbial Nitrogen Use Efficiency to Carbon:Nitrogen Imbalances Regulates Soil Nitrogen Cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]

| Total C (g·kg−1) | Total N (g·kg−1) | P Olsen (mg·kg−1) | C:N | C:P | N:P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VS | RS | VS | RS | VS | RS | VS | RS | VS | RS | VS | RS | |
| Wheat | 11.77 ± 0.18 b | 11.37 ± 0.28 ab | 1.77 ± 0.47 b | 1.29 ± 0.02 ab | 143.5 ± 3.3 a | 137.7 ± 2.3 b | 6.94 ± 1.56 a | 8.80 ± 0.28 a | 82.1 ± 1.8 b | 82.6 ± 3.1 b | 12.40 ± 3.36 b | 9.39 ± 0.24 b |
| Faba bean | 11.64 ± 0.22 ab | 11.73 ± 0.10 bc | 1.36 ± 0.10 a | 1.33 ± 0.03 b | 147.8 ± 3.2 ab | 100.6 ± 5.7 a | 8.56 ± 0.52 b | 8.78 ± 0.18 a | 78.8± 2.3 ab | 116.8± 6.0 c | 9.22 ± 0.59 a | 13.30 ± 0.56 c |
| Pea | 11.25 ± 0.36 a | 11.01 ± 0.24 a | 1.29 ± 0.11 a | 1.28 ± 0.04 a | 151.5 ± 3.5 ab | 130.9 ± 10.5 b | 8.73 ± 0.56 b | 8.58 ± 0.28 a | 74.3 ± 3.0 a | 84.5 ± 7.0 b | 8.54 ± 0.80 a | 9.83 ± 0.57 b |
| White clover | 11.51 ± 0.18 ab | 11.81 ± 0.26 cd | 1.25 ± 0.02 a | 1.34 ± 0.02 b | 150.9 ± 4.1 ab | 88.6 + 5.1 a | 9.14 ± 0.11 b | 8.81 ± 0.14 a | 76.3 ± 2.9 a | 133.6 ± 6.4 d | 8.35 ± 0.37 a | 15.16 ± 0.65 d |
| Crimson clover | 11.39 ± 0.26 ab | 12.20 ± 0.29 d | 1.33 ± 0.07 a | 1.39 ± 0.03 c | 155.4 ± 7.8 b | 92.9 ± 1.9 a | 8.54 ± 0.60 ab | 8.74 ± 0.12 a | 73.4 ± 3.6 a | 131.4 ± 3.3 d | 8.62 ± 0.49 a | 15.02 ± 0.30 d |
| Plants species × development stages | F = 9.35 *** | F = 5.43 ** | F = 55.42 *** | F = 5.17 ** | F = 93.85 *** | F = 29.64 *** | ||||||
| Shoot Dry Mass (g) | Root Dry Mass (g) | Shoot:Root Ratio | Total Root Length (cm) | |||||
|---|---|---|---|---|---|---|---|---|
| VS | RS | VS | RS | VS | RS | VS | RS | |
| Wheat | 4.14 ± 0.35 c | 4.34 ± 0.34 a | 0.55 ± 0.26 a | 0.94 ± 0.09 a | 8.63 ± 3.46 b | 4.59 ± 0.37 a | 2435 ± 1031 a | 8961 ± 5223 ab |
| Faba bean | 2.08 ± 0.31 b | 13.95 ± 7.77 b | 1.52 ± 0.28 b | 6.46 ± 2.98 b | 1.37 ± 0.13 a | 2.12 ± 0.38 a | 4569 ± 1070 a | 24,329± 13,953 b |
| Pea | 2.25 ± 0.41 b | 3.75 ± 0.97 a | 0.55 ± 0.26 a | 0.39 ± 0.18 a | 4.42 ± 1.03 a | 10.98 ± 4.79 b | 4441 ± 1941 a | 3199± 15,292 a |
| White clover | 2.4 ± 0.24 b | 25.88 ± 1.60 c | 0.28 ± 0.04 a | 6.31 ± 1.66 b | 8.48 ± 1.14 b | 4.31 ± 1.08 a | 2379 ± 438 a | 82,655± 21,032 c |
| Crimson clover | 1.19 ± 0.18 a | 27.21 ± 2.03 c | 0.28 ± 0.04 a | 5.03 ± 0.24 b | 4.19 ± 0.52 a | 5.41 ± 0.44 a | 2617 ± 860 a | 60,859± 6339 c |
| Plants species × development stages | F = 52.54 *** | F = 17.04 *** | F = 12.55 *** | F = 42.31 *** | ||||
| Shoot C:N | Root C:N | |||
|---|---|---|---|---|
| VS | RS | VS | RS | |
| Wheat | 34.44 ± 2.16 c | 115.92 ± 27.10 b | 44.75 ± 6.09 b | 49.80 ± 9.46 b |
| Faba bean | 13.67 ± 1.07 a | 33.11 ± 7.90 a | 12.61 ± 0.29 a | 15.61 ± 1.11 a |
| Pea | 17.89 ± 3.10 b | 48.15 ± 18.89 a | 12.17 ± 0.31 a | 18.19 ± 2.78 a |
| White clover | 12.44 ± 1.05 a | 21.14 ± 1.90 a | 14.37 ± 0.74 a | 17.47 ± 1.08 a |
| Crimson clover | 13.05 ± 2.18 a | 19.38 ± 1.05 a | 14.75 ± 0.66 a | 17.12 ± 1.01 a |
| Plants species × development stages | F = 19.99 *** | F = 0.43 ns | ||
| Shoot Dry Mass | Root Dry Mass | Shoot:Root | Root Length | CdfR | Soil C:N | Soil C:P | Soil N:P | ACP | NAG | BG | GMea | BG:NAG | BG:ACP | NAG:ACP | Vector Length | Vector Angle | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoot dry mass | 1.00 | ||||||||||||||||
| Root dry mass | 0.87 *** | 1.00 | |||||||||||||||
| Shoot:Root | −0.16 | −0.39 ** | 1.00 | ||||||||||||||
| Root length | 0.94 *** | 0.84 *** | −0.23 | 1.00 | |||||||||||||
| CdfR | 0.94 *** | 0.81 *** | −0.16 | 0.86 *** | 1.00 | ||||||||||||
| Soil C:N | 0.14 | 0.17 | −0.12 | 0.17 | 0.14 | 1.00 | |||||||||||
| Soil C:P | 0.95 *** | 0.88 *** | −0.21 | 0.90 *** | 0.93 *** | 0.15 | 1.00 | ||||||||||
| Soil N:P | 0.84 *** | 0.75 *** | −0.12 | 0.77 *** | 0.81 *** | −0.32 * | 0.88 *** | 1.00 | |||||||||
| ACP | 0.61 *** | 0.63 *** | −0.19 | 0.55 *** | 0.65 *** | −0.11 | 0.62 *** | 0.64 *** | 1.00 | ||||||||
| NAG | 0.87 *** | 0.81 *** | −0.17 | 0.83 *** | 0.87 *** | 0.15 | 0.88 *** | 0.76 *** | 0.56 *** | 1.00 | |||||||
| BG | 0.67 *** | 0.76 *** | −0.16 | 0.54 *** | 0.65 *** | 0.09 | 0.64 *** | 0.56 *** | 0.59 *** | 0.57 *** | 1.00 | ||||||
| GMea | 0.90 *** | 0.87 *** | −0.18 | 0.83 *** | 0.91 *** | 0.11 | 0.91 *** | 0.81 *** | 0.70 *** | 0.97 *** | 0.72 *** | 1.00 | |||||
| BG:NAG | −0.69 *** | −0.62 *** | 0.09 | −0.66 *** | −0.71 *** | −0.04 | −0.73 *** | −0.65 *** | −0.42 ** | −0.84 *** | −0.33 * | −0.82 *** | 1.00 | ||||
| BG:ACP | 0.19 | 0.26 | −0.01 | 0.10 | 0.13 | 0.22 | 0.14 | 0.03 | −0.29 * | 0.13 | 0.59 *** | 0.16 | 0.03 | 1.00 | |||
| NAG:ACP | 0.83 *** | 0.75 *** | −0.15 | 0.79 *** | 0.82 *** | 0.17 | 0.84 *** | 0.72 *** | 0.40 ** | 0.98 *** | 0.50 ** | 0.92 *** | −0.84 *** | 0.20 | 1.00 | ||
| Vector lenght | −0.69 *** | −0.62 *** | 0.09 | −0.65 *** | −0.71 *** | −0.04 | −0.73 *** | −0.65 *** | −0.42 ** | −0.84 | −0.33 * | −0.82 *** | 0.99 *** | 0.03 | −0.84 *** | 1.00 | |
| Vector angle | −0.83 *** | −0.75 *** | 0.15 | −0.79 *** | −0.82 *** | −0.17 | −0.84 *** | −0.71 *** | −0.40 ** | −0.98 *** | −0.51 ** | −0.92 *** | 0.84 *** | −0.20 | −0.99 *** | 0.84 *** | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanté, M.; Riah-Anglet, W.; Cliquet, J.-B.; Trinsoutrot-Gattin, I. Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy 2021, 11, 2131. https://doi.org/10.3390/agronomy11112131
Kanté M, Riah-Anglet W, Cliquet J-B, Trinsoutrot-Gattin I. Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy. 2021; 11(11):2131. https://doi.org/10.3390/agronomy11112131
Chicago/Turabian StyleKanté, Mohamed, Wassila Riah-Anglet, Jean-Bernard Cliquet, and Isabelle Trinsoutrot-Gattin. 2021. "Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition" Agronomy 11, no. 11: 2131. https://doi.org/10.3390/agronomy11112131
APA StyleKanté, M., Riah-Anglet, W., Cliquet, J.-B., & Trinsoutrot-Gattin, I. (2021). Soil Enzyme Activity and Stoichiometry: Linking Soil Microorganism Resource Requirement and Legume Carbon Rhizodeposition. Agronomy, 11(11), 2131. https://doi.org/10.3390/agronomy11112131







