It should be emphasized that the basic microbiological parameters used in the assessment of the ecological condition of soils include the total number of bacteria [
19]. According to Novak et al. [
20], soil is the environment where the biodiversity of bacteria is high. They play an important role as soil quality indicators due to their involvement in the processes occurring in agroecosystems [
21]. However, even the most uniform soil is not a homogeneous environment for bacteria.
3.1. Vegetative Forms
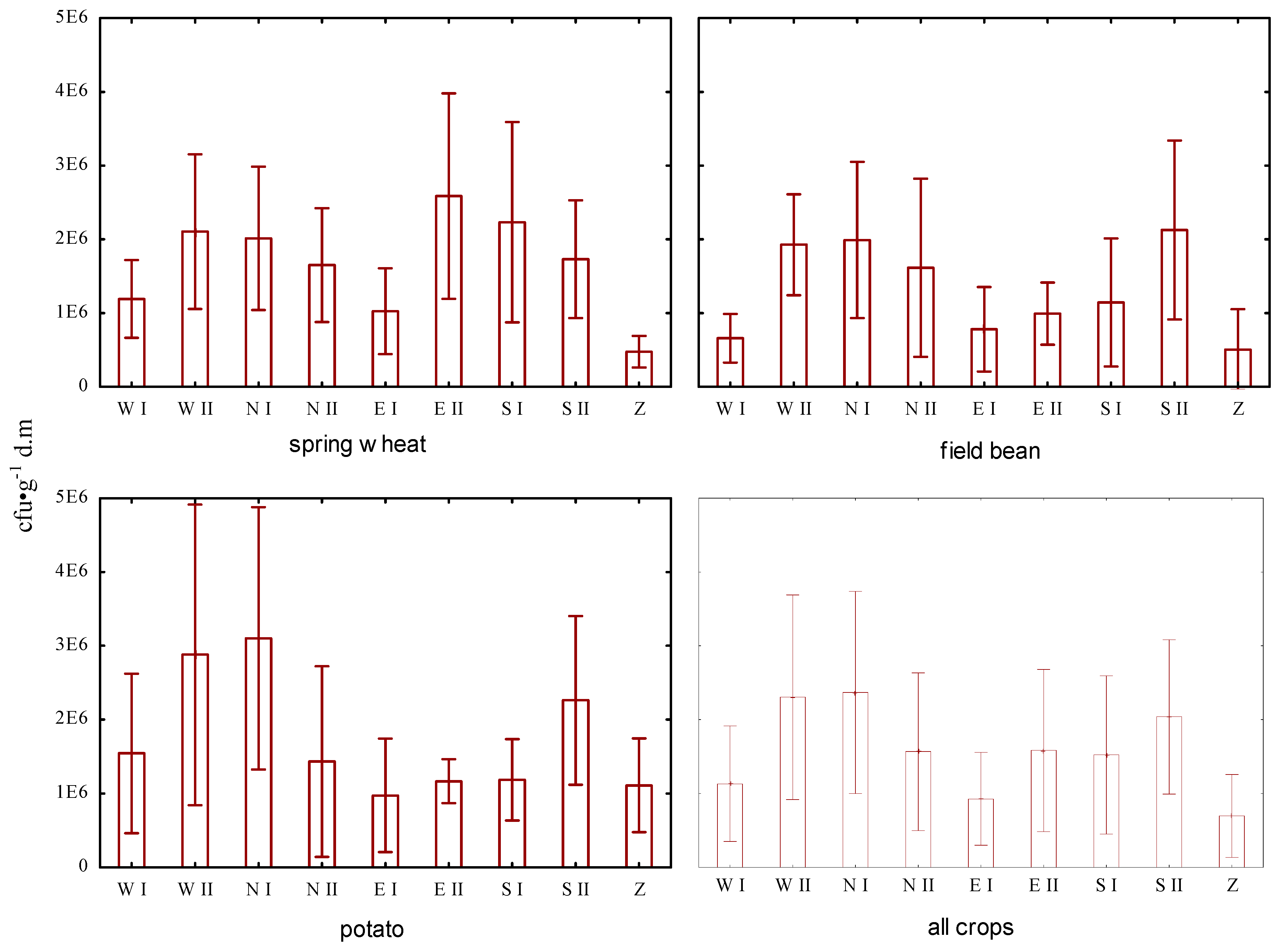
The bacterial numbers in the vegetative state ranged from 13.2 to 578.2 × 10
4 cfu g
−1 dry soil (
Figure 1). Their highest value was found in plot WII for the cultivation of potatoes, and the lowest number in plot Z for the cultivation of horse beans. In the case of plots located outside the landfill site, the numbers of bacterial vegetative forms were in the range of 27.1–492.2 × 10
4 cfu g
−1 dry soil for the cultivation of wheat, 13.6–416.0 × 10
4 cfu g
−1 dry soil for the cultivation of horse beans and from 29.6 190 to 578.2 × 10
4 cfu g
−1 dry soil for potato cultivation. The bacterial abundance in the soil of the experimental plot located in the landfill area was in the range of 18.2–80.3 × 10
4 cfu g
−1 dry soil for wheat cultivation, 13.2–179.2 × 10
4 cfu g
−1 dry soil for the cultivation of horse bean and 51.3–186.1 × 10
4 cfu g
−1 dry soil for potato cultivation. Comparing the median numbers of vegetative bacteria, the highest differences were observed between the plot in the field of horse bean cultivation located in the reclaimed sector (median: 31.8 × 10
4 cfu g
−1 dry soil) and the plot in the cultivation of wheat, located at a distance of 250 to 500 m from the landfill area (median: 192.3 × 10
4 cfu g
−1 dry soil) (
Table 2).
A one-way analysis of variance (ANOVA) was performed to assess the impact of the location of the plot in relation to the landfill site on the bacterial numbers in the vegetative and sporulating stage for the cultivation of agricultural plants near the municipal waste landfill. The ANOVA results together with the Newman–Keuls test (
p < 0.05) showed a significant effect of the cultivated plot (indirectly of municipal waste landfill) on the bacterial numbers in vegetative forms (
p < 0.05). It was found that their average abundance in soil for the cultivation of all crops (wheat, field beans, potato) in plot Z, located in the landfill area, was significantly lower than the average numbers recorded for plots located 250–500 m from the landfill site (Newman–Keuls test:
p < 0.05). However, no significant differences were found between the plots located outside the landfill area, in two zones, both located at a distance of 50–250 m from its area (zone I) and 250–500 m (zone II) (Newman–Keuls test:
p > 0.05) (
Figure 1). The results of the comparisons showed that the mean values of the bacterial vegetative forms for the studied soil differed the most when the mean value of the Z plot was compared with that of the WII, SII and NI plots (Newman–Keuls test:
p < 0.05). A statistically significant predominance of the average numbers of vegetative bacteria in the NI plot was also observed over the Z, EI, EII, WI, Si and NII plots. However, there were no significant differences for the means between the EI, WI and SI, NII, EII plots (Newman–Keuls test:
p > 0.05). The analysis clearly showed that there were statistically significant differences in the vegetative bacterial numbers in the soil depending on the distance of the plot from the landfill site (Newman–Keuls test:
p < 0.05). For the wheat cultivation, significant differences for the means were observed between plot Z and plot EII (Newman–Keuls test:
p < 0.05); regarding the potatoes, there were significant differences for means between plots EI and Z and NI and WII (Newman–Keuls test:
p < 0.05).
Regardless of the plot’s location, the grain size composition of the soils did not have a significant effect on the recorded numbers of vegetative bacteria (p > 0.05). The means that individual soil types did not differ significantly (Newman–Keuls test: p > 0.05). The highest average was recorded for the heavy clay sand, and it was not significantly higher than the lowest average for the light clay.
The impact of the element contents in the soil on the numbers of bacteria in vegetative forms observed in the plots was assessed using the Pearson correlation coefficient. For most cases, there was no significant correlation between the presence of elements in the soil and the number of vegetative bacteria in the soil. The numbers of vegetative bacteria were only weakly negatively correlated (p < 0.05) with the presence of calcium (R = −0.39 p < 0.05) and chlorine: (R = −0.40 p < 0.05), and weakly positively correlated with the pH values (r = 0.37 p < 0.05) and heavy metals cadmium (r = 0.35 p < 0.05) and lead (r = 0.38 p < 0.05).
It is also worth noting that the statistical analysis showed that the total numbers of bacterial vegetative forms differed insignificantly (
p > 0.05) depending on the type of cultivated plant. In this case, their highest mean values were recorded in the plots of wheat cultivation, but it was non-significantly higher than the lowest mean value recorded for the horse beans (Newman–Keuls test:
p > 0.05). The conducted analyses showed that the type of the plant cultivated in the plots did not have a significant effect on the number of bacterial vegetative forms present in the soil (
p > 0.05). These results were confirmed by Wielgosz et al. [
22], who indicated that plants only slightly stimulated the growth of the number of bacteria in the soil. However, the authors draw attention to the fact that the survival and activity of soil microorganisms are closely related to the growing plants [
23,
24].
The simultaneous effect of the sampling site (first factor) and the month of the year (second factor) on the occurrence of vegetative forms of bacteria in the soil was investigated by performing a two-way analysis of variance (ANOVA). The results of the analysis showed that the impact of the site (plot) on the number of bacterial vegetative forms was stronger than that of the month. In general, the vegetative bacteria were more numerous in the summer months than in spring or early autumn (
p < 0.05). In this case, the means of vegetative bacteria for May and July were the highest—significantly higher compared to the months when these microorganisms were least frequent (March and September, respectively) (Newman–Keuls test:
p < 0.05). Wielgosz and Szember [
24] have claimed that there are two periods of increased microbial growth during the year: The first with the occurrence of higher temperatures, and the second with the supply of organic matter to the soil. Similarly, Kulig [
4] has indicated that significant differences in the intensity and range of the impact of municipal facilities are caused by meteorological conditions.
3.2. Sporulating Forms
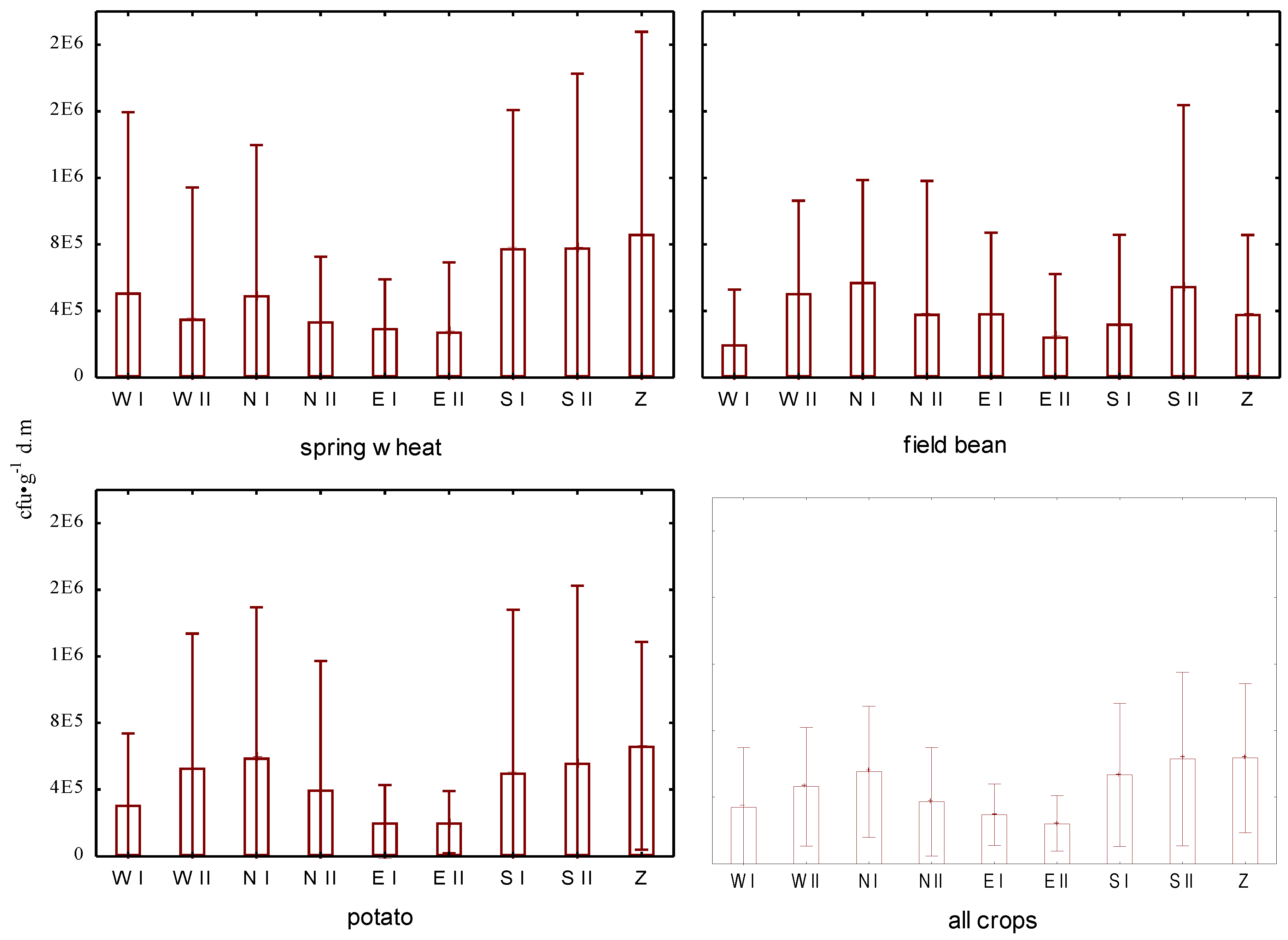
In the analysed soils, the numbers of bacteria in the sporulating stage ranged from 3.8 to 196.8 × 10
4 cfu g
−1 dry soil (
Figure 2). Their highest counts were recorded in the SII plot for the cultivation of wheat, and the lowest numbers were found for the NII plot under the cultivation of horse beans. For the plots located outside the landfill, the numbers of these bacterial forms were in the range of 5.3–196.8 × 10
4 cfu g
−1 dry soil, 3.8 to 134.8 × 10
4 cfu g
−1 dry soil and from 4.9 to 167.6 × 10
4 cfu g
−1 dry soil under the cultivation of wheat, horse beans and potatoes, respectively. The data allowed us to conclude that the ranges of their numbers in the plot located in the reclaimed sector were from 19.2 to 174.6 × 10
4 cfu g
−1 dry soil, from 17.8 to 83.8 × 10
4 cfu g
−1 dry soil and from 33.6 to 127.0 × 10
4 cfu g
−1 dry soil under wheat, horse beans and potato cultivation, respectively. When comparing the median number of surviving bacteria, the highest differences were observed between the plots in the field of horse bean cultivation located within 250 m from the landfill site (median: 25.5 × 10
4 cfu g
−1 dry soil) (
Table 3), and the plot located in the landfill under the wheat cultivation (median: 89.6 × 10
4 cfu g
−1 dry soil).
The one-way ANOVA showed, as in the case of vegetative bacteria, a significant effect of the position of the plot to the landfill on the numbers of sporulating bacteria in the soil (
p < 0.05). In this case, the greatest differences in average values were observed between the plot located in the landfill area in the reclaimed sector, compared to the plot located a considerable distance from the landfill (250–500 m) outside its area, on the eastern side. The highest mean for plot Z was significantly higher than the recorded mean of the lowest for plot EII (Newman–Keuls test:
p < 0.05). However, no statistically significant differences were observed between the plots located near the landfill, that is WI, NII, WII, SI and NI (Newman–Keuls test:
p > 0.05) (
Figure 2). Statistical analysis showed no significant differences in the abundance of sporulating bacteria in the soil depending on the distance between the plots and the landfill area (Newman–Keuls test:
p > 0.05).
It should be emphasized that the numbers of surviving soil bacteria in the plots, also due to the type of soil present there, did not differ significantly in their levels from each other (
p > 0.05). As the analyses showed, the differences in the mean values between the different soil types found in the experimental plots were not statistically significant (Newman–Keuls test:
p > 0.05). The highest average was recorded for the light clayey sand, but it was not significantly higher than that of the lowest average for heavy clay sand. Apart from determining the granulometric composition of the tested soils, tests of their chemical properties (pH, elemental composition) were also carried out in each selected plot. These are factors that, depending on local conditions, may have a significant impact on the number of microorganisms present in the soil. The impact of the measured chemical factors of the studied soils on the presence of sporulating bacteria was assessed based on the correlation coefficient. As the analyses showed, only the presence of copper Cu (r = 0.32
p < 0.05) and nickel Ni (r = 0.35
p < 0.05) in the soil was of significant importance in this respect. In other cases, there was no significant correlation between the presence of elements in the soil and the presence of sporulating bacteria. It should be emphasized that microorganisms can both contribute to increasing the content of metals in the environment and reduce their mobility or toxicity [
25].
The results of the statistical analysis did not reveal a significant effect of the cultivated plant on the numbers of sporulating bacteria in the plot experiment, as the means for individual plants differed non-significantly (
p > 0.05). In this case, the highest average was recorded in the soil under the wheat cultivation and did not differ significantly from the lowest average recorded under the cultivation of field beans. For the wheat cultivation, the highest average was recorded for plot Z; it was non-significantly higher than that of the lowest plot for EII (Newman–Keuls test:
p > 0.05). Additionally, a high average was recorded for the SI and SII plots (slightly lower than the highest average). For the plots under the horse bean cultivation, the highest mean was recorded for the NI plot compared to that of the lowest average for the WI plot, while in the cultivation of potatoes, the greatest differences were recorded between the mean for the plot Z and the lowest mean recorded for the plot EI. The recorded means were not significantly different (Newman–Keuls test:
p > 0.05). The plant is one of the elements in the entire biocenotic system and all physiological changes that occur during the growing season are reflected in the features of the soil microbial community [
22,
23].
The simultaneous impact of the sampling place and the month of the year on the occurrence of sporulating soil bacteria in the plots was investigated by performing a two-way ANOVA. Overall, it was shown that the effect of the site on the numbers of sporulating bacteria was stronger than that of the month of the year. The comparison of ANOVA results using the Newman–Keuls test (p < 0.05) indicated that the means for the individual months of the year did not differ significantly. The highest average numbers of these bacteria were recorded in the summer months (May, July). These values were not significantly higher than the lowest means recorded in spring, e.g., in March (p > 0.05).
3.3. Bacteriological Indicator
If the highest average number of bacteria of vegetative forms in the soil under the cultivation of wheat in the E II plot is taken as 100%, the calculated percentage of bacteria in the remaining plots located at different distances from the landfill was as follows: At the experimental sites located up to 250 m from the landfill (WI, NI, E, I and SI plots), it ranged from 39.6 to 86.2, and at the plots located in zone II, i.e., at a distance of 250 to 500 m, that is In II, N II and S II, it remained at the level of 64.9–80.9%. For the soil of the plot located in the reclaimed sector (plot number Z), this ratio was 18.4%. For the horse bean cultivation, the highest average number of bacterial vegetative forms was recorded in the S II plot and was assumed as 100%; the calculated percentage of bacteria in the studied sites located in zone I was 30.9 to 93.6, while for the experimental plots located in zone II, it remained at the level of 46.6–90.6%. In the soil in the plot located within the landfill site, this ratio was 23.7%. For the potato cultivation, the highest average number of bacterial vegetative forms was recorded for the NI plot, and the calculated percentage of bacteria in the soil under potato cultivation in the plots located in zone I was from 31.3 to 45.7%, and the research sites located in zone II (plots W II, N II, E II) remained at the level of 37.6–91.0%. For the soil of the plot located in the reclaimed sector, the value was 35.8%.
Based on the research results, the highest average number of sporulating bacteria under the cultivation of wheat was found in the Z plot located in the reclaimed sector and it was taken as 100%. Considering this assumption, it was calculated that in the soils of the plots located within 250 m from the landfill (plots: WI, NI, E, I and SI), there were 34.1% to 90.1% of these bacteria, and in plots located in zone II (plots: W II, N II, E II), 30.0% to 90.6%. For the horse bean cultivation, the highest average number of sporulating bacteria was recorded to the north of the landfill in the N I plot and was assumed to be 100%. Based on this assumption, it was calculated that for the plots located in zone I, bacteria ranged from 43.3% to 67.2%, whereas for the plots of the second zone, they ranged from 42.8% to 95.6%. For the plot located in the reclaimed sector, this ratio was 66.5%. On the other hand, under potato cultivation, the highest average number of sporulating bacteria was recorded in plot Z, i.e., located in the landfill, and it was assumed to be 100%. Considering this, it was calculated that in the soils of plots located at a distance of 250 m around the dump, bacteria ranged from 30.6% to 89.1%, and in the plots located at a distance of 250 to 500 m, the range was from 30.8% to 84.6%.
Analysing the results, the ratio of the number of sporulating bacteria to the number of vegetative bacteria was calculated, assuming it as a bacteriological index for the assessment of soil quality near the landfill (
Table 4). Considering the results, the highest ratio of sporulating versus vegetative bacteria was found in the soil of the experimental plot located in the reclaimed sector. This indicates that the deposited waste in this sector may have affected the bacteriological conditions of the soil in the plot under study.
The statistical analysis showed the significant effect of deposited municipal waste (landfills) on the ratio of the sporulating to vegetative bacteria in the studied soil (
p < 0.05). It was found that the value of the bacteriological index in the soil under the cultivation of all crops in the landfill area was significantly higher than those recorded values outside the landfill area, both at a distance of 50–250 m from the landfill area (zone I) and between 250 and 500 m (zone II) (Newman–Keuls test:
p < 0.05) (
Table 5). However, no significant differences were observed between the ratio of the sporulating to vegetative bacteria in the soil outside the landfill area (Newman–Keuls test:
p > 0.05).
Plot (Z) is located in the area of the landfill site, which is characterized by a relatively short time since it was recultivated (10 years) and a relatively shallow soil layer (c.a. 100 cm). The other plots were established on arable land, which was under cultivation for at least several dozen years and has never been transformed to such an extent by humans (waste disposal and later recultivation). Despite the fact that in terms of physicochemical properties, it does not differ significantly from the other studied sites (arable land), the degradation of soil expressed by the harm of its ecological function was clearly visible.
The obtained results indicate that a wide range of environmental research should be carried out around municipal waste dumps, allowing for the selection of agricultural plants that positively affect the soil microorganisms, which may contribute to the improvement of biological activity of soils surrounding municipal facilities.