Profile of Semiquinone Radicals, Phytohormones and Sugars in Pistacia vera L. cv. Kirmizi Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Climatic Data of Research Area
2.3. Electron Paramagnetic Resonance (EPR)
2.4. Detection of Phytohormones
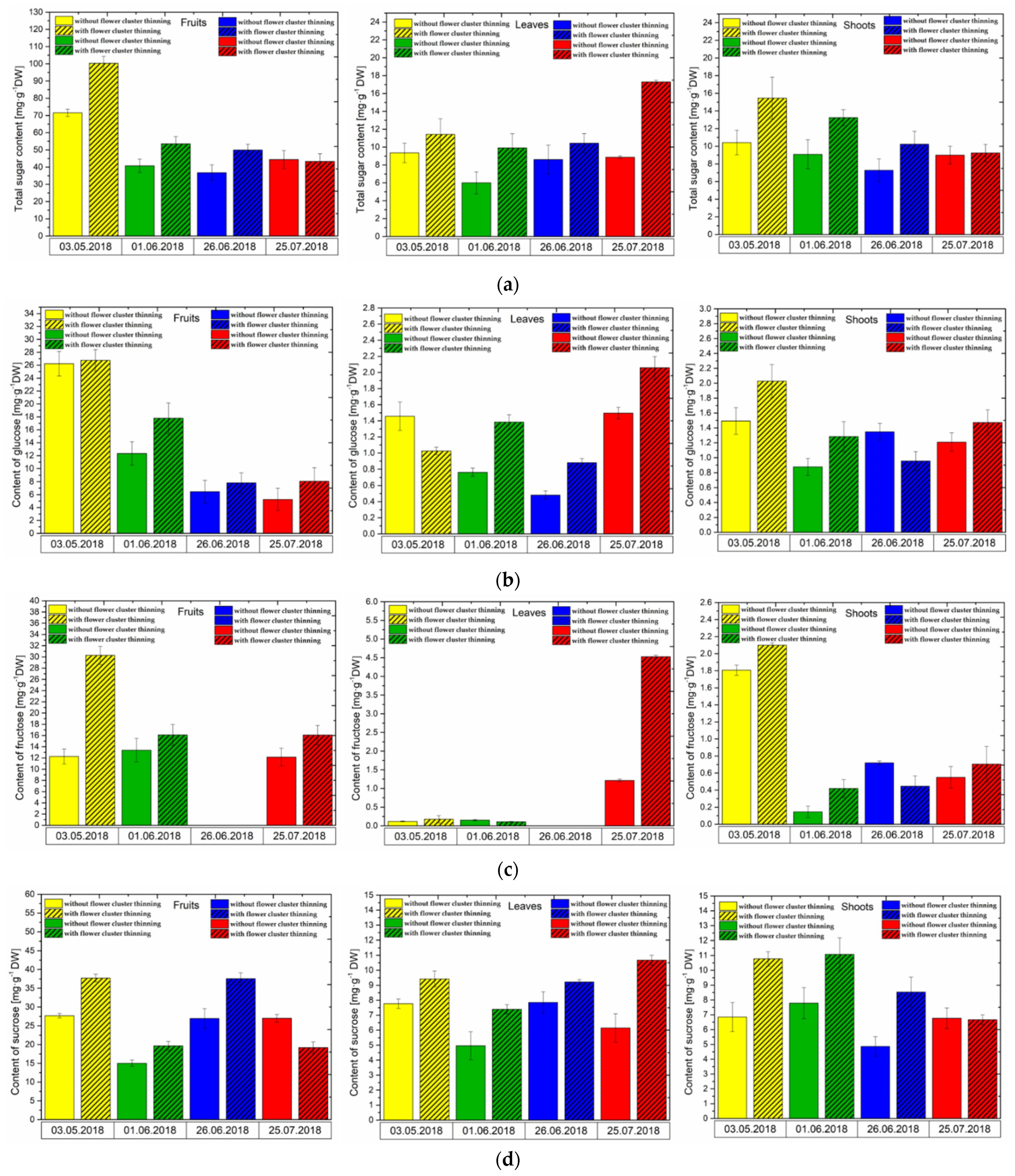
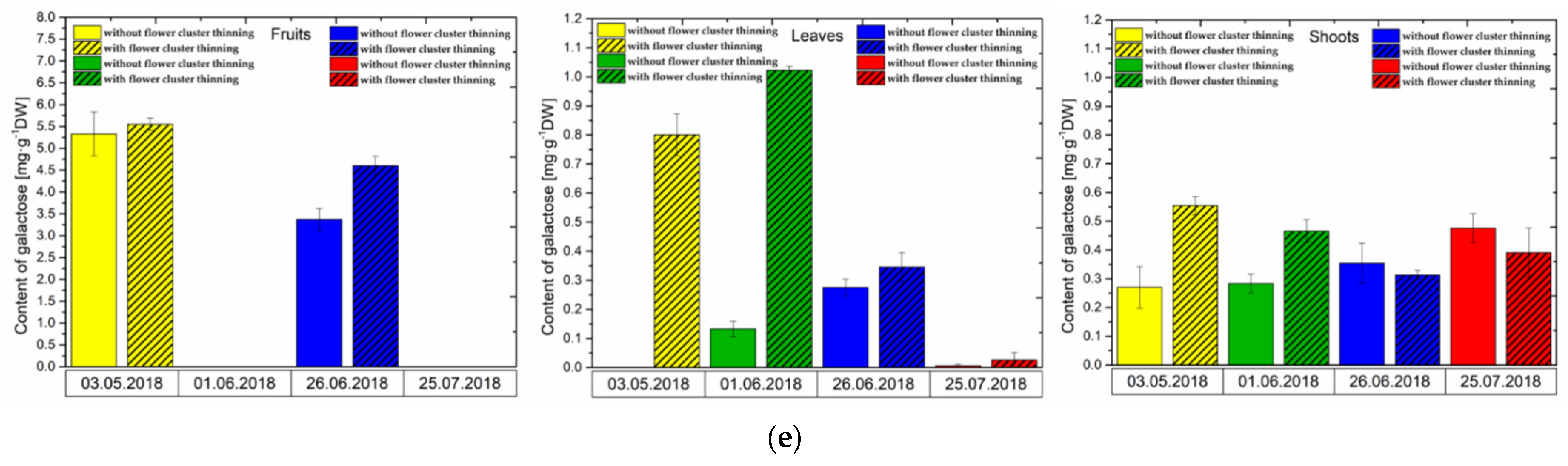
2.5. Determination of Soluble Sugar Content
2.6. Nitrogen Analysis
2.7. Phosphorus Analysis
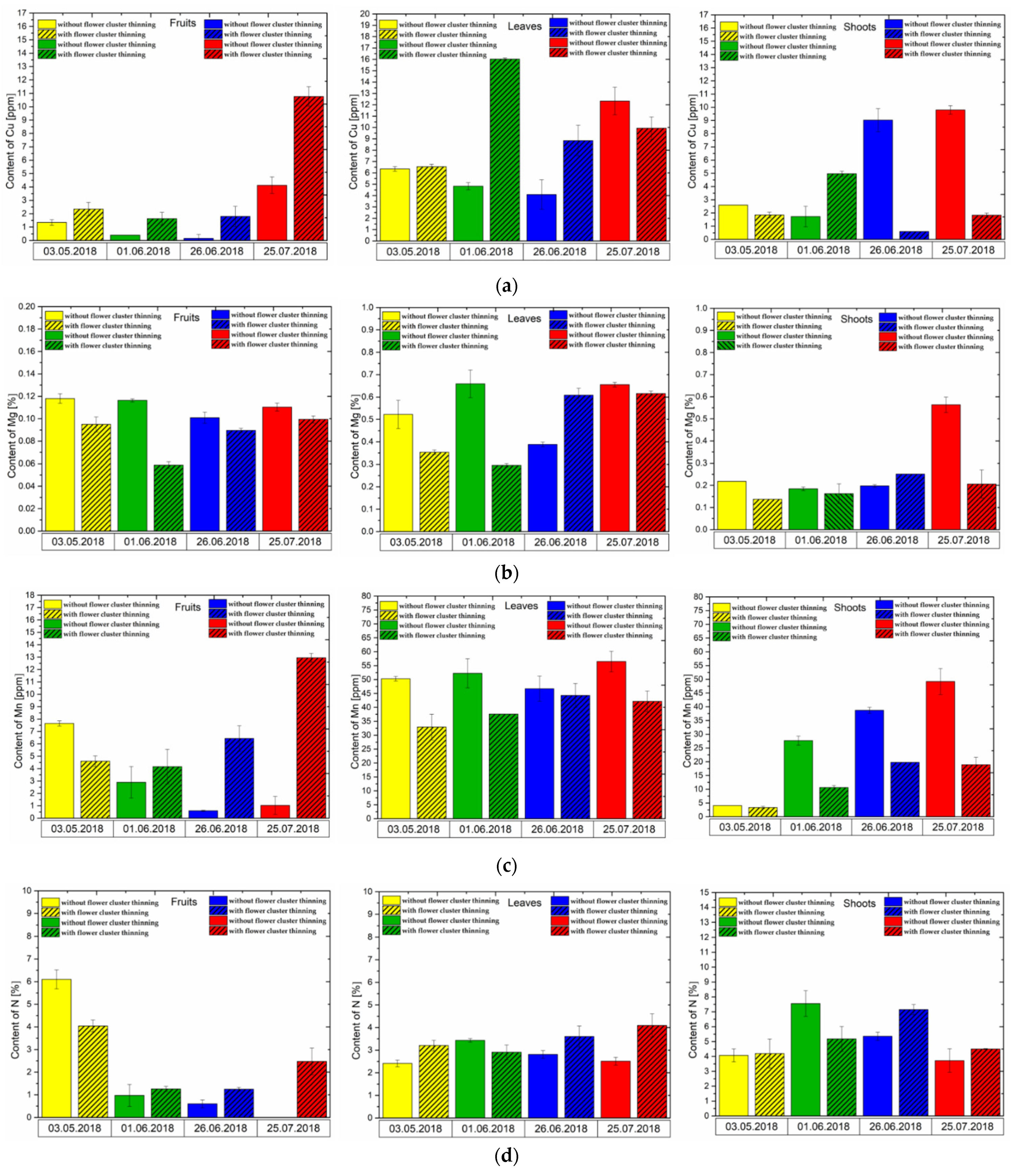
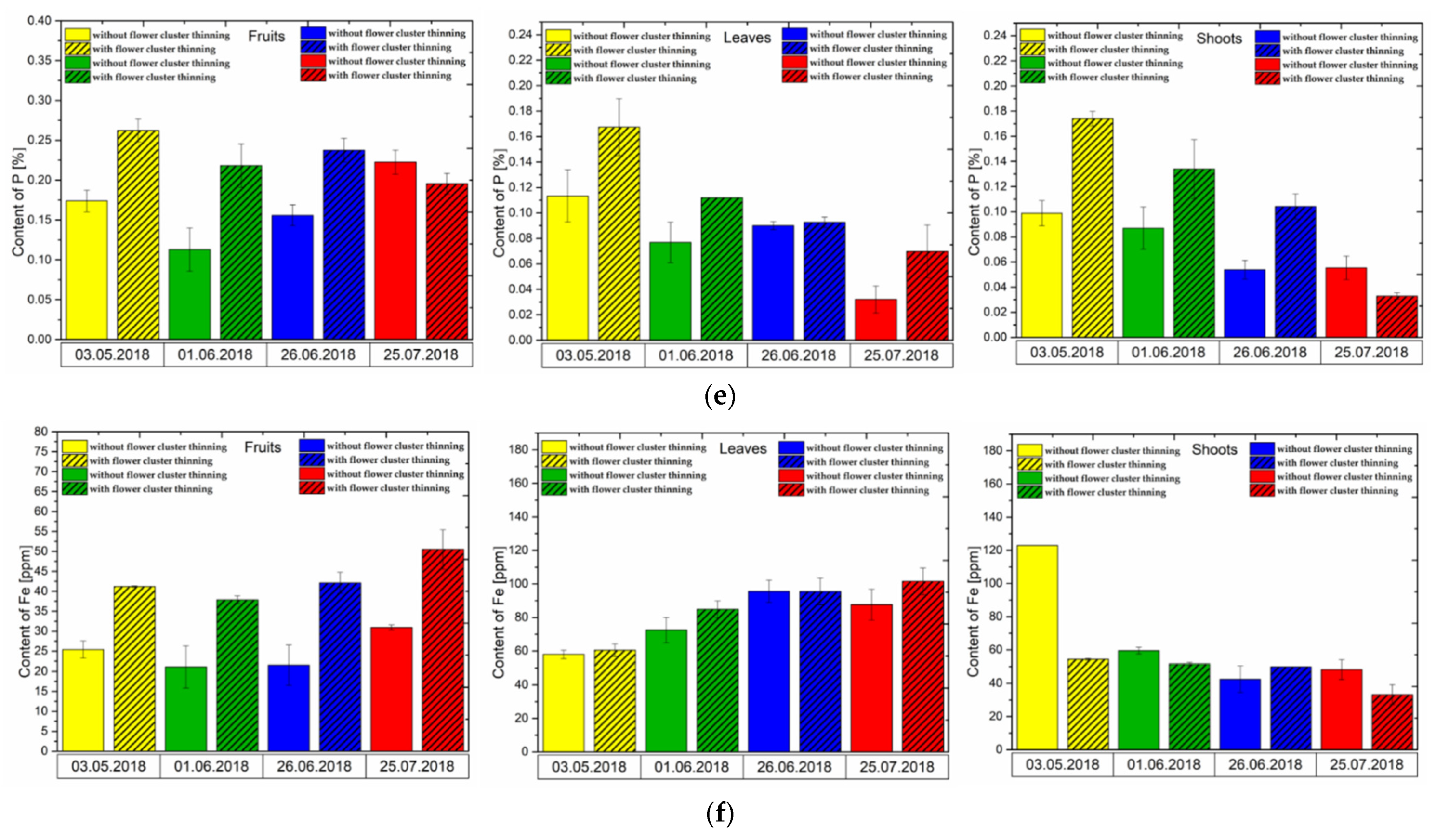
2.8. Macro and Micronutrient Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Flower Cluster Thinning Application on the Concentration of Semiquinone Radicals and Mn2+ in Organs of Pistachio Trees
3.2. Changes in the Concentration of Phytohormones after Flower Cluster Thinning Application in Pistachio Organs
3.2.1. IAA Content
3.2.2. ABA Content
3.2.3. SA Content
3.3. Changes in Sugar Levels after the Flower Cluster Thinning Application in Pistachio Organs
3.4. Effect of Flower Cluster Thinning Application on the Concentration of Mineral Elements in Pistachio Organs
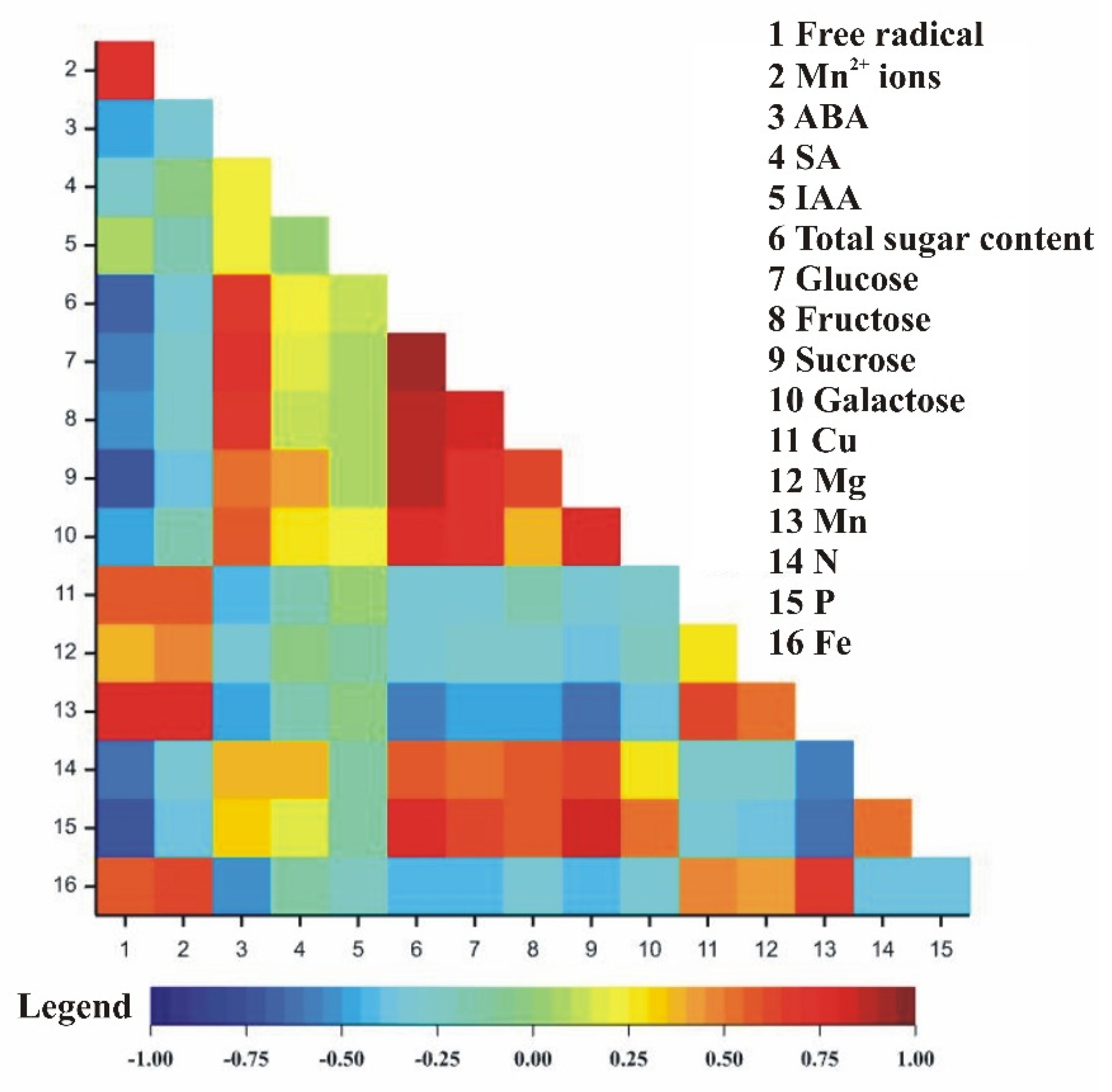
3.5. Correlation Coefficients between Observed Traits in Relation to Pistachio Organs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Saghir, M.G. Evolutionary History of the Genus Pistacia (Anacardiaceae). Int. J. Bot. 2009, 5, 255–257. [Google Scholar] [CrossRef]
- Stevens, P.F. Angiosperm Phylogeny Website. Version 14 July 2017. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 22 July 2021).
- AL-Saghir, M.G.; Porter, D.M. Random Amplified Polymorphic DNA (RAPD) study of Pistacia species (Anacardiaceae). Asian J. Plant Sci. 2006, 5, 1002–1006. [Google Scholar] [CrossRef]
- Zohary, M. A Monographical Study of the Genus Pistacia. Palest. J. Bot. Jerus. Ser. 1952, 5, 187–228. [Google Scholar]
- Kafkas, S. SSR Markers in the Genus Pistacia. In Sustainable Crop Production; IntechOpen: Rijeka, Croatia; London, UK, 2019; ISBN 978-1-78985-318-6. [Google Scholar]
- Karcι, H.; Paizila, A.; Topçu, H.; İlikçioğlu, E.; Kafkas, S. Transcriptome Sequencing and Development of Novel Genic SSR Markers From Pistacia vera L. Front. Genet. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Kafkas, S. Phylogenetic analysis of the genus Pistacia by AFLP markers. Plant Syst. Evol. 2006, 262, 113–124. [Google Scholar] [CrossRef]
- Belhadj, S.; Derridj, A.; Aigouy, T.; Gers, C.; Gauquelin, T.; Mévy, J.-P. Comparative Morphology of Leaf Epidermis in Eight Populations of Atlas Pistachio (Pistacia atlantica Desf., Anacardiaceae). Microsc. Res. Tech. 2007, 70, 837–846. [Google Scholar] [CrossRef]
- Behboudian, M.; Walker, R.; Törökfalvy, E. Effects of water stress and salinity on photosynthesis of pistachio. Sci. Hortic. 1986, 29, 251–261. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Tabil, L.G. Pistachio (Pistacia vera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruit; Woodhead Publishing Series in Food Science, Technology and Nutrition: Oxford, UK, 2011; pp. 218–247. ISBN 978-1-84569-735-8. [Google Scholar]
- Isam, A.M. Risk Attitude, Risk Perceptions and Risk Management Strategies: An Empirical Analysis of Syrian Wheat-Cotton and Pistachio Farmers. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2014. [Google Scholar]
- Baazeem, A.; Garcia-Cela, E.; Medina, A.; Magan, N. Interacting Abiotic Factors Affect Growth and Aflatoxin B1 Production Profiles of Aspergillus flavus Strains on Pistachio-Based Matrices and Pistachio Nuts. Front. Microbiol. 2021, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, M.; Dimou, A.; Yanniotis, S. Aflatoxin contamination in pistachio nuts: A farm to storage study. Food Control. 2012, 26, 580–586. [Google Scholar] [CrossRef]
- Kaminiaris, M.D.; Leggieri, M.C.; Tsitsigiannis, D.I.; Battilani, P. AFLA-PISTACHIO: Development of a Mechanistic Model to Predict the Aflatoxin Contamination of Pistachio Nuts. Toxins 2020, 12, 445. [Google Scholar] [CrossRef]
- Maggs, D.H. Genetic Resources in Pistachio. In Plant Genetic Resources Newsletter; Roma, Italy, 1973. [Google Scholar]
- Hormaza, I.; Dollo, L.; Polito, V.S. Determination of relatedness and geographical movements of Pistacia vera (Pistachio; Anacardiaceae) germplasm by RAPD analysis. Econ. Bot. 1994, 48, 349–358. [Google Scholar] [CrossRef]
- Hormaza, I.; Plnney, K.; Polito, V.S. Genetic diversity of Pistachio (Pistacia vera, Anacardiaceae) Germplasm based on Randomly Amplified Polymorphic DNA (RAPD) markers. Econ. Bot. 1998, 52, 78–87. [Google Scholar] [CrossRef]
- Al-Shareef, M. Comparative Advantages of Pistachio; Ministry of Agriculture and Agrarian Reform National Agricultural Policy Center (NAPC): Damascus, Syria, 2007; pp. 1–42.
- Gundesli, M.A.; Kafkas, S.; Zarifikhosroshahi, M.; Kafkas, N.E. Role of endogenous polyamines in the alternate bearing phenomenon in pistachio. Turk. J. Agric. For. 2019, 43, 265–274. [Google Scholar] [CrossRef]
- Okay, Y.; Kouml, A.İ. Free Endogenous Growth Regulators in Pistachio (Pistacia vera L.). Afr. J. Agric. Res. 2011, 6, 1161–1169. [Google Scholar]
- Benny, J.; Marra, F.P.; Giovino, A.; Balan, B.; Caruso, T.; Martinelli, F.; Marchese, A. Transcriptome Analysis of Pistacia vera Inflorescence Buds in Bearing and Non-Bearing Shoots Reveals the Molecular Mechanism Causing Premature Flower Bud Abscission. Genes 2020, 11, 851. [Google Scholar] [CrossRef]
- Barbehenn, R.; Poopat, U.; Spencer, B. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Biol. 2002, 33, 125–130. [Google Scholar] [CrossRef]
- Pearce, N.J.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A Compilation of New and Published Major and Trace Element Data for NIST SRM 610 and NIST SRM 612 Glass Reference Materials. Geostand. Geoanalytical Res. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of Endogenous Signals and Fusarium oxysporum on the Mechanism Regulating Genistein Synthesis and Accumulation in Yellow Lupine and Their Impact on Plant Cell Cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef]
- Woźniak, A.; Bednarski, W.; Dancewicz, K.; Gabryś, B.; Borowiak-Sobkowiak, B.; Bocianowski, J.; Samardakiewicz, S.; Rucińska-Sobkowiak, R.; Morkunas, I. Oxidative stress links response to lead and Acyrthosiphon pisum in Pisum sativum L. J. Plant Physiol. 2019, 240, 152996. [Google Scholar] [CrossRef]
- Durak, R.; Bednarski, W.; Formela-Luboińska, M.; Woźniak, A.; Borowiak-Sobkowiak, B.; Durak, T.; Dembczyński, R.; Morkunas, I. Defense responses of Thuja orientalis to infestation of anholocyclic species aphid Cinara tujafilina. J. Plant Physiol. 2018, 232, 160–170. [Google Scholar] [CrossRef]
- Morkunas, I.; Gmerek, J. The Possible Involvement of Peroxidase in Defence of Yellow Lupine Embryo Axase Agains Fusarium Oxysporum. J. Plant Physiol. 2007, 164, 185–194. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-K.; Kleiber, T.; Zydlik, Z.; Rutkowski, K.; Woźniak, A.; Świerczyński, S.; Bednarski, W.; Kęsy, J.; Marczak, Ł.; Seo, J.-H.; et al. A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.). Agronomy 2020, 10, 458. [Google Scholar] [CrossRef] [Green Version]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Morkunas, I.; Borek, S.; Formela, M.; Ratajczak, L. Plant Responses to Sugar Starvation. In Carbohydrates—Comprehensive Studies on Glycobiology and Glycotechnology; Chang, C.-F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 409–438. ISBN 978-953-51-0864-1. [Google Scholar]
- Morkunas, I.; Waplak, S.; Bednarski, W.; Ratajczak, L.; Garnczarska, M. The Effect of Carbohydrate Nutrition on Free Radical Formation in Pea Embryo Axes Cultivated In Vitro. Plant Physiol. Biochem. 2000, 38. [Google Scholar]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-Talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Polat, R.; Aydin, C.; Ak, B.E. Some Physical and Mechanical Properties of Pistachio Nut. Bulg. J. Agric. Sci. 2007, 13, 237. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. Available online: http://www.fao.org/faostat/en/#home (accessed on 22 July 2021).
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, L.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-Talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar] [CrossRef] [Green Version]
- Bednarski, W.; Ostrowski, A.; Waplak, S. Low temperature short-range ordering caused by Mn2+ doping of Rb3H(SO4)2. J. Phys. Condens. Matter 2010, 22, 225901. [Google Scholar] [CrossRef]
- Morkunas, I.; Garnczarska, M.; Bednarski, W.; Ratajczak, W.; Walpak, S. Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J. Plant Physiol. 2003, 160, 311–319. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W.; Kozłowska, M. Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2004, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Vine, J.H.; Noiton, D.; Plummer, J.A.; Baleriola-Lucas, C.; Mullins, M.G. Simultaneous Quantitation of Indole 3-Acetic Acid and Abscisic Acid in Small Samples of Plant Tissue by Gas Chromatography/Mass Spectrometry/Selected Ion Monitoring. Plant Physiol. 1987, 85, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Miron, D.; Schaffer, A.A. Sucrose Phosphate Synthase, Sucrose Synthase, and Invertase Activities in Developing Fruit of Lycopersicon esculentum Mill. and the Sucrose Accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol. 1991, 95, 623–627. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, M.B. Micro-Kjeldahl Method for Biologicals. J. Am. Pharm. Assoc. 1951, 40, 151–153. [Google Scholar] [CrossRef]
- Barton, C.J. Photometric Analysis of Phosphate Rock. Anal. Chem. 1948, 20, 1068–1073. [Google Scholar] [CrossRef]
- Pratt, P.F.; Chapman, H.D. Gains and losses of mineral elements in an irrigated soil during a 20-year lysimeter investigation. Hilgardia 1961, 30, 445–467. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Foster, M.A. Magnetic Resonance in Medicine and Biology, 1st ed.; Pergamon: Oxford, UK, 1984; ISBN 0-08-030770-1. [Google Scholar]
- Tao, Y.; Chen, R.; Li, H.; Yuan, J.; Wan, Y.; Jiang, H.; Chen, C.; Si, Y.; Zheng, C.; Yang, B.; et al. Resonance-Activated Spin-Flipping for Efficient Organic Ultralong Room-Temperature Phosphorescence. Adv. Mater. 2018, 30, e1803856. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A. Free Radicals and the Role of Plant Phytochemicals as Antioxidants Against Oxidative Stress-Related Diseases; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78984-378-1. [Google Scholar]
- Yamasaki, H.; Grace, S.C. EPR detection of phytophenoxyl radicals stabilized by zinc ions: Evidence for the redox coupling of plant phenolics with ascorbate in the H2O2-peroxidase system. FEBS Lett. 1998, 422, 377–380. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. Free Radicals as Mediators of Tissue Injury and Disease. Crit. Rev. Toxicol. 1993, 23, 21–48. [Google Scholar] [CrossRef]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar] [CrossRef]
- Barbehenn, R.; Cheek, S.; Gasperut, A.; Lister, E.; Maben, R. Phenolic Compounds in Red Oak and Sugar Maple Leaves Have Prooxidant Activities in the Midgut Fluids of Malacosoma disstria and Orgyia leucostigma Caterpillars. J. Chem. Ecol. 2005, 31, 969–988. [Google Scholar] [CrossRef] [Green Version]
- Woźniak, A.; Formela, M.; Bilman, P.; Grześkiewicz, K.; Bednarski, W.; Marczak, Ł.; Narożna, D.; Dancewicz, K.; Mai, V.C.; Borowiak-Sobkowiak, B.; et al. The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation. Int. J. Mol. Sci. 2017, 18, 329. [Google Scholar] [CrossRef] [Green Version]
- Morkunas, I.; Woźniak, A.; Mai, V.C.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammel, K.; Kalyanaraman, B.; Kirk, T.K. Substrate free radicals are intermediates in ligninase catalysis. Proc. Natl. Acad. Sci. USA 1986, 83, 3708–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood: Its Biology and Ecology. In Fungal Decomposition of Wood. Its Biology and Ecology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1988. [Google Scholar]
- Kalyanaraman, B. Radical intermediates during degradation of lignin-model compounds and environmental pollutants: An electron spin resonance study. Xenobiotica 1995, 25, 667–675. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—The Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Stokes, M.E.; Chattopadhyay, A.; Wilkins, O.; Nambara, E.; Campbell, M.M. Interplay between Sucrose and Folate Modulates Auxin Signaling in Arabidopsis. Plant Physiol. 2013, 162, 1552–1565. [Google Scholar] [CrossRef]
- Grossman, Y.L. Maximum Fruit Growth Potential Following Resource Limitation During Peach Growth. Ann. Bot. 1995, 75, 561–567. [Google Scholar] [CrossRef]
- Miller, S.S.; Schupp, J.R.; Baugher, T.A.; Wolford, S.D. Performance of Mechanical Thinners for Bloom or Green Fruit Thinning in Peaches. HortScience 2011, 46, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Rajput, V.; Bhatia, S.K. Changes in Fruit Quality Parameters in Japanese Plum CV. Kala Amritsari with Chemical Flower Thinning. J. Pharmacogn. Phytochem. 2017, 6, 2220–2223. [Google Scholar]
- Pereira, J.F.M.; Raseira, A. Raleio. In Pessegueiro; DF: Embrapa, Brasília, 2014; pp. 309–327. [Google Scholar]
- Giovanaz, M.A.; Fachinello, J.C.; Spagnol, D.; Weber, D.; Carra, B. Gibberellic Acid Reduces Flowering and Time of Manual Thinning in ‘Maciel’ Peach Trees. Rev. Bras. De Frutic. Jaboticabal 2016, 38, e-692. [Google Scholar] [CrossRef] [Green Version]
- Stein, O.; Granot, D. Plant Fructokinases: Evolutionary, Developmental, and Metabolic Aspects in Sink Tissues. Front. Plant Sci. 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, D.T.; Blakeley, S.D. Carbohydrate metabolism. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 676–728. [Google Scholar]
- Marra, F.; Roxas, A.A.; Marino, G.; Caruso, T. Seasonal changes in starch content in pistachio organs as related to crop load. Acta Hortic. 2018, 1229, 171–176. [Google Scholar] [CrossRef]
- Lee, T.-H.; Sugiyama, A.; Ofosu-Anim, J.; Takeno, K.; Ohno, H.; Yamaki, S. Activation of sucrose-metabolizing enzymes and stimulation of sucrose uptake by auxin and sucrose in eggplant (Solanum melongena L.). J. Plant Physiol. 1997, 150, 297–301. [Google Scholar] [CrossRef]
- Nitsch, J.P. The Role of Plant Hormones in Fruit Development. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 1951. [Google Scholar]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Takeda, F.; Crane, J. Abscisic Acid in Pistachio as Related to Inflorescence Bud Abscission. J. Am. Soc. Hortic. Sci. 1980, 105, 573–576. [Google Scholar]
- Marino, F.; Greene, D.W. Involvement of Gibberellins in the Biennial Bearing of Apples Malus Domestica Cultivar Early Mcintosh. J. Am. Soc. Hortic. Sci. 1981, 106, 593–596. [Google Scholar]
- Vemmos, S.N. Carbohydrate content of inflorescent buds of defruited and fruiting pistachio (Pistacia vera L) branches in relation to biennial bearing. J. Hortic. Sci. Biotechnol. 1999, 74, 94–100. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Chauret, G.; Swift, D.E.; Duchesne, I. Effects of precommercial thinning on tree growth and lumber quality in a jack pine stand in New Brunswick, Canada. Can. J. For. Res. 2006, 36, 945–952. [Google Scholar] [CrossRef]
- Kazankaya, A.; Balta, M.F.; Yörük, I.H.; Balta, F.; Battal, P. Analysis of Sugar Composition in Nut Crops. Asian J. Chem. 2008, 20, 1519–1525. [Google Scholar]
- Whiting, M.D.; Lang, G.A. ‘Bing’ Sweet Cherry on the Dwarfing Rootstock ‘Gisela 5’: Thinning Affects Fruit Quality and Vegetative Growth but Not Net CO2 Exchange. I. Amer. Soc. Hort. Sci. 2004, 129, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Usenik, V.; Orazem, P.; Stampar, F. Low leaf to fruit ratio delays fruit maturity of ‘Lapins’ sweet cherry on Gisela 5. Sci. Hortic. 2010, 126, 33–36. [Google Scholar] [CrossRef]
- DeJong, T.; Johnson, R.; Castagnoli, S. Computer simulation of the carbohydrate economy of peach crop growth. Acta Hortic. 1990, 276, 97–104. [Google Scholar] [CrossRef]
- Bai, Q.; Shen, Y.; Huang, Y. Advances in Mineral Nutrition Transport and Signal Transduction in Rosaceae Fruit Quality and Postharvest Storage. Front. Plant Sci. 2021, 12, 68. [Google Scholar] [CrossRef]


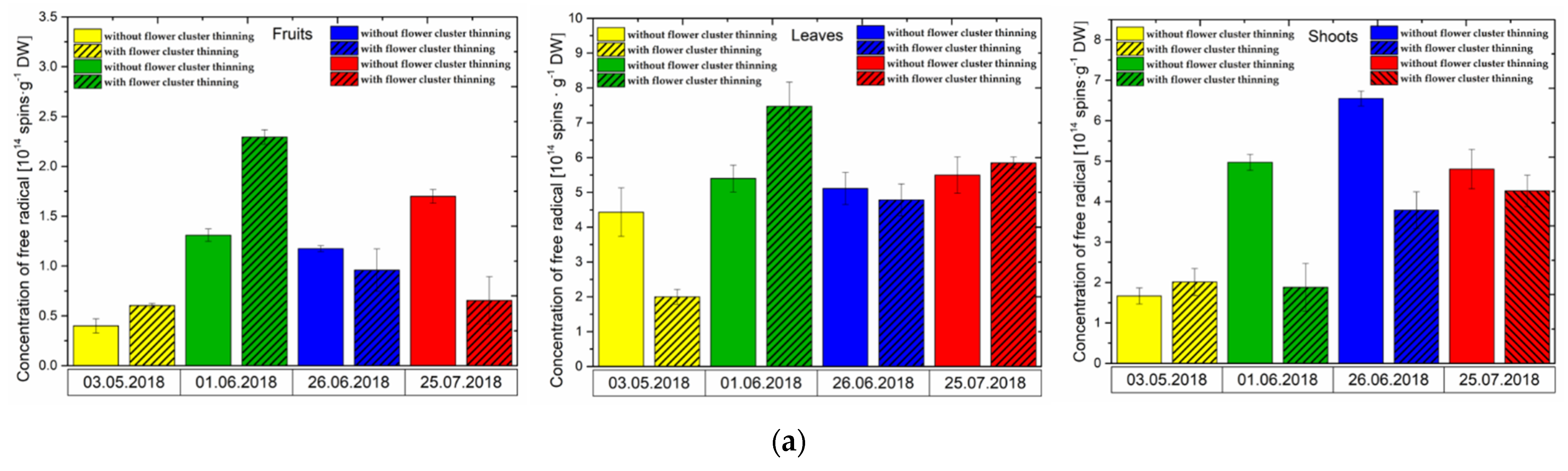
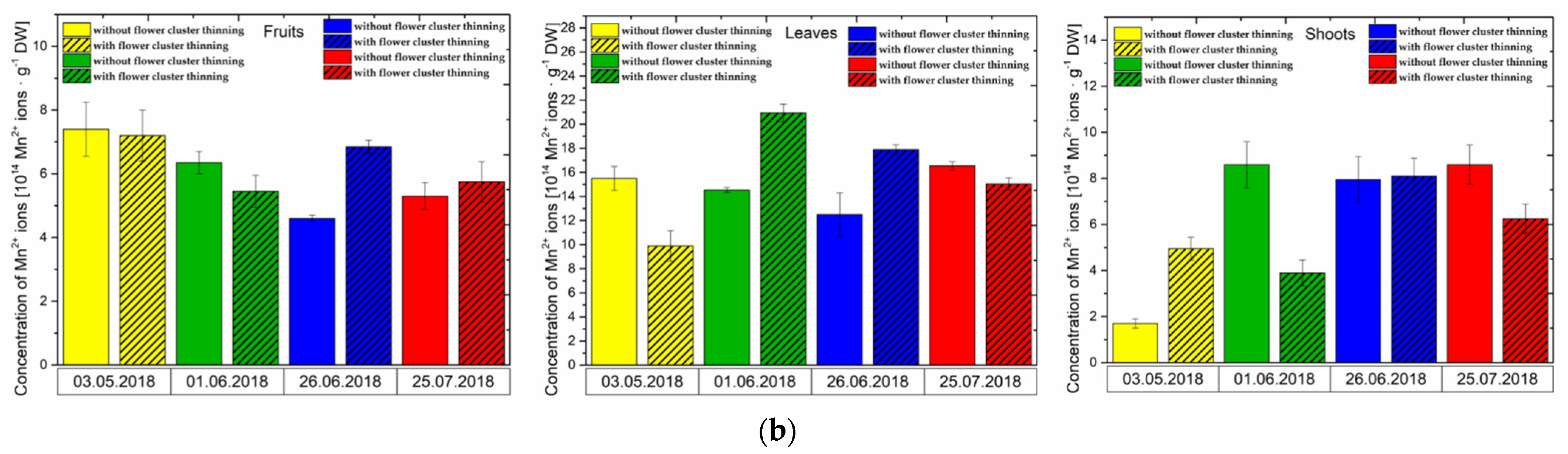

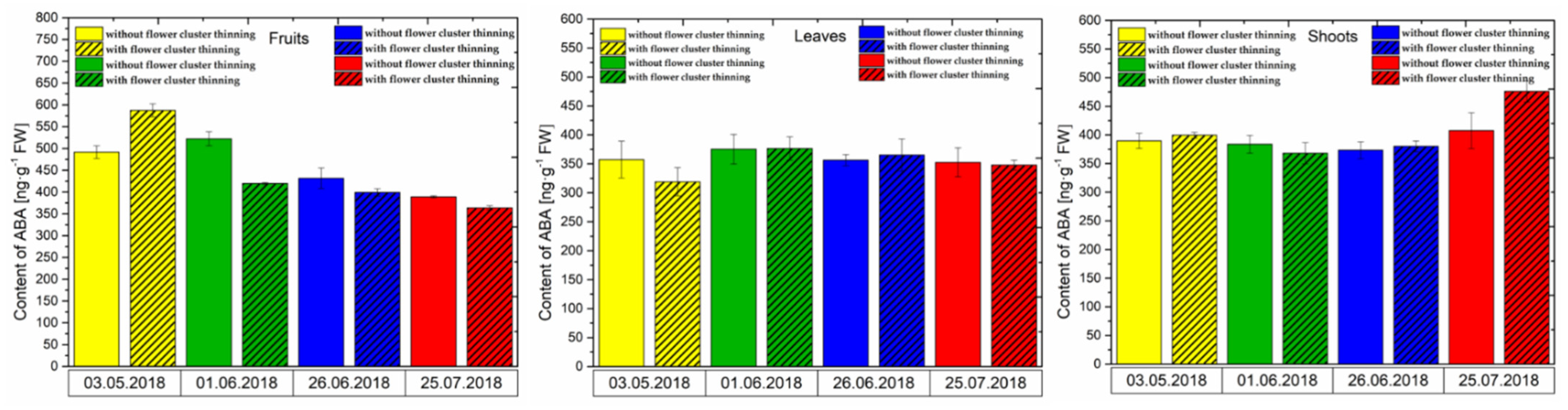
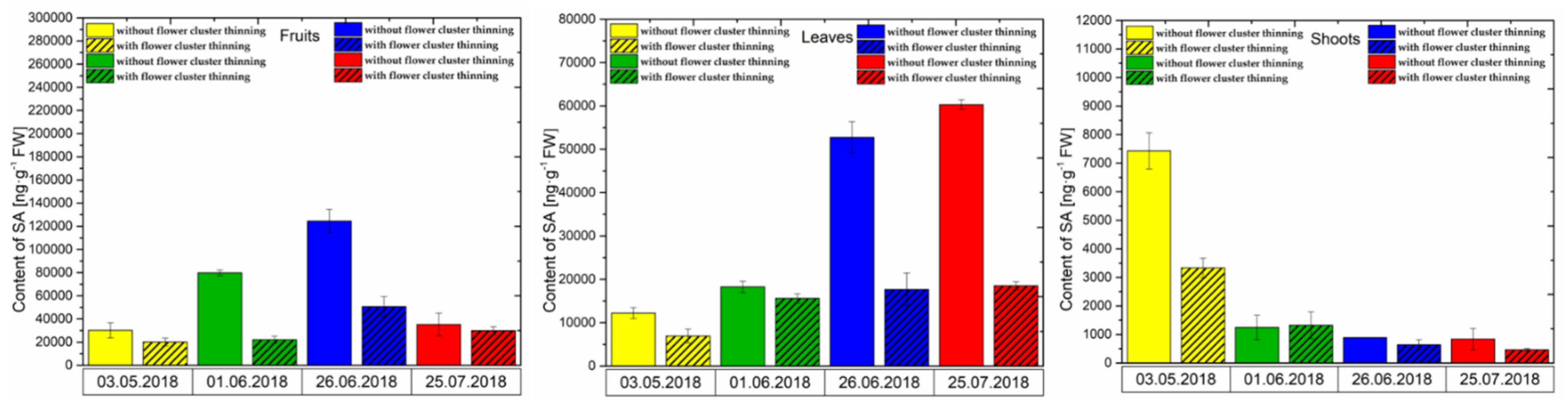
| Months | Average Temperature (°C) | Average Maximum Temperature (°C) | Average Minimum Temperature (°C) | Average Hours of Sunshine (Hours) | Total Rainfall (mm) |
|---|---|---|---|---|---|
| January | 3.0 | 7.4 | −0.7 | 3.6 | 102.8 |
| February | 4.4 | 9.3 | 0.1 | 4.4 | 83.4 |
| March | 8.1 | 13.8 | 2.9 | 5.5 | 72.5 |
| April | 13.3 | 19.6 | 7.2 | 7.0 | 52.7 |
| May | 18.7 | 25.4 | 11.8 | 8.4 | 31.3 |
| June | 24.2 | 31.1 | 17.0 | 10.3 | 8.5 |
| July | 27.9 | 35.1 | 21.0 | 10.6 | 7.1 |
| August | 27.7 | 35.2 | 20.9 | 10.0 | 5.2 |
| September | 23.3 | 31.0 | 16.1 | 8.7 | 7.2 |
| October | 16.6 | 24.1 | 9.9 | 6.9 | 37.3 |
| November | 9.8 | 16.2 | 4.4 | 5.3 | 62.0 |
| December | 4.9 | 9.6 | 1.0 | 3.5 | 98.5 |
| Annual | 15.2 | 21.5 | 9.3 | 84.2 | 568.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morkunas, I.; Doğu, M.Z.; Woźniak, A.; Bednarski, W.; Kęsy, J.; Bocianowski, J.; Atar, Ş.H.; Ürün, İ.D.; Labudda, M.; Zydlik, Z.; et al. Profile of Semiquinone Radicals, Phytohormones and Sugars in Pistacia vera L. cv. Kirmizi Development. Agronomy 2021, 11, 2115. https://doi.org/10.3390/agronomy11112115
Morkunas I, Doğu MZ, Woźniak A, Bednarski W, Kęsy J, Bocianowski J, Atar ŞH, Ürün İD, Labudda M, Zydlik Z, et al. Profile of Semiquinone Radicals, Phytohormones and Sugars in Pistacia vera L. cv. Kirmizi Development. Agronomy. 2021; 11(11):2115. https://doi.org/10.3390/agronomy11112115
Chicago/Turabian StyleMorkunas, Iwona, Mehmet Zafer Doğu, Agnieszka Woźniak, Waldemar Bednarski, Jacek Kęsy, Jan Bocianowski, Şule Hilal Atar, İpek Değirmenci Ürün, Mateusz Labudda, Zofia Zydlik, and et al. 2021. "Profile of Semiquinone Radicals, Phytohormones and Sugars in Pistacia vera L. cv. Kirmizi Development" Agronomy 11, no. 11: 2115. https://doi.org/10.3390/agronomy11112115
APA StyleMorkunas, I., Doğu, M. Z., Woźniak, A., Bednarski, W., Kęsy, J., Bocianowski, J., Atar, Ş. H., Ürün, İ. D., Labudda, M., Zydlik, Z., Kafkas, N. E., Kafkas, S., & Jeandet, P. (2021). Profile of Semiquinone Radicals, Phytohormones and Sugars in Pistacia vera L. cv. Kirmizi Development. Agronomy, 11(11), 2115. https://doi.org/10.3390/agronomy11112115










