Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Condition
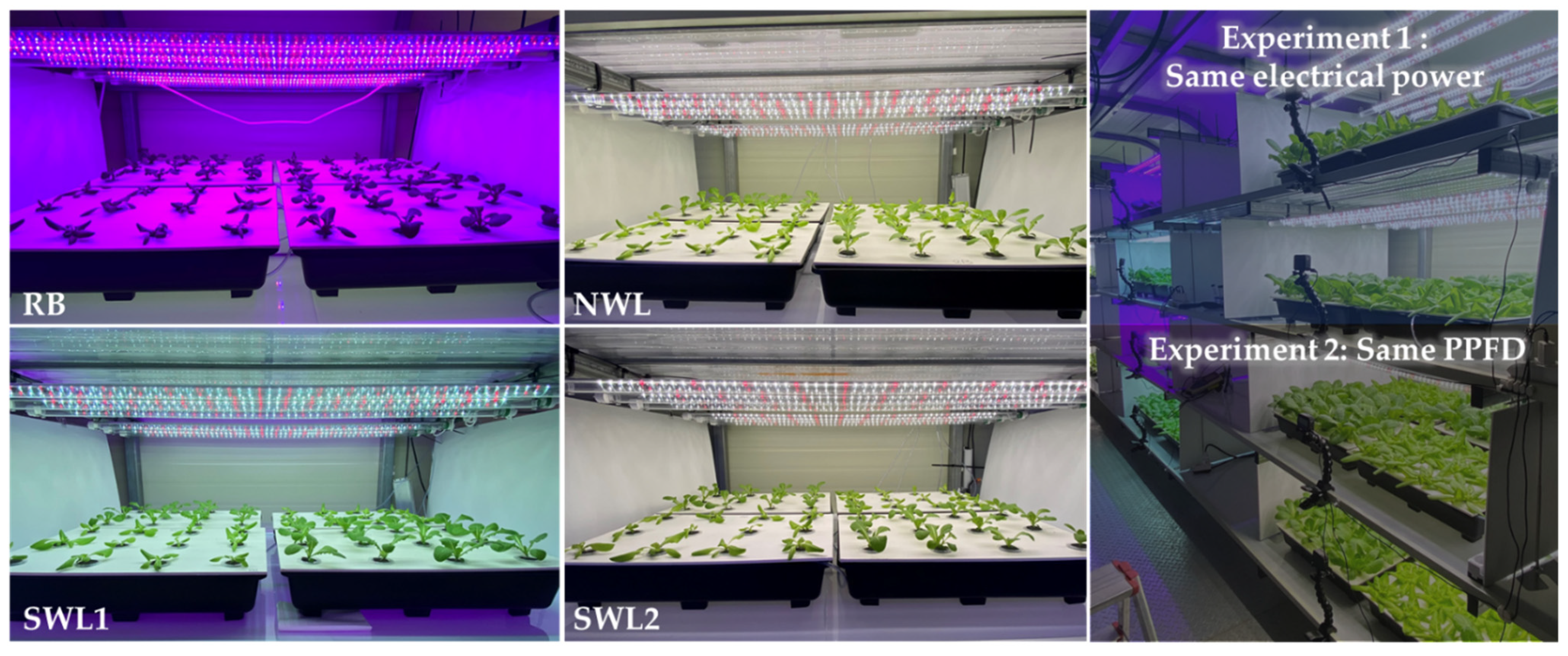
2.2. Light Treatments
2.3. Growth Characteristics
2.4. Absorbance and Transmittance
2.5. Individual Phenolic Acid and Flavonol Analysis
2.6. Light and Energy Use Efficiency
2.7. Statistical Analysis
3. Results
3.1. Experiment 1
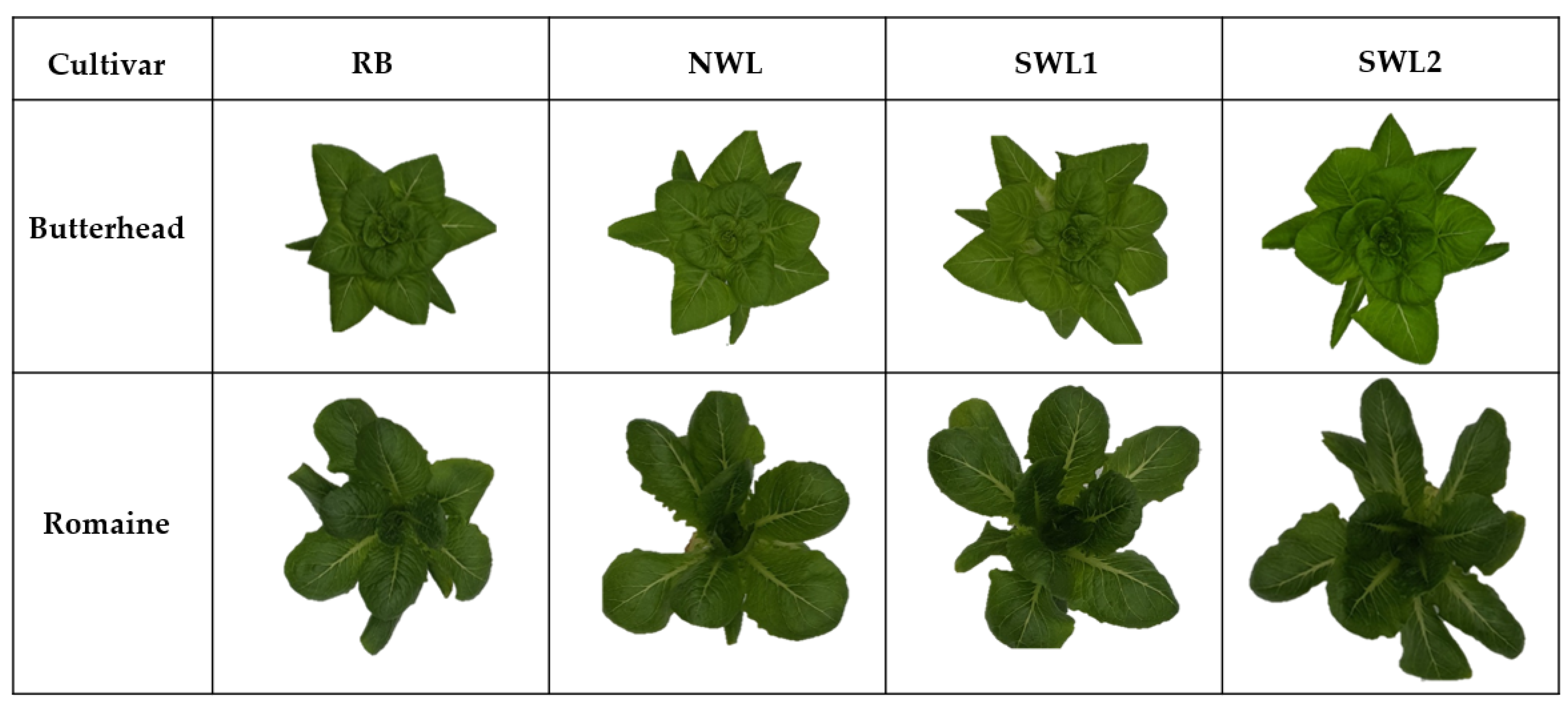
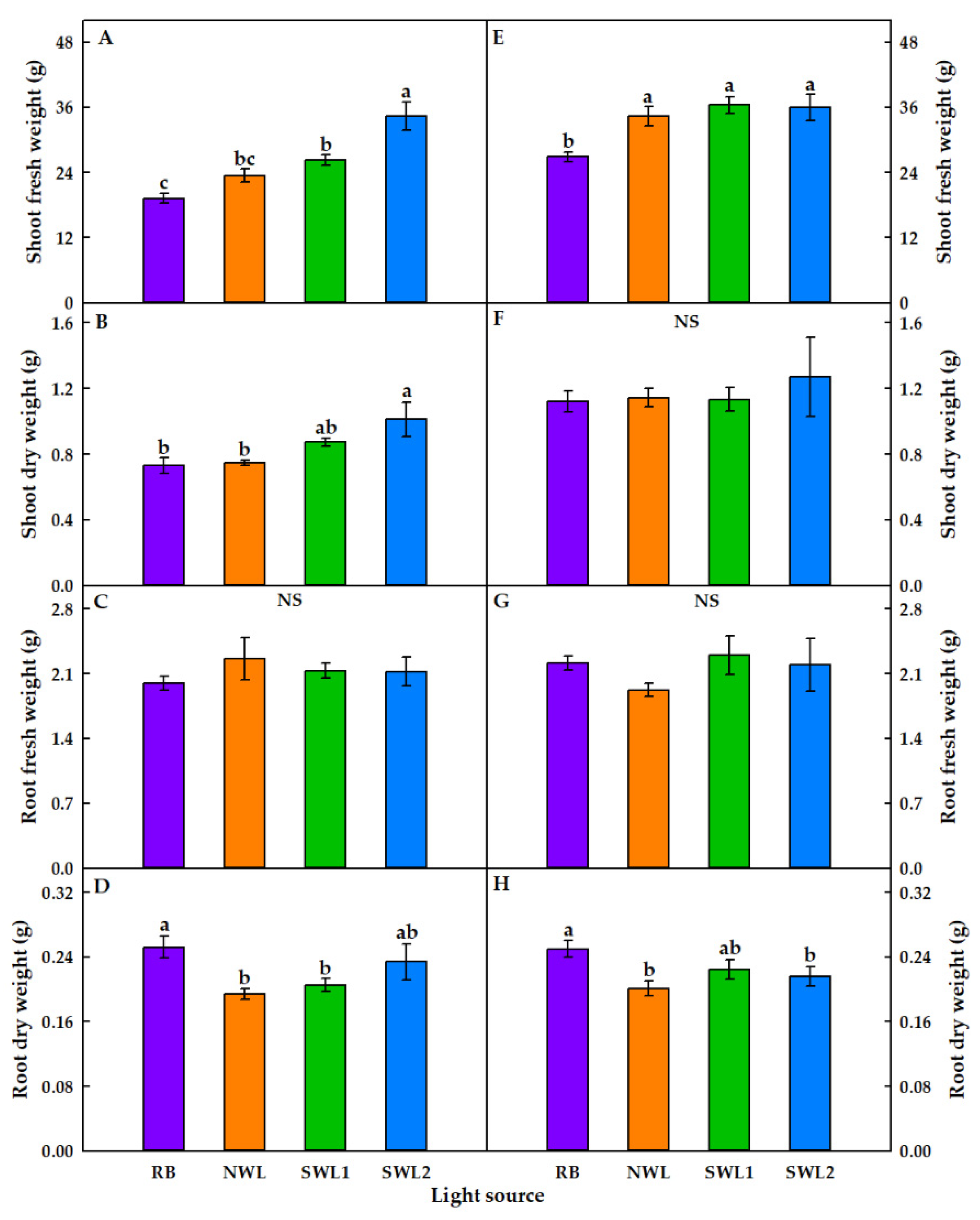
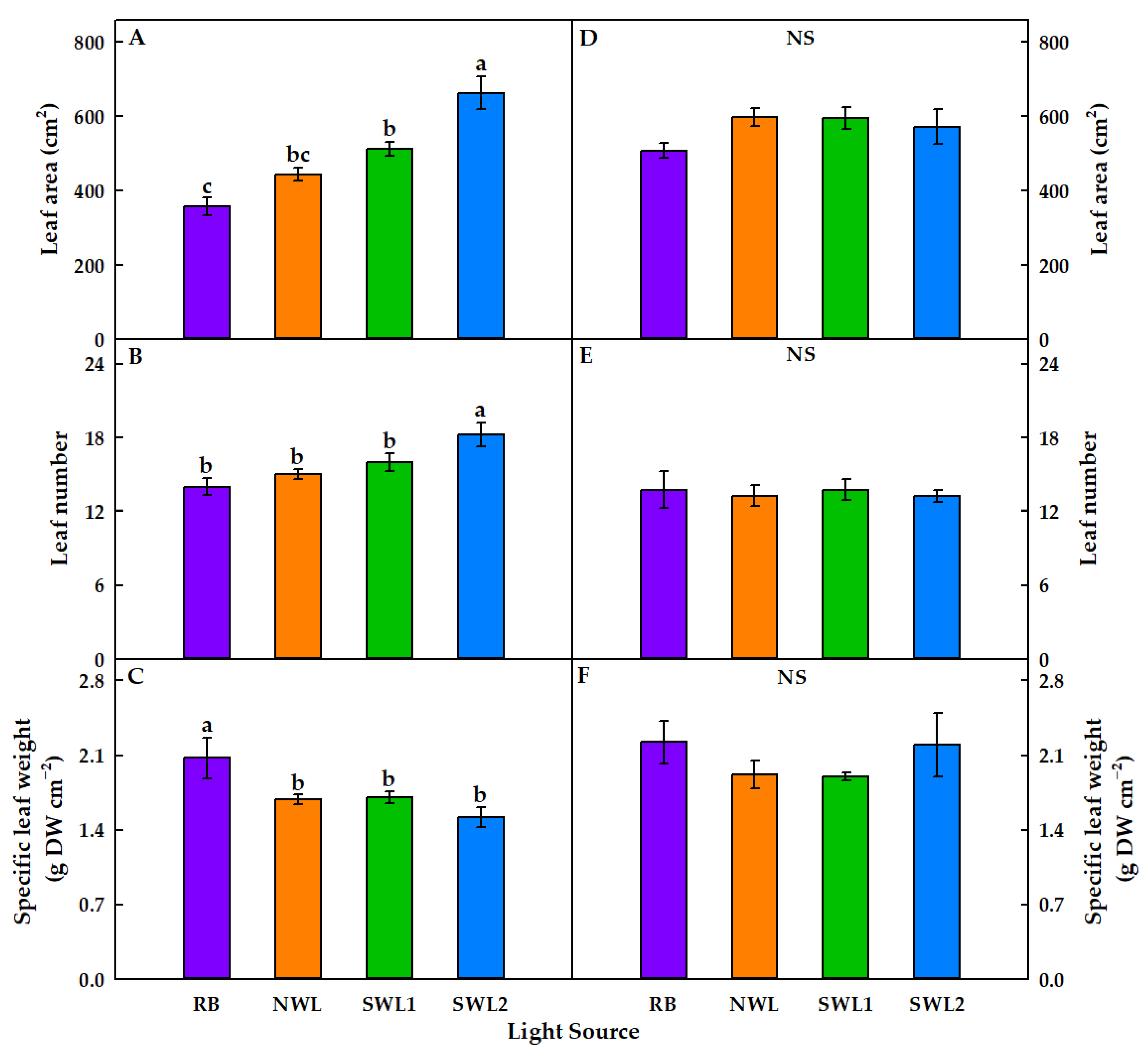
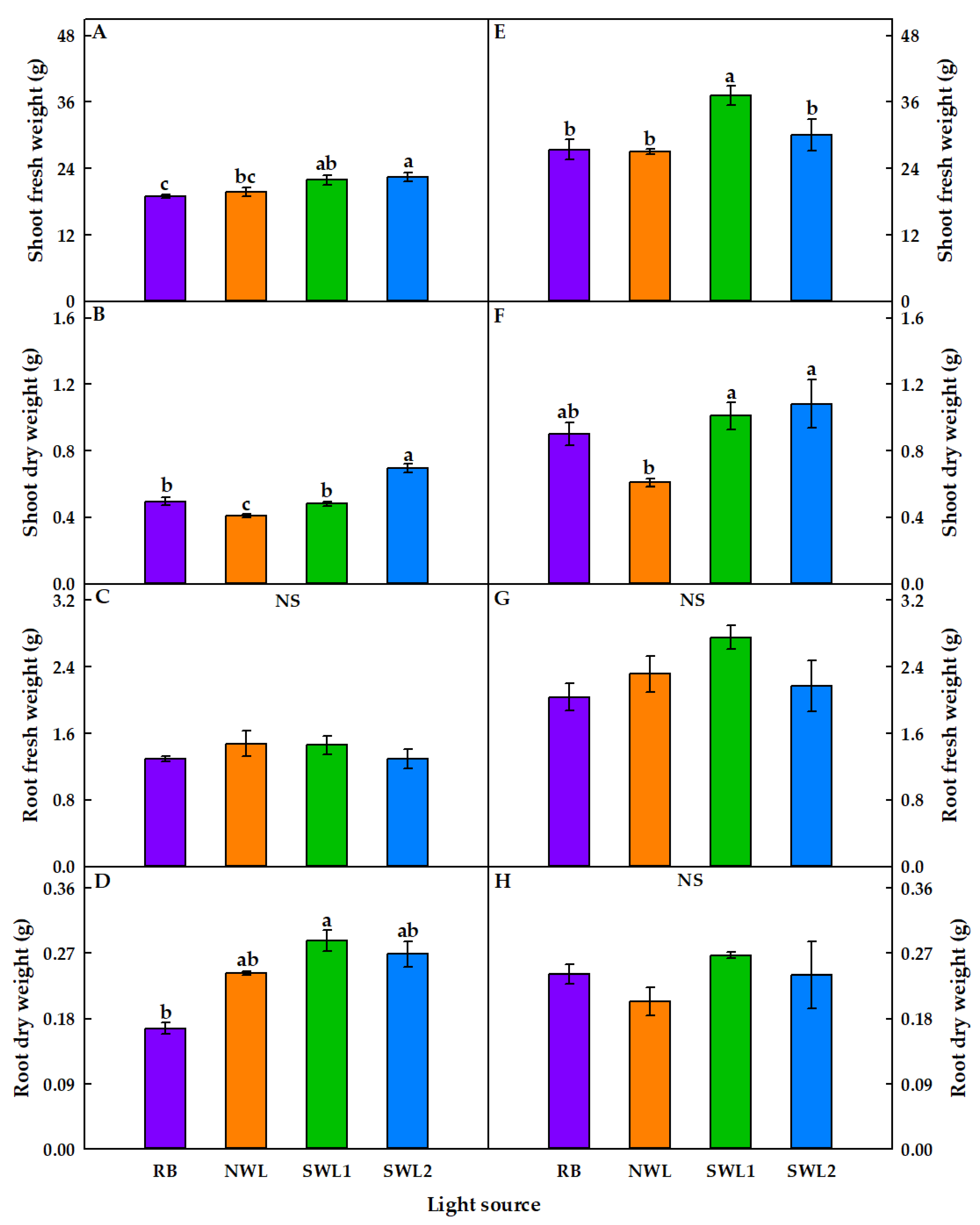
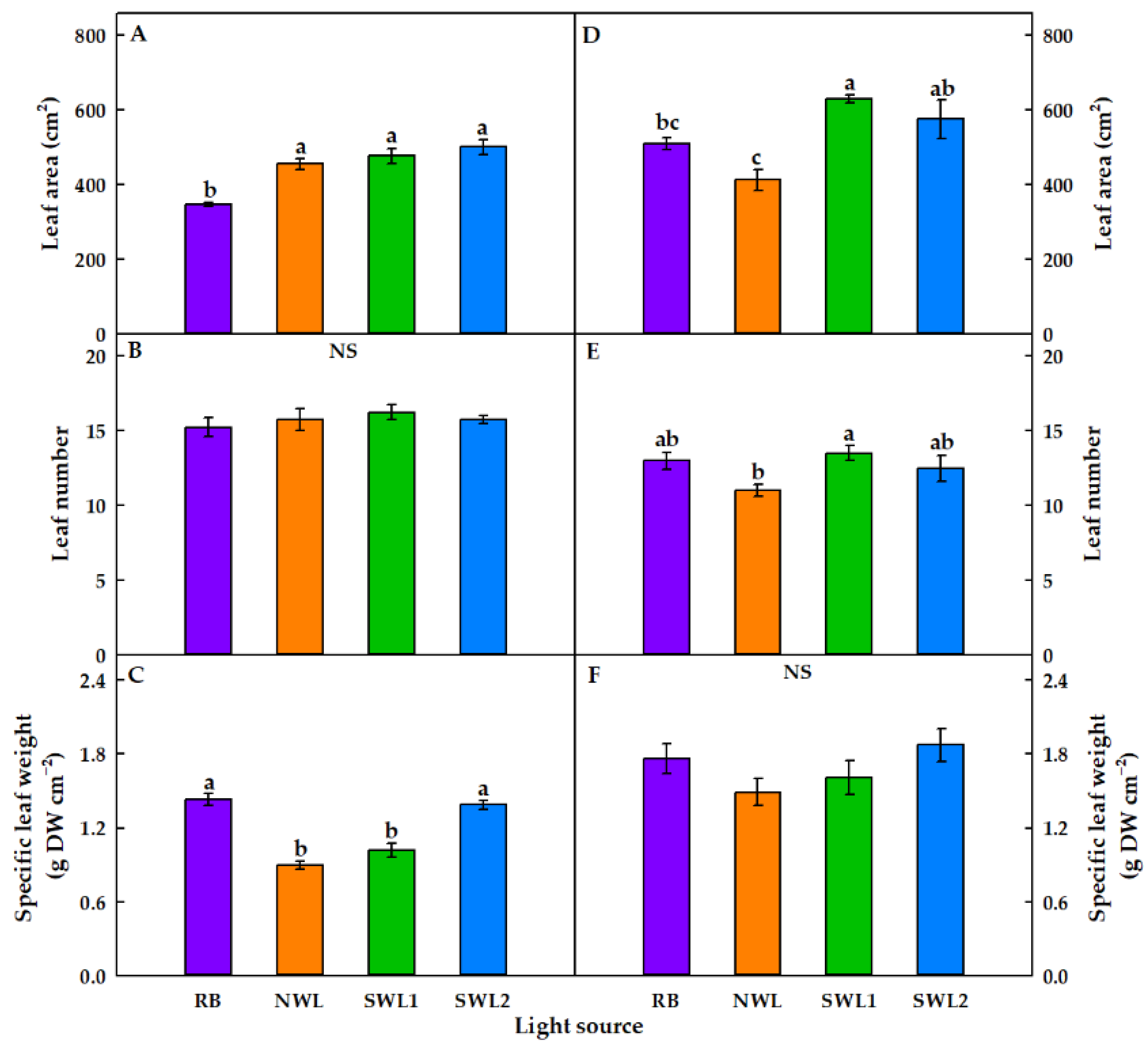
3.1.1. Growth Characteristics
3.1.2. Absorbance and Transmittance
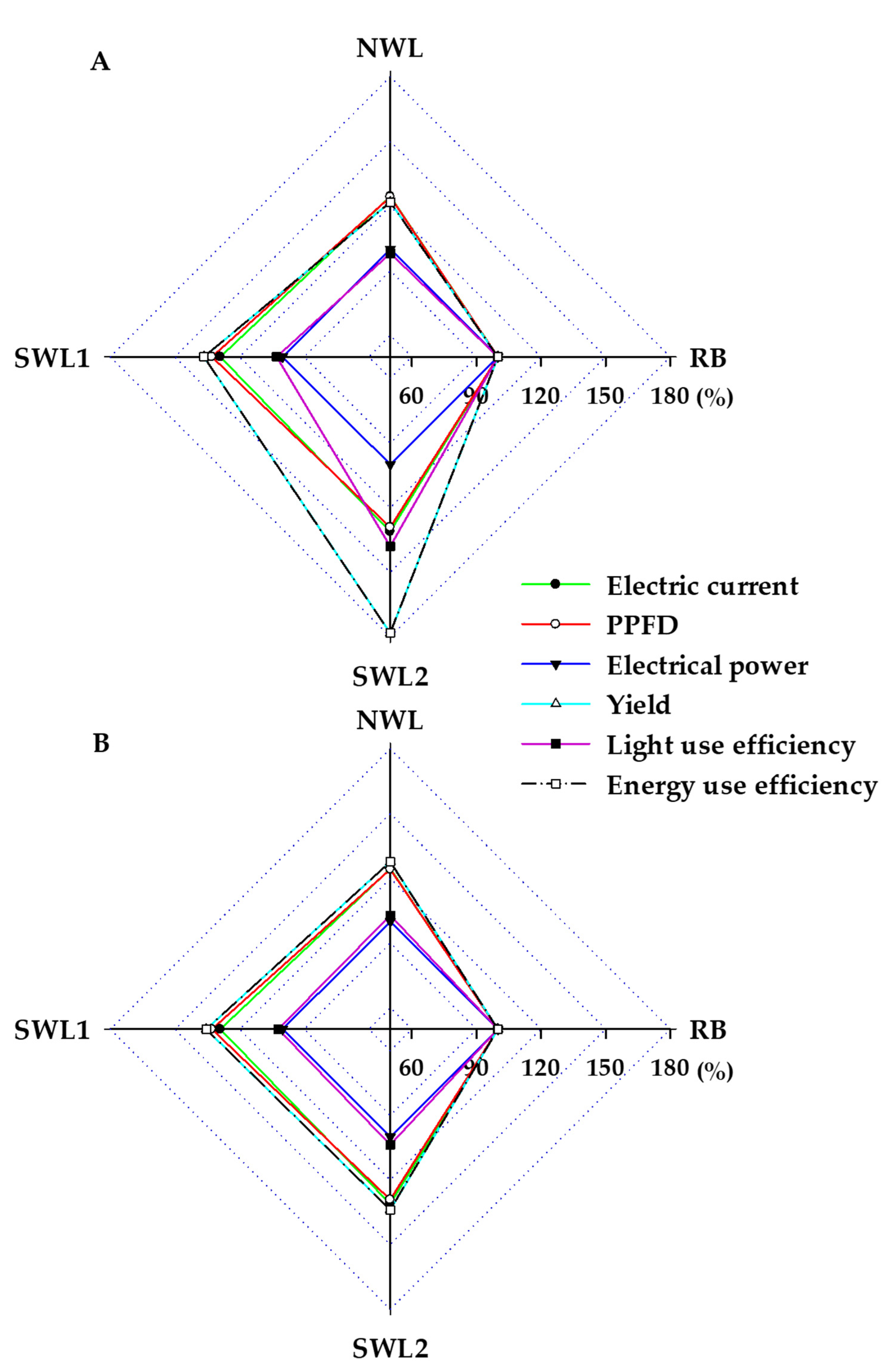
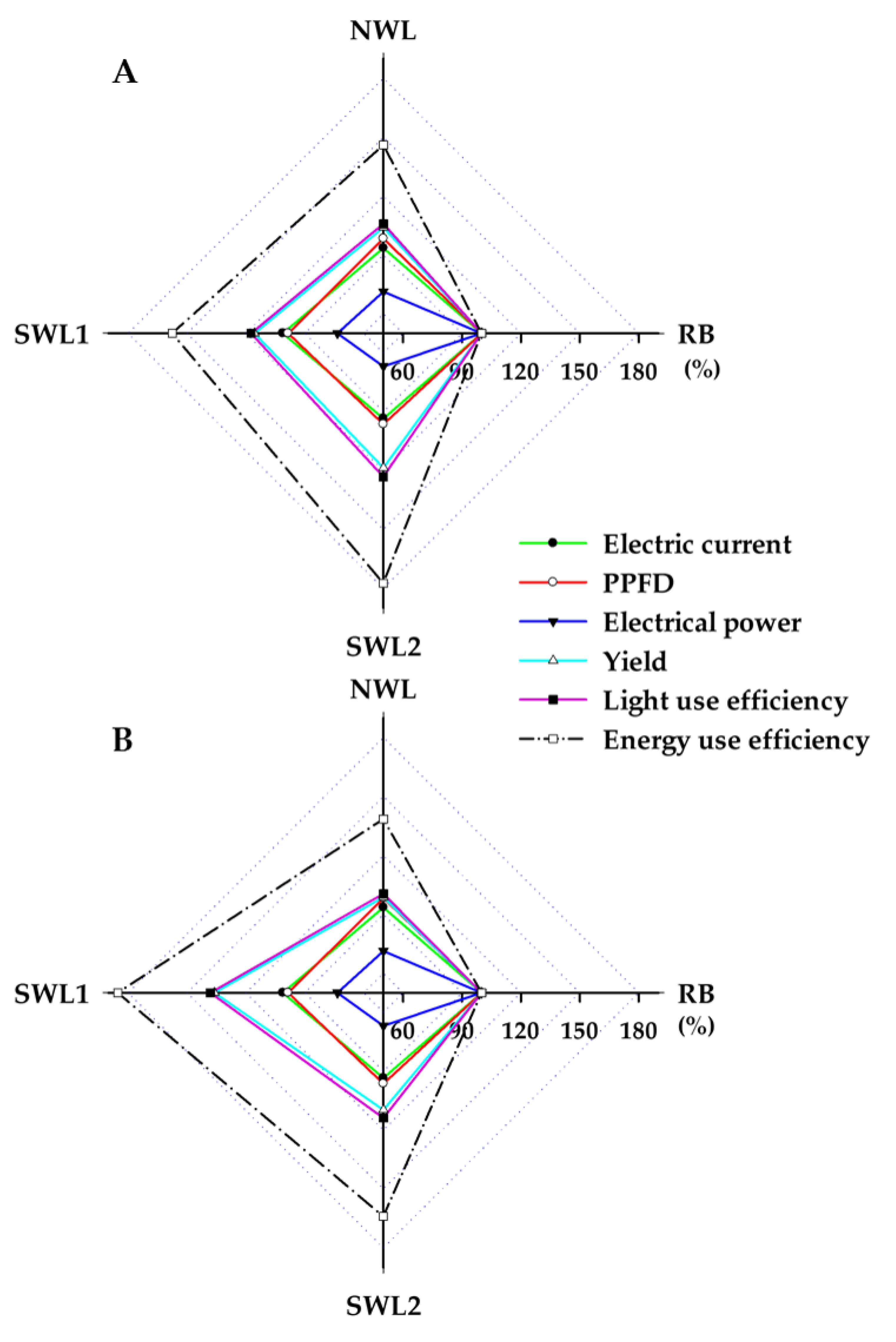
3.1.3. Light and Energy Use Efficiency
3.2. Experiment 2
3.2.1. Growth Characteristics
3.2.2. Absorbance and Transmittance
3.2.3. Individual Phenolic Acid and Flavonol Contents
3.2.4. Light and Energy Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golzar, F.; Heeren, N.; Hellweg, S.; Roshandel, R. A comparative study on the environmental impact of greenhouses: A probabilistic approach. Sci. Total Environ. 2019, 675, 560–569. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Martineau, V.; Lefsrud, M.; Naznin, M.T.; Kopsell, D. Comparison of Light-emitting Diode and High-pressure Sodium Light Treatments for Hydroponics Growth of Boston Lettuce. HortScience 2012, 47, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y. Nature and Source of Light for Plant Factory. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–69. [Google Scholar]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant Productivity in Response to LED Lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Son, K.-H.; Park, J.-H.; Kim, D.; Oh, M.-M. Leaf Shape Index, Growth, and Phytochemicals in Two Leaf Lettuce Cultivars Grown under Monochromatic Light-emitting Diodes. Korean J. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.-N.; Yoshihara, T. Blue Light-emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of Green, Red and Blue Light Emitting Diodes on Multiprotein Complex Proteins and Photosynthetic Activity under Different Light Intensities in Lettuce Leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.-X.; Wang, X.; Gao, L.-H.; Chen, Q.-Y.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2014, 53, 213–222. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-H.; Wheeler, R.M.; Sager, J.C.; Yorio, N.C.; Goins, G.D. Light-emitting diodes as an illumination source for plants: A review of research at Kennedy Space Center. Habitation 2005, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.L.; McAusland, L.; Murchie, E. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral Effects of Three Types of White Light-emitting Diodes on Plant Growth and Development: Absolute versus Relative Amounts of Blue Light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Chang, M.-H.; Das, D.; Varde, P.; Pecht, M. Light emitting diodes reliability review. Microelectron. Reliab. 2012, 52, 762–782. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; Denbaars, S.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Bian, Z.-H.; Cheng, R.-F.; Yang, Q.-C.; Wang, J.; Lu, C. Continuous Light from Red, Blue, and Green Light-emitting Diodes Reduces Nitrate Content and Enhances Phytochemical Concentrations and Antioxidant Capacity in Lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological Interactions of Blue Light and Photosynthetic Photon Flux: Effects of Monochromatic and Broad-Spectrum Light Sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environ. Exp. Bot. 2012, 75, 128–133. [Google Scholar] [CrossRef]
- Son, K.-H.; Jeon, Y.-M.; Oh, M.-M. Application of supplementary white and pulsed light-emitting diodes to lettuce grown in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2016, 57, 560–572. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, Nutritional Quality, and Energy Use Efficiency of Hydroponic Lettuce as Influenced by Daily Light Integrals Exposed to White versus White Plus Red Light-emitting Diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, N.; Fujita, M.; Ohta, Y.; Sase, S.; Nishimura, S.; Ezura, H. Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci. Hortic. 2008, 115, 176–182. [Google Scholar] [CrossRef]
- Woltering, E.; Witkowska, I. Effects of pre- and postharvest lighting on quality and shelf life of fresh-cut lettuce. Acta Hortic. 2016, 1134, 357–366. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Sago, Y. Effects of Light Intensity and Growth Rate on Tipburn Development and Leaf Calcium Concentration in Butterhead Lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Nájera, C.; Gea, M.D.M. Effect of the Spectral Quality and Intensity of Light-emitting Diodes on Several Horticultural Crops. HortScience 2016, 51, 268–271. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Steinger, T.; Roy, B.A.; Stanton, M.L. Evolution in stressful environments II: Adaptive value and costs of plasticity in response to low light in Sinapis arvensis. J. Evol. Biol. 2003, 16, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Son, K.-H.; Oh, M.-M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-emitting Diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Phansurin, W.; Jamaree, T.; Sakhonwasee, S. Comparison of Growth, Development, and Photosynthesis of Petunia Grown Under White or Red-blue LED lights. Korean J. Hortic. Sci. 2017, 35, 689–699. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010, 37, 403–412. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV—A irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Park, S.-Y.; Bae, J.-H.; Oh, M.-M.; Hoon, B.-J. Manipulating light quality to promote shoot growth and bioactive compound biosynthesis of Crepidiastrum denticulatum (Houtt.) Pak & Kawano cultivated in plant factories. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100237. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Oh, M. Physiological and biochemical responses of green and red perilla to LED -based light. J. Sci. Food Agric. 2021, 101, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Taulavuori, K.; Hyöky, V.; Oksanen, J.; Taulavuori, E.; Julkunen-Tiitto, R. Species-specific differences in synthesis of flavonoids and phenolic acids under increasing periods of enhanced blue light. Environ. Exp. Bot. 2016, 121, 145–150. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Lee, K.B.; Kim, J.K.; Park, J.S.; Lee, J.-W.; Park, S.U. Metabolic Analysis of Vigna unguiculata Sprouts Exposed to Different Light-Emitting Diodes. Nat. Prod. Commun. 2018, 13, 1934578X1801301029. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.; Chivkunova, O.; Zhigalova, T.; Solovchenko, A. In situ optical properties of foliar flavonoids: Implication for non-destructive estimation of flavonoid content. J. Plant Physiol. 2017, 218, 258–264. [Google Scholar] [CrossRef]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A light wavelengths positively affected accumulation profiles of healthy compounds in pak-choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Constantino, L.F.D.S.; Nascimento, L.B.D.S.; Casanova, L.M.; Moreira, N.D.S.; Menezes, E.A.; Esteves, R.L.; Costa, S.S.; Tavares, E.S. Responses of Crepis japonica induced by supplemental blue light and UV-A radiation. Photochem. Photobiol. Sci. 2017, 16, 238–245. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G.P. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Poulet, L.; Massa, G.; Morrow, R.; Bourget, C.; Wheeler, R.; Mitchell, C. Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sci. Space Res. 2014, 2, 43–53. [Google Scholar] [CrossRef]

| Light Source | Range (nm) | Peak Wavelength (nm) | Ratio (%) |
|---|---|---|---|
| RB | Blue (400–500) | 444 | 47 |
| Green (500–600) | - | 2 | |
| Red (600–700) | 665 | 51 | |
| NWL | Blue (400–500) | 453 | 21 |
| Green (500–600) | 586 | 42 | |
| Red (600–700) | 665 | 37 | |
| SWL1 | Blue (400–500) | 437 | 26 |
| Green (500–600) | 526 | 41 | |
| Red (600–700) | 665 | 33 | |
| SWL2 | Blue (400–500) | 437 | 19 |
| Green (500–600) | 578 | 43 | |
| Red (600–700) | 664 | 38 |
| Experiment | Light Source | PPFD (μmol m−2 s−1) | Electrical Power (W) |
|---|---|---|---|
| Experiment 1 | RB | 129.73 | 80 |
| NWL | 161.15 | ||
| SWL1 | 172.28 | ||
| SWL2 | 167.55 | ||
| Experiment 2 | RB | 147.50 ± 2.89 | 88.59 |
| NWL | 63.10 | ||
| SWL1 | 65.01 | ||
| SWL2 | 59.24 |
| Cultivar | Light Source | Absorbance (%) | Transmittance (%) | ||||
|---|---|---|---|---|---|---|---|
| Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | ||
| Butterhead | RB | 97 | - | 92 | 3 | - | 8 |
| NWL | 98 | 82 | 90 | 2 | 18 | 10 | |
| SWL1 | 96 | 77 | 88 | 4 | 23 | 12 | |
| SWL2 | 96 | 73 | 85 | 4 | 27 | 15 | |
| Romaine | RB | 98 | - | 96 | 2 | - | 4 |
| NWL | 97 | 84 | 90 | 3 | 16 | 10 | |
| SWL1 | 99 | 87 | 93 | 1 | 13 | 7 | |
| SWL2 | 98 | 83 | 91 | 2 | 17 | 9 | |
| Cultivar | Light Source | Absorbance (%) | Transmittance (%) | ||||
|---|---|---|---|---|---|---|---|
| Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | Blue (380–499 nm) | Green (500–599 nm) | Red (600–700 nm) | ||
| Butterhead | RB | 98 | - | 94 | 2 | - | 6 |
| NWL | 96 | 77 | 86 | 4 | 23 | 14 | |
| SWL1 | 96 | 77 | 86 | 4 | 23 | 14 | |
| SWL2 | 96 | 73 | 84 | 4 | 27 | 16 | |
| Romaine | RB | 99 | - | 95 | 1 | - | 5 |
| NWL | 97 | 83 | 90 | 3 | 17 | 10 | |
| SWL1 | 98 | 85 | 92 | 2 | 15 | 8 | |
| SWL2 | 98 | 84 | 91 | 2 | 16 | 9 | |
| Individual Compound Content (μg Plant−1) | Light Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RB | NWL | SWL1 | SWL2 | ||||||
| Phenolic acids | Gallic acid | 193.77 | b z | 167.50 | c | 185.05 | bc | 241.56 | a |
| Protocatechuic acid | 69.46 | a | 42.74 | b | 44.64 | b | 47.89 | b | |
| Chlorogenic acid | 729.54 | a | 433.24 | c | 509.25 | b | 691.87 | a | |
| p-Hydrobenzoic acid | 98.28 | b | 75.82 | d | 86.12 | c | 113.50 | a | |
| Vanillic acid | 11.45 | c | 20.10 | b | 9.78 | c | 23.47 | a | |
| p-Coumaric acid | 13.70 | n.d. | n.d. | n.d. | |||||
| Ferulic acid | 45.52 | a | 16.33 | b | 17.06 | b | 19.42 | b | |
| Veratric acid | 65.10 | a | 28.37 | c | 32.64 | c | 47.34 | b | |
| Benzoic acid | 280.00 | a | 174.96 | b | 180.61 | b | 183.77 | b | |
| t-Cinnamic acid | 5.81 | b | 4.13 | d | 4.80 | c | 6.72 | a | |
| Total | 1512.65 | 963.18 | 1069.96 | 1375.56 | |||||
| Flavonols | Epigallocatechin | 522.10 | c | 637.60 | b | 586.33 | bc | 749.57 | a |
| Catechin | 390.01 | a | 224.36 | c | 206.86 | c | 270.07 | b | |
| Epicatechin | 156.22 | a | 130.20 | b | 120.18 | b | 152.37 | a | |
| Epigallocatechin gallate | 80.26 | a | 67.96 | b | 63.11 | b | 87.26 | a | |
| Vanillin | n.d. | n.d. | n.d. | n.d. | |||||
| Rutin | 69.59 | a | 8.33 | c | 9.51 | c | 17.92 | b | |
| Catechin gallate | 99.92 | a | 1.85 | d | 70.26 | c | 81.77 | b | |
| Quercetin | 1052.95 | b | 847.18 | c | 977.60 | b | 1422.73 | a | |
| Naringin | 80.62 | a | 27.91 | d | 43.30 | c | 64.00 | b | |
| Naringenin | 101.25 | b | 79.49 | c | 105.93 | b | 148.37 | a | |
| Formononetin | 25.58 | a | n.d. | 24.38 | a | 13.64 | b | ||
| Total | 2578.51 | 2024.88 | 2207.46 | 3007.69 | |||||
| Individual Compound Content (μg Plant−1) | Light Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RB | NWL | SWL1 | SWL2 | ||||||
| Phenolic acids | Gallic acid | 342.04 | a z | 176.88 | b | 346.16 | a | 254.25 | b |
| Protocatechuic acid | 43.02 | a | 17.35 | b | n.d. | 55.21 | a | ||
| Chlorogenic acid | 1624.00 | a | 820.43 | b | 1470.84 | a | 1583.90 | a | |
| p-Hydrobenzoic acid | n.d. | 84.44 | b | 151.41 | a | 136.80 | a | ||
| Vanillic acid | n.d. | n.d. | n.d. | n.d. | |||||
| p-Coumaric acid | 208.15 | a | 50.95 | c | 110.53 | b | n.d. | ||
| Ferulic acid | 198.47 | a | 39.22 | c | 78.94 | b | 64.94 | bc | |
| Veratric acid | 190.54 | a | 45.46 | c | 87.44 | b | 87.61 | b | |
| Benzoic acid | 1006.31 | a | 301.05 | c | 529.17 | b | 542.88 | b | |
| t-Cinnamic acid | 5.16 | b | 5.11 | b | 9.22 | a | 9.11 | a | |
| Total | 3617.68 | 1540.89 | 2783.61 | 2734.70 | |||||
| Flavonols | Epigallocatechin | 870.92 | a | 484.09 | b | 823.99 | a | 934.25 | a |
| Catechin | 381.06 | a | 241.71 | b | 395.73 | a | 475.51 | a | |
| Epicatechin | 64.94 | c | 63.73 | c | 133.48 | b | 203.23 | a | |
| Epigallocatechin gallate | 159.13 | a | 86.05 | b | 139.56 | a | 152.78 | a | |
| Vanillin | n.d. | n.d. | n.d. | n.d. | |||||
| Rutin | 278.24 | a | 60.85 | c | 115.14 | b | 114.22 | b | |
| Catechin gallate | 367.73 | a | 104.30 | c | 197.29 | b | 182.16 | b | |
| Quercetin | 1578.36 | bc | 1180.10 | c | 2038.07 | ab | 2263.71 | a | |
| Naringin | n.d. | 21.61 | c | 47.49 | b | 110.38 | a | ||
| Naringenin | 22.48 | c | 64.18 | b | 107.16 | a | 113.49 | a | |
| Formononetin | n.d. | n.d. | n.d. | n.d. | |||||
| Total | 3722.85 | 2306.61 | 3997.90 | 4549.73 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy 2021, 11, 2111. https://doi.org/10.3390/agronomy11112111
Nguyen TKL, Cho KM, Lee HY, Cho DY, Lee GO, Jang SN, Lee Y, Kim D, Son K-H. Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy. 2021; 11(11):2111. https://doi.org/10.3390/agronomy11112111
Chicago/Turabian StyleNguyen, Thi Kim Loan, Kye Man Cho, Hee Yul Lee, Du Yong Cho, Ga Oun Lee, Seong Nam Jang, Yongki Lee, Daesup Kim, and Ki-Ho Son. 2021. "Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System" Agronomy 11, no. 11: 2111. https://doi.org/10.3390/agronomy11112111
APA StyleNguyen, T. K. L., Cho, K. M., Lee, H. Y., Cho, D. Y., Lee, G. O., Jang, S. N., Lee, Y., Kim, D., & Son, K.-H. (2021). Effects of White LED Lighting with Specific Shorter Blue and/or Green Wavelength on the Growth and Quality of Two Lettuce Cultivars in a Vertical Farming System. Agronomy, 11(11), 2111. https://doi.org/10.3390/agronomy11112111







