Effects of Insect Growth Regulators on Mortality, Survival, and Feeding of Euprosterna elaeasa (Lepidoptera: Limacodidae) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Concentration–Mortality Bioassay
2.3. Time–Mortality Bioassay
2.4. Feeding Inhibition
2.5. Mortality in Semifield Conditions
2.6. Statistical Analysis
3. Results
3.1. Concentration–Mortality Bioassay
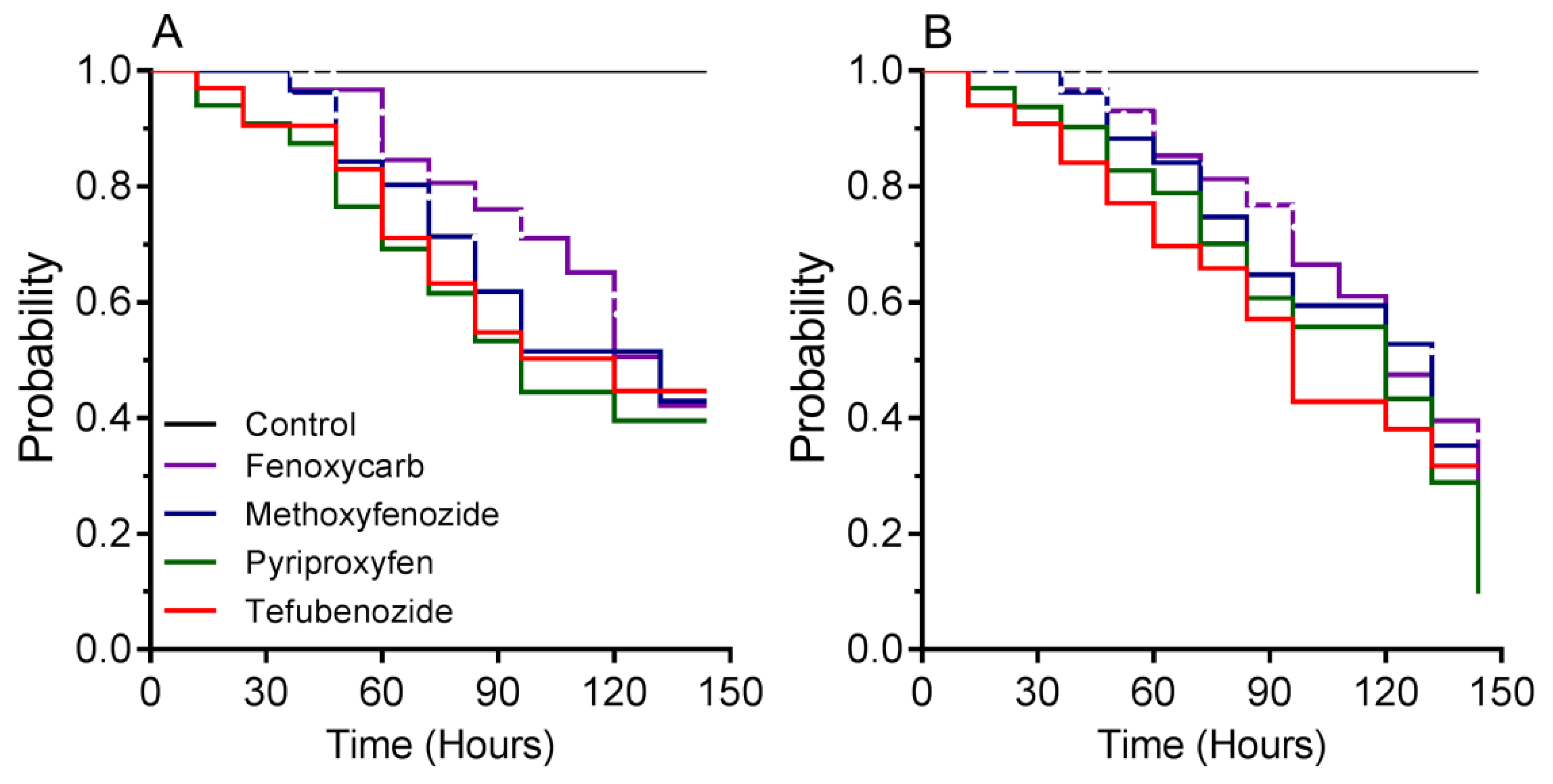
3.2. Time–Mortality Bioassay
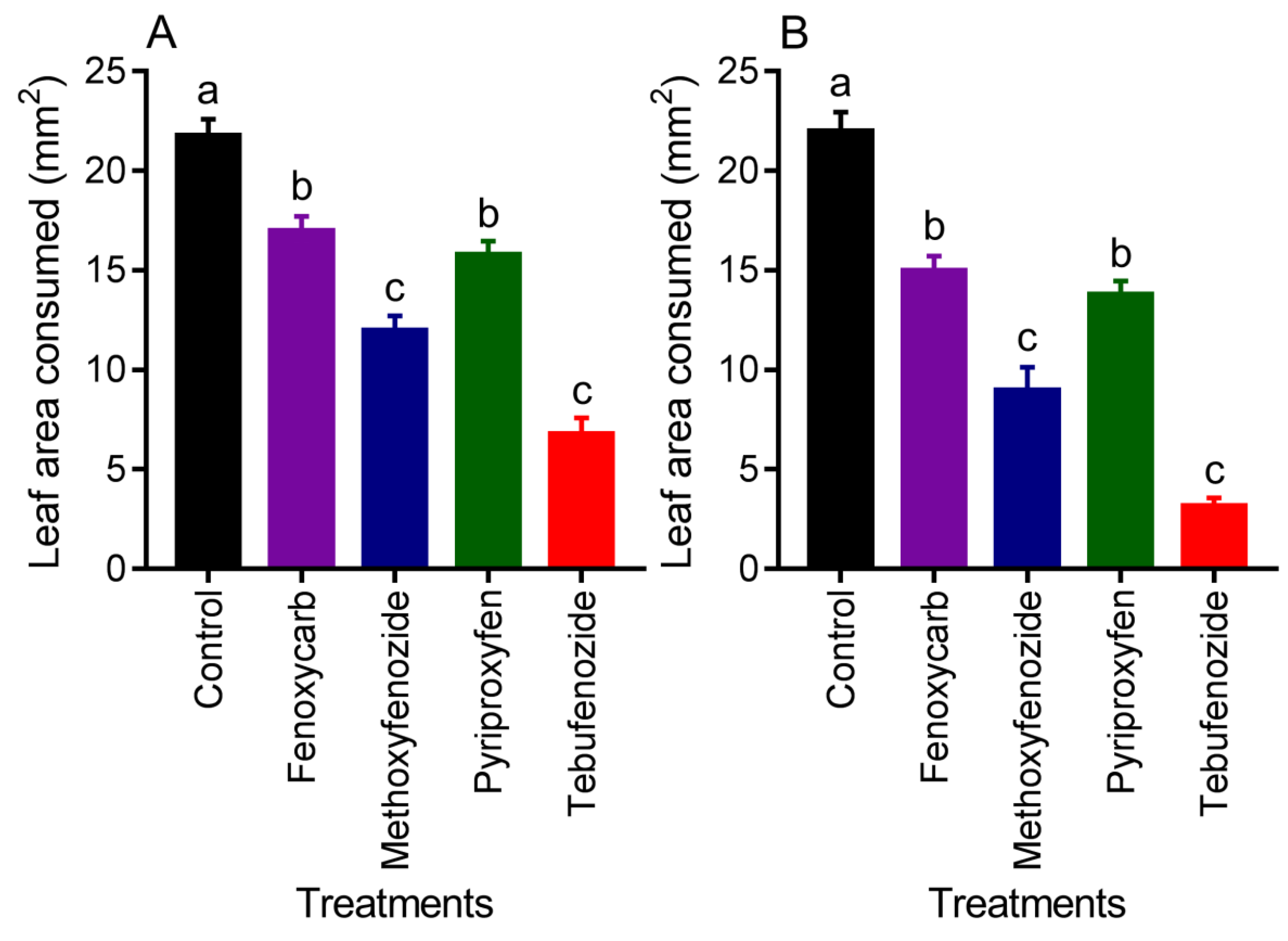
3.3. Feeding Inhibition
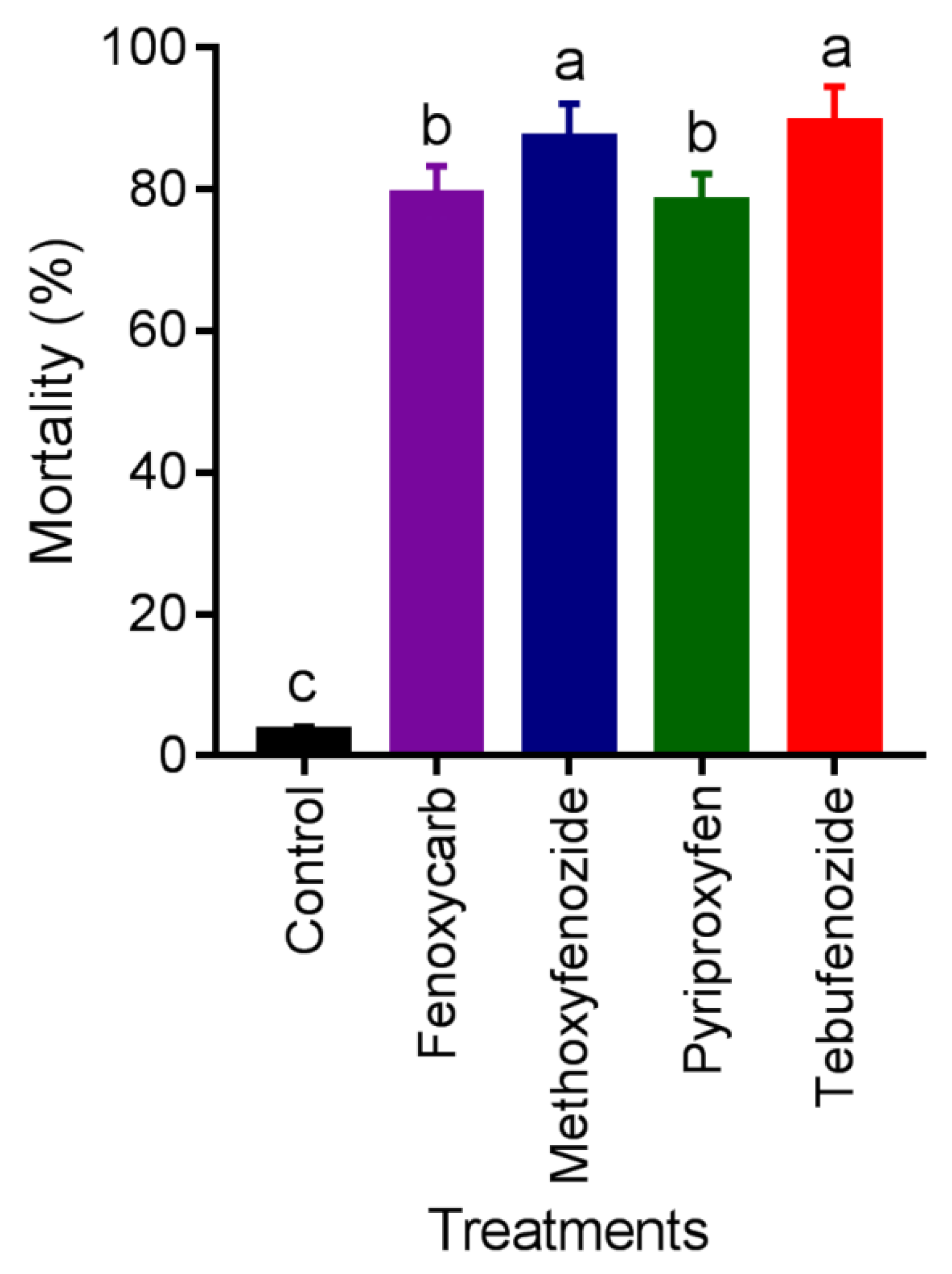
3.4. Mortality in Semifield Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genty, P.; Desmier de Chenon, R.; Morin, J.P. Les ravageurs du palmier a huile en Amerique latine. Oléagineux 1978, 33, 325–419. [Google Scholar]
- Alvarado, H.; de La Torre, R.A.; Barrera, E.; Martínez, L.; Bustillo, A. Ciclo de vida y tasa de consumo de Euprosterna elaeasa Dyar (Lepidoptera: Limacodidae) defoliador de la palma de aceite. Rev. Palmas 2014, 35, 41–51. [Google Scholar]
- Martínez, L.C.; Plata-Rueda, A. Lepidoptera vectors of Pestalotiopsis fungal disease: First records in oil palm plantations from Colombia. Int. J. Trop. Insect Sci. 2013, 33, 239–246. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Serrao, J.E.; Zanuncio, J.C. Life history traits and damage potential of an invasive pest Acharia fusca (Lepidoptera: Limacodidae) on oil palm. Ann. Entomol. Soc. Am. 2014, 107, 1086–1093. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Comparative toxicity of six insecticides on the rhinoceros beetle (Coleoptera: Scarabaeidae). Fla. Entomol. 2014, 97, 1056–1062. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Rodríguez-Dimaté, F.A.; Campos, J.M.; Santos Júnior, V.C.D.; Rolim, G.D.S.; Fernandes, F.L.; Silva, W.M.; Wilcken, C.F.; Zanuncio, J.C.; et al. Exposure to insecticides reduces populations of Rhynchophorus palmarum in oil palm plantations with Bud Rot disease. Insects 2019, 10, 111. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.R.; Cruz, M.A.; Genty, P. The root absorption technique for controlling oil-palm pests. Oléagineux 1988, 43, 363–370. [Google Scholar]
- Yeoh, C.B.; Kuntom, A.; Dorasamy, S.; Omar, M.R.; Nor, M.Y.M.; Noh, M.R.M. Determination of acephate, methamidophos and monocrotophos in crude palm oil. Eur. J. Lipid Sci. Technol. 2006, 108, 960–964. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Last, J.M. Global change: Ozone depletion, greenhouse warming, and public health. Annu. Rev. Public Health 1993, 14, 115–136. [Google Scholar] [CrossRef]
- Shipp, J.L.; Wang, K.; Ferguson, G. Residual toxicity of avermectin b1 and pyridaben to eight commercially produced beneficial arthropod species used for control of greenhouse pests. Biol. Control 2000, 17, 125–131. [Google Scholar] [CrossRef]
- Samantsidis, G.R.; O’Reilly, A.O.; Douris, V.; Vontas, J. Functional validation of target-site resistance mutations against sodium channel blocker insecticides (SCBIs) via molecular modeling and genome engineering in Drosophila. Insect Biochem. Mol. Biol. 2019, 104, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; da Silva Neves, G.; Gonçalves, W.G.; Zanuncio, J.C.; Bozdoğan, H.; Serrão, J.E. Permethrin induces histological and cytological changes in the midgut of the predatory bug, Podisus nigrispinus. Chemosphere 2018, 212, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.L.; Plata-Rueda, A.; Martínez, L.C. Oil palm plantations as an agroecosystem: Impact on integrated pest management and pesticide use. Outlooks Pest Manag. 2013, 24, 225–229. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Quintero, H.A.; Serrão, J.E.; Martínez, L.C. Insecticidal activity of Bacillus thuringiensis strains on the nettle caterpillar, Euprosterna elaeasa (Lepidoptera: Limacodidae). Insects 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Ramírez, A.; Serrão, J.E. Susceptibility of Demotispa neivai (Ceoleoptera Chrysomelidae) to Beauveria bassiana and Metarhizium anisopliae entomopathogenic fungal isolates. Pest Manag.Sci. 2021. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Serrão, J.E. Effect of benzoylphenyl ureas on survival and reproduction of the lace bug, Leptopharsa gibbicarina. Insects 2021, 12, 34. [Google Scholar] [CrossRef]
- Amaral, K.D.; Martínez, L.C.; Lima, M.A.P.; Serrão, J.E.; Della Lucia, T.M.C. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut. 2018, 243, 809–814. [Google Scholar] [CrossRef]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Cossolin, J.F.S.; Serra, R.S.; Martins, G.F.; Serrão, J.E. Behavioral and ultrastructural effects of novaluron on Aedes aegypti larvae. Infect. Genet. Evol. 2021, 93, 104974. [Google Scholar] [CrossRef]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; de Souza, D.L.L.; Cossolin, J.F.S.; Carvalho, P.E.G.R.; Martins, G.F.; Serrão, J.E. Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae. PeerJ 2019, 7, e7489. [Google Scholar] [CrossRef] [Green Version]
- Fiaz, M.; Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Shareef, M.; Zanuncio, J.C.; Serrão, J.E. Toxicological and morphological effects of tebufenozide on Anticarsia gemmatalis (Lepidoptera: Noctuidae) larvae. Chemosphere 2018, 212, 337–345. [Google Scholar] [CrossRef]
- Müller, A.; Silva, A.D.B.; de Souza, L.A.; Buecke, J.; Guimarães, L.G.; Silva, J.D.O.; Vale, M.P.D.; Lins, P.M.P.; Ohashi, O.S. Controle químico de lagartas de Eupalamides dedalus em dendezeiros. Embrapa Amaz. Orient. Comun. Técnico 2000, 38, 1–4. [Google Scholar]
- Llácer, E.; Dembilio, O.; Jacas, J.A. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 402–408. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, L.; Liu, X.; Peng, Y.; Liang, G.; Xiao, H. Dissecting the roles of FTZ-F1 in larval molting and pupation, and the sublethal effects of methoxyfenozide on Helicoverpa armigera. Pest Manag. Sci. 2020, 77, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, E.A.; Kamita, S.G.; Hammock, B.D. Effects of juvenile hormone (JH) analog insecticides on larval development and JH esterase activity in two spodopterans. Pestic. Biochem. Physiol. 2016, 128, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zou, H.; Geng, N.; Ding, N.; Wang, Y.; Zhang, J.; Zoua, C. Fenoxycarb and methoxyfenozide (RH-2485) affected development and chitin synthesis through disturbing glycometabolism in Lymantria dispar larvae. Pestic. Biochem. Physiol. 2020, 163, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Crossthwaite, A.L.; Nauen, R.; Banbad, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification–a tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Leucothyreus femoratus (Coleoptera: Scarabaeidae): Feeding and behavioral activities as an oil palm defoliator. Fla. Entomol. 2013, 96, 55–63. [Google Scholar] [CrossRef]
- GraphPad Prism User Guide, 2015. GraphPad Software, Inc. Available online: http://www.graphpad.com/guides/prism/6/user-guide/ (accessed on 15 December 2015).
- SAS Institute. The SAS System for Windows, Release 9.0.; SAS Institute: Cary, NC, USA, 2002; Available online: http://www.sas.com (accessed on 6 January 2003).
- Sial, A.A.; Brunner, J.F. Lethal and sublethal effects of an insect growth regulator, pyriproxyfen, on obliquebanded leafroller (Lepidoptera: Tortricidae). J. Econ. Entomol. 2010, 103, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, M.; Moharramipour, S. Sublethal effects of fenoxycarb on the Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2015, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Boina, D.R.; Rogers, M.E.; Wang, N.; Stelinski, L.L. Effect of pyriproxyfen, a juvenile hormone mimic, on egg hatch, nymph development, adult emergence and reproduction of the asian citrus psyllid, Diaphorina citri Kuwayama. Pest Manag. Sci. 2010, 66, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Smagghe, G.; Dhadialla, T.S.; Lezzi, M. Comparative toxicity and ecdysone receptor affinity of non-steroidal ecdysone agonists and 20-hydroxyecdysone in Chironomus tentans. Insect Biochem. Mol. Biol. 2002, 32, 187–192. [Google Scholar] [CrossRef]
- Jindra, M.; Bittova, L. The juvenile hormone receptor as a target of juvenoid “insect growth regulators”. Arch. Insect Biochem. Physiol. 2020, 103, e21615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauchamp, B.; Malosse, C.; Saroglia, P. Biological effects and metabolism of fenoxycarb after treatment of the fourth and the fifth instars of the tobacco budworm, Heliothis virescens F. Pestic. Sci. 1989, 26, 283–301. [Google Scholar] [CrossRef]
- Ono, E.K.; Zanardi, O.Z.; Santos, K.F.A.; Yamamoto, P.T. Susceptibility of Ceraeochrysa cubana larvae and adults to six insect growth regulator insecticides. Chemosphere 2017, 168, 49–57. [Google Scholar] [CrossRef]
- Critchley, B.R.; Campion, D.G. Effects of a juvenile hormone analogue on growth and reproduction in the cotton stainer Dysdercus fasciatus Say. Bull. Entomol. Res. 2009, 61, 49–53. [Google Scholar] [CrossRef]
- Farder-Gomes, C.; Saravanan, M.; Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Azadirachtin affects the respiration and digestion in Anticarsia gemmatalis caterpillars. Toxin Rev. 2021, 1–10. [Google Scholar] [CrossRef]
- Rimoldi, F.; Fogel, M.N.; Schneider, M.I.; Ronco, A.E. Lethal and sublethal effects of cypermethrin and methoxyfenozide on the larvae of Rachiplusia nu (Guenee) (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 2012, 56, 200–208. [Google Scholar] [CrossRef]
- Cook, S.P. Laboratory and field evaluation of tebufenozide, diflubenzuron, and Bacillus thurengiensis var. kurstaki for suppression of Douglas-fir tussock moth (Orgyia pseudotsugata (McDunnough)) in Idaho: A case study. J. Econ. Entomol. 2003, 96, 396–400. [Google Scholar] [CrossRef]
- Jiang, Z.; Shang, Z.; Zhao, S.; Chiu, S. The relationship between insect growth regulators with juvenile hormone activity and insect feeding behavioural response. Insect Sci. 1999, 6, 71–76. [Google Scholar] [CrossRef]
- Nasr, H.M.; Badawy, M.E.; Rabea, E.I. Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littoralis. Pestic. Biochem. Physiol. 2010, 98, 198–205. [Google Scholar] [CrossRef]
- Santos Junior, V.C.; Martínez, L.C.; Plata-Rueda, A.; Fernandes, F.L.; Tavares, W.S.; Zanuncio, J.C.; Serrão, J.E. Histopathological and cytotoxic changes induced by spinosad on midgut cells of the non-target predator Podisus nigrispinus Dallas (Heteroptera: Pentatomidae). Chemosphere 2020, 238, 124585. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Martínez, L.C.; Da Silva, B.K.R.; Zanuncio, J.C.; Sena Fernandes, M.E.; Serrão, J.E.; Guedes, R.N.C.; Fernandes, F.L. Exposure to cyantraniliprole causes mortality and disturbs behavioral and respiratory response in the coffee berry borer (Hypothenemus hampei). Pest Manag. Sci. 2019, 75, 2236–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Qin, D.-Q.; Zhang, P.-W.; Liu, B.-J.; Chen, X.-T.; Zhang, Z.-X. The comparative metabolic response of Bactrocera dorsalis larvae to azadirachtin, pyriproxyfen and tebufenozide. Ecotox. Environ. Saf. 2020, 189, 110020. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.D.C.; Martinez, L.C.; Barbosa, S.G.; Serrão, J.E.; Wilcken, C.F.; Soares, M.A.; Silva, A.A.D.; Carvalho, A.G.D.; Zanuncio, J.C. Toxicity and cytopathology mediated by Bacillus thuringiensis in the midgut of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 6667. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.M.C.; Martínez, L.C.; Plata-Rueda, A.; Soares, M.A.; Wilcken, C.F.; Zanuncio, A.J.V.; Fiaz, M.; Zanuncio, J.C.; Serrão, J.E. Exposure to chlorantraniliprole reduces locomotion, respiration, and causes histological changes in the midgut of velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2021, 263, 128008. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, M.; Martínez, L.C.; da Silva Costa, M.; Cossolin, J.F.S.; Plata-Rueda, A.; Gonçalves, W.G.; Sant’Ana, A.E.G.; Zanuncio, J.C.; Serrão, J.E. Squamocin induce histological and ultrastructural changes in the midgut cells of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Ecotoxicol. Environ. Saf. 2018, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Menezes, C.H.M.; Cunha, W.S.; Alvarenga, T.M.; Barbosa, B.F.; Zanuncio, J.C.; Martínez, L.C.; Serrão, J.E. Side-effects caused by chlorpyrifos in the velvetbean caterpillar Anticarsia gemmatalis (Lepidoptera: Noctuidae). Chemosphere 2020, 259, 127530. [Google Scholar] [CrossRef]
- Vinha, G.L.; Plata-Rueda, A.; Soares, M.A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Deltamethrin-mediated effects on locomotion, respiration, feeding, and histological changes in the midgut of Spodoptera frugiperda Caterpillars. Insects 2021, 12, 483. [Google Scholar] [CrossRef]
- Guillebeau, L.P.; All, J.N.; Javid, A.M. Influence of weather on efficacy of pyrethroid insecticides for boll weevil (Coleoptera: Curculionidae) and bollworm (Lepidoptera: Noctuidae) in cotton. J. Econ. Entomol. 1989, 82, 291–296. [Google Scholar] [CrossRef]
- Hayasaka, D.; Korenaga, T.; Sánchez-Bayo, F.; Goka, K. Differences in ecological impacts of systemic insecticides with different physicochemical properties on biocenosis of experimental paddy fields. Ecotoxicology 2012, 21, 191–201. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. 2009, 86, 27–35. [Google Scholar] [CrossRef]
| IGRs | No. Insects | LC | EC (g L−1) | 95% CI (g L−1) | Slope ± SE | χ2 (p-Value) |
|---|---|---|---|---|---|---|
| Fenoxycarb | 120 | LC25 | 0.109 | 0.079–0.137 | 1.815 ± 0.23 | 1.66 (0.64) |
| 120 | LC50 | 0.199 | 0.162–0.241 | |||
| 120 | LC75 | 0.363 | 0.297–0.470 | |||
| 120 | LC95 | 0.859 | 0.630–1.378 | |||
| Methoxyfenozide | 120 | LC25 | 0.120 | 0.086–0.152 | 1.486 ± 0.20 | 4.42 (0.21) |
| 120 | LC50 | 0.233 | 0.188–0.285 | |||
| 120 | LC75 | 0.451 | 0.362–0.603 | |||
| 120 | LC95 | 1.167 | 0.824–1.997 | |||
| Pyriproxyfen | 120 | LC25 | 0.079 | 0.061–0.096 | 2.281 ± 0.29 | 6.23 (0.11) |
| 120 | LC50 | 0.141 | 0.117–0.171 | |||
| 120 | LC75 | 0.251 | 0.204–0.333 | |||
| 120 | LC95 | 0.578 | 0.419–0.942 | |||
| Tebufenozide | 120 | LC25 | 0.154 | 0.121–0.185 | 1.760 ± 0.23 | 5.77 (0.12) |
| 120 | LC50 | 0.259 | 0.217–0.308 | |||
| 120 | LC75 | 0.434 | 0.360–0.554 | |||
| 120 | LC95 | 0.914 | 0.690–1.387 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.C.; Plata-Rueda, A.; Serrão, J.E. Effects of Insect Growth Regulators on Mortality, Survival, and Feeding of Euprosterna elaeasa (Lepidoptera: Limacodidae) Larvae. Agronomy 2021, 11, 2002. https://doi.org/10.3390/agronomy11102002
Martínez LC, Plata-Rueda A, Serrão JE. Effects of Insect Growth Regulators on Mortality, Survival, and Feeding of Euprosterna elaeasa (Lepidoptera: Limacodidae) Larvae. Agronomy. 2021; 11(10):2002. https://doi.org/10.3390/agronomy11102002
Chicago/Turabian StyleMartínez, Luis Carlos, Angelica Plata-Rueda, and José Eduardo Serrão. 2021. "Effects of Insect Growth Regulators on Mortality, Survival, and Feeding of Euprosterna elaeasa (Lepidoptera: Limacodidae) Larvae" Agronomy 11, no. 10: 2002. https://doi.org/10.3390/agronomy11102002
APA StyleMartínez, L. C., Plata-Rueda, A., & Serrão, J. E. (2021). Effects of Insect Growth Regulators on Mortality, Survival, and Feeding of Euprosterna elaeasa (Lepidoptera: Limacodidae) Larvae. Agronomy, 11(10), 2002. https://doi.org/10.3390/agronomy11102002








