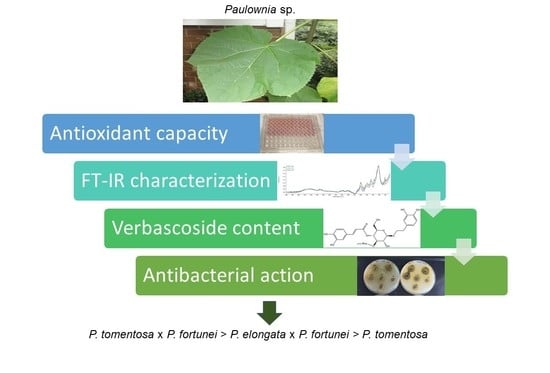
Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Extracts Preparation
2.3.1. Crude Extract
2.3.2. Freeze-Dried Extract
2.4. Antioxidant Activity Determination
2.4.1. FRAP Assay
2.4.2. DPPH Test
2.4.3. ABTS Test
2.5. Total Phenolic Content (TPC) Determination
2.6. Total Flavonoid Content (TFC) Determination
2.7. FT-IR Analysis
2.8. Polyphenolic Profiles by HPTLC and Verbascoside Quantification
2.9. Antibacterial Activity
2.10. Statistical Analyses
3. Results
3.1. Screening for the Clone of the Best Antioxidant Potential
3.2. Detailed Characteristic of Biological Activity of Selected Paulownia Clones Leaves
3.2.1. Antioxidant Activity
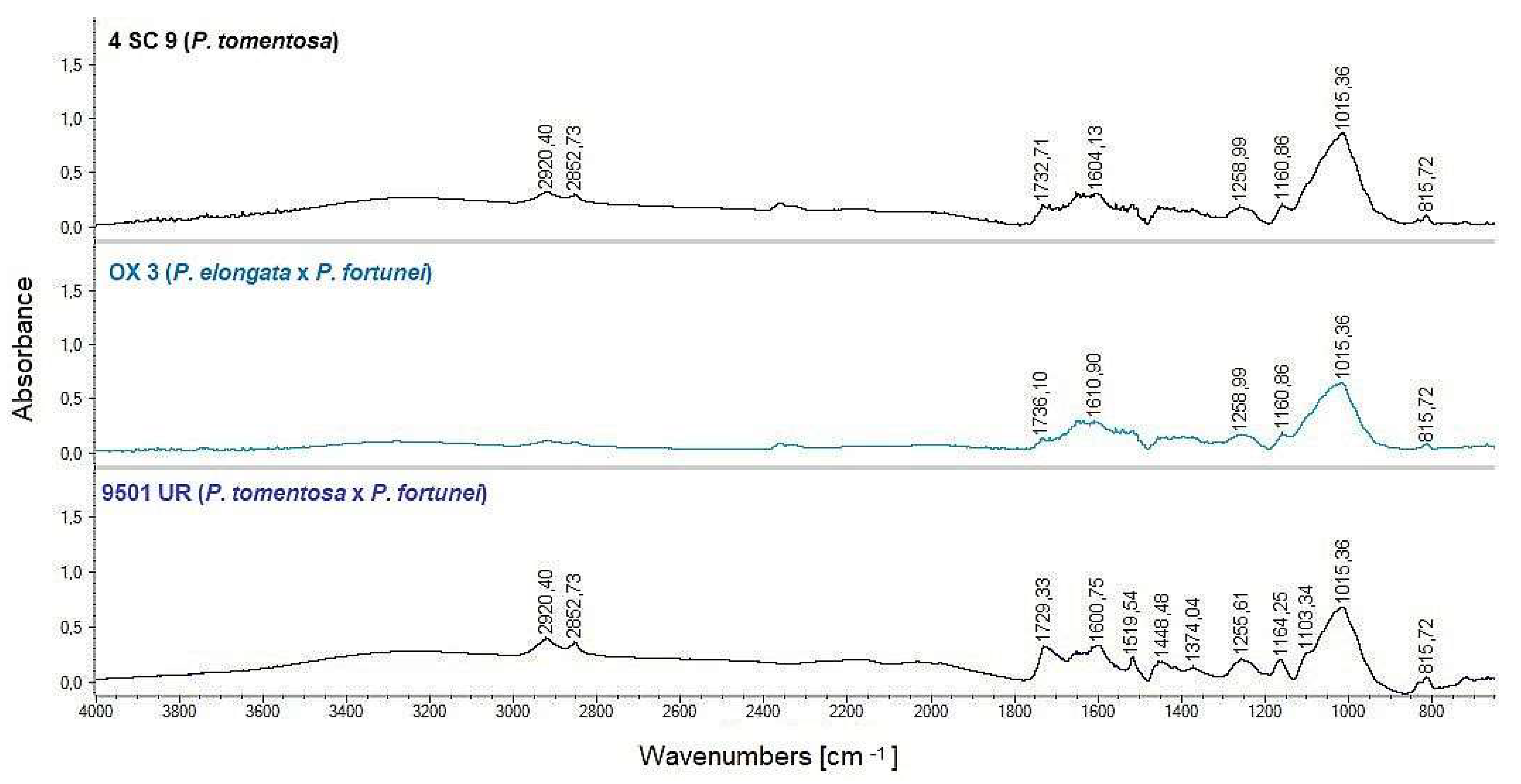
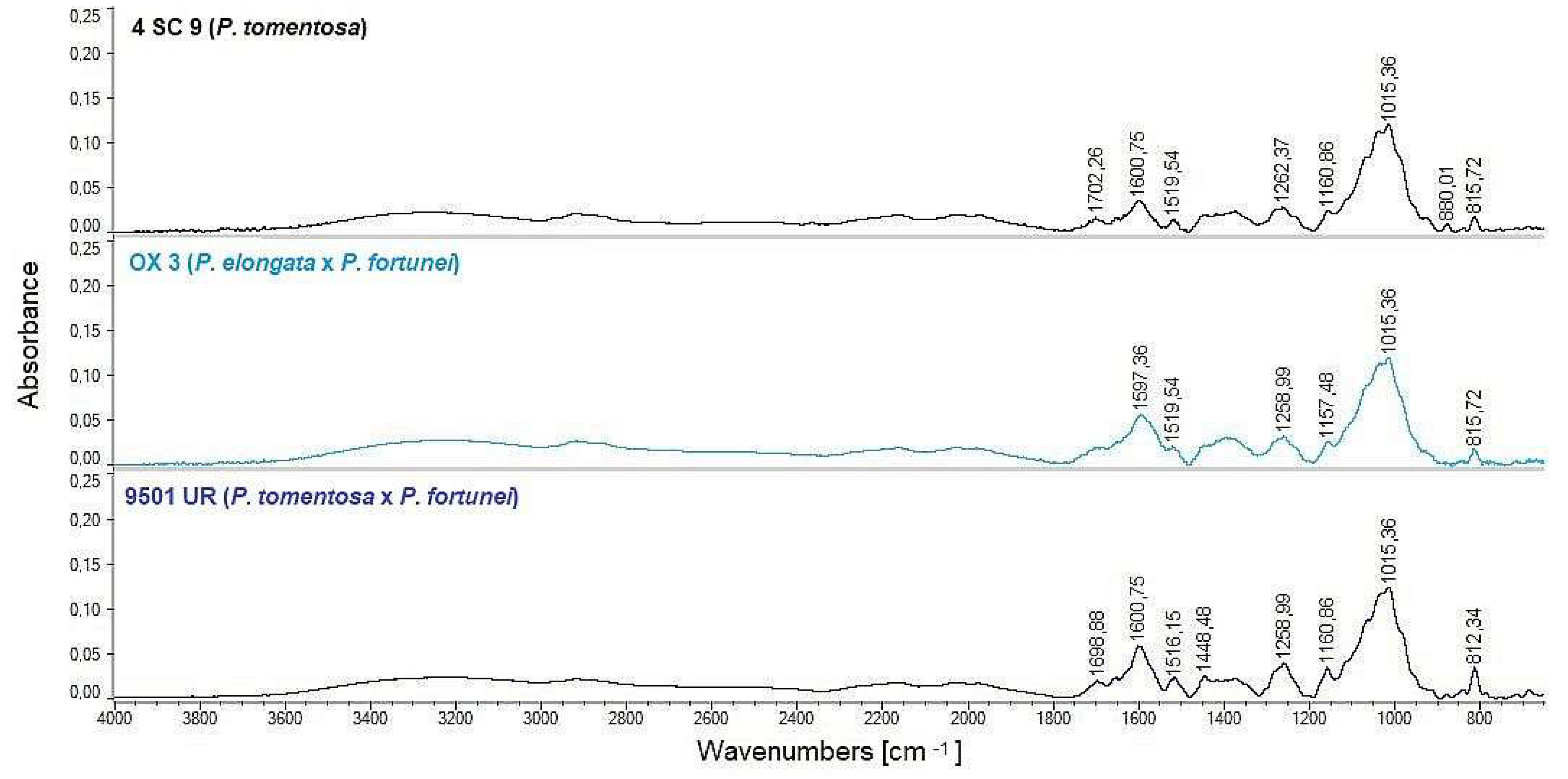
3.2.2. FT-IR Spectra Analysis
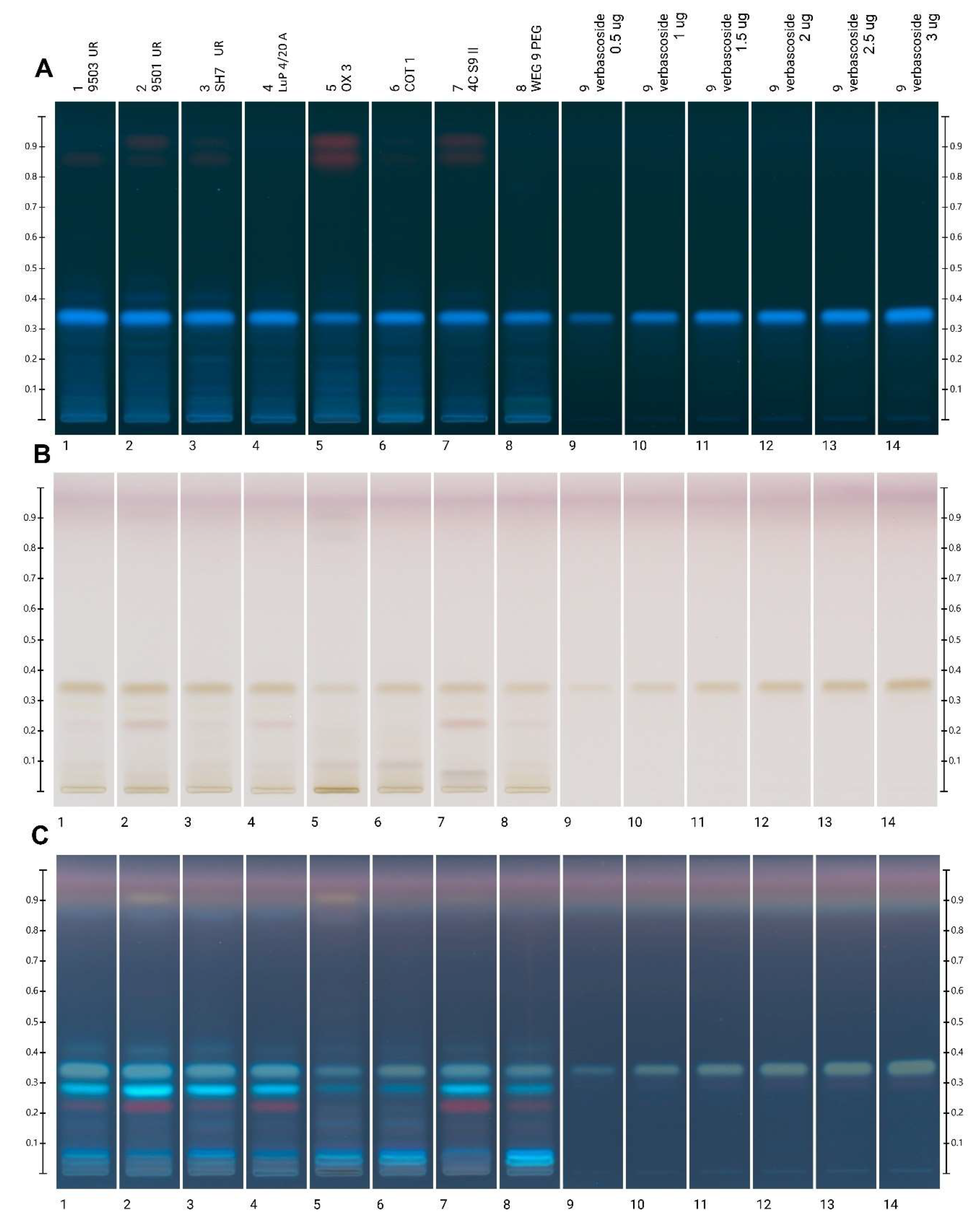
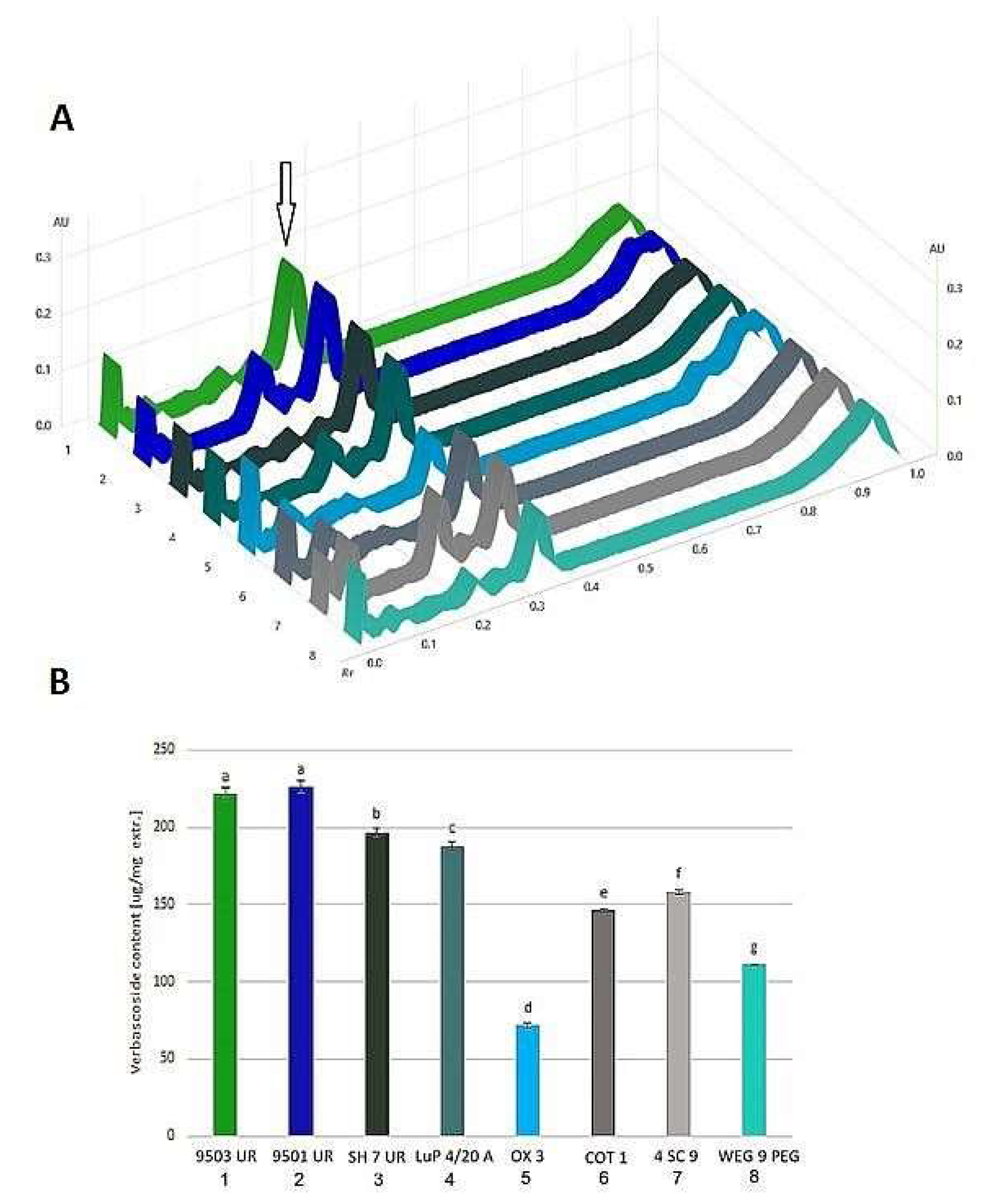
3.2.3. HPTLC Analysis
3.2.4. Antibacterial Activity
4. Discussion
4.1. Clone-Specific Antioxidant Activity of Leaves
4.2. FT-IR Comparison of Chemical Composition
4.3. HPTLC Polyphenolic Profile and Verbascoside Quantification
4.4. Clone-Specific Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Icka, P.; Damo, R.; Icka, E. Paulownia Tomentosa, a Fast Growing Timber. Ann. Valahia Univ. Targoviste Agric. 2016, 10, 14–19. [Google Scholar] [CrossRef]
- Woods, V.B. Paulownia as a Novel Bio-Mass Crop for Northern Ireland? A Review of Current Knowledge; Occasional Publ. Agri-Food and Biosciences Institute: Hillsborough, FL, USA, 2008. [Google Scholar]
- Akyildiz, M.H.; Kol, H.S. Some technological properties and uses of paulownia (Paulownia tomentosa Steud.) wood. J. Environ. Biol. 2010, 31, 351–355. [Google Scholar] [PubMed]
- Zhu, Z.H.; Chao, C.J.; Lu, X.Y.; Xiong, Y.G. Paulownia in China: Cultivation and Utilization; Asian Network for Biological Sciences: Singapore, 1986; pp. 1–65. [Google Scholar]
- Barton, I.L.; Nicholas, I.D.; Ecroyd, C.E. Paulownia. For. Res. Bull. 2007, 231, 5–68. [Google Scholar]
- Essl, F. From ornamental to detrimental? The incipient invasion of Central Europe by Paulownia tomentosa. Preslia 2007, 79, 377–389. [Google Scholar]
- Yadav, N.K.; Vaidya, B.N.; Henderson, K.; Lee, J.F.; Stewart, W.M.; Dhekney, S.A.; Joshee, N. A Review of PaulowniaBiotechnology: A Short Rotation, Fast Growing Multipurpose Bioenergy Tree. Am. J. Plant Sci. 2013, 4, 2070–2082. [Google Scholar] [CrossRef]
- Snow, W.A. Ornamental, crop, or invasive? The history of the Empress tree (Paulownia) in the USA. For. Trees Livelihoods 2015, 24, 85–96. [Google Scholar] [CrossRef]
- TISI 2019. The Texas Invasive Species Institute. Available online: http://www.tsusinvasives.org/home/database/paulownia-tomentosa (accessed on 29 April 2021).
- DAISIE. Delivering Alien Invasive Species Inventories for Europe. 2018. Available online: http://www.europe-aliens.org/speciesFactsheet.do?speciesId=18222 (accessed on 29 April 2021).
- GISD. Global Invasive Species Database. 2018. Available online: http://www.iucngisd.org/gisd/search.php (accessed on 29 April 2021).
- Berg, E.C.; Zarnoch, S.J.; McNab, W.H. Survivorship, attained diameter, height and volume of three Paulownia species after 9 years in the southern Appalachians, USA. J. For. Res. 2020, 31, 2181–2191. [Google Scholar] [CrossRef]
- Doumett, S.; Lamperi, L.; Checchini, L.; Azzarello, E.; Mugnai, S.; Mancuso, S.; Petruzzelli, G.; Del Bubba, M. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: Influence of different complexing agents. Chemosphere 2008, 72, 1481–1490. [Google Scholar] [CrossRef]
- Stankovic, D.; Nikolic, M.S.; Krstic, B.; Vilotic, D. Heavy metals in the leaves of tree species paulownia elongata s.y.hu in the region of the city of Belgrade. Biotechnol. Biotechnol. Equip. 2009, 23, 1330–1336. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.B.; Jin, Z.X. The distribution and phytoavailability of heavy metal fractions in rhizosphere soils of Paulowniu fortunei (seem) Hems near a Pb/Zn smelter in Guangdong, PR China. Geoderma 2009, 148, 299–306. [Google Scholar] [CrossRef]
- Azzarello, E.; Pandolfi, C.; Giordano, C.; Rossi, M.; Mugnai, S.; Mancuso, S. Ultramorphological and physiological modifications induced by high zinc levels in Paulownia tomentosa. Environ. Exp. Bot. 2012, 81, 11–17. [Google Scholar] [CrossRef]
- Ayan, S.; Sivacioglu, A.; Bilir, N. Growth variation of Paulownia Sieb, and Zucc. species and origins at the nursery stage in Kastamonu-Turkey. J. Environ. Biol. 2006, 27, 499–504. [Google Scholar]
- López, F.; Pérez, A.; Zamudio, M.A.M.; De Alva, H.E.; García, J.C. Paulownia as raw material for solid biofuel and cellulose pulp. Biomass Bioenergy 2012, 45, 77–86. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Jiménez Bocanegra, J.A.; Perea Torres, F.; Rodríguez Pleguezuelo, C.R.; Francia Martínez, J.R. Biomass Yield Potential of Paulownia Trees in a Semi-Arid Mediterranean Environment (S Spain). Int. J. Renew. Energy Res. 2013, 3, 789–793. [Google Scholar]
- Ayan, S.; Sağlam, İ.; Sıvacıoğlu, A. A new exotic genus for multi-purpose uses in Kastamonu-Turkey. Decision Support for Multiple Purpose. Forestry 2003, 4, 23–25. [Google Scholar]
- Stewart, W.M.; Vaidya, B.N.; Mahapatra, A.K.; Terrill, T.H.; Joshee, N. Potential Use of Multipurpose Paulownia elongata Tree as an Animal Feed Resource. Am. J. Plant Sci. 2018, 9, 1212–1227. [Google Scholar] [CrossRef]
- Popova, T.P.; Baykov, B.D. Antimicrobial activity of aqueous extracts of leaves and silage from Paulownia elongata. Am. J. Biol. Chem. Pharm. Sci. 2013, 1, 8–15. [Google Scholar]
- He, T.; Vaidya, B.; Perry, Z.; Parajuli, P.; Joshee, N. Paulownia as a Medicinal Tree: Traditional Uses and Current Advances. Eur. J. Med. Plants 2016, 14, 1–15. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałazka, A.; Grzadziel, J.; Frac, M. Microbial diversity of Paulownia spp. leaves-A new source of green manure. BioResources 2019, 13, 4807–4819. [Google Scholar] [CrossRef]
- Yamauchi, T.; Date, H.; Saihara, Y.; Osada, K. Deodorants Containing Hydrogen Peroxide and Plant Extracts. Jpn. Kokai Tokkyo Koho 1987, 62–357. [Google Scholar]
- Si, C.L.; Liu, S.C.; Hu, H.Y.; Jiang, J.Z.; Yu, G.J.; Ren, X.D.; Xu, G.H. Activity-guided screening of the antioxidants from Paulownia tomentosa var. Tomentosa bark. BioResources 2013, 8, 628–637. [Google Scholar] [CrossRef]
- Smejkal, K.; Holubova, P.; Zima, A.; Muselik, J.; Dvorska, M. Antiradical activity of Paulownia tomentosa (Scrophulariaceae) extracts. Molecules 2007, 12, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Lee, D.-C.; Hong, J.-E.; Chang, I.-S.; Cho, H.-Y.; Kwon, Y.-K.; Kim, H.-Y. Antimicrobial Effects of Ethanol Extracts from Korean and Indonesian Plants. Korean J. Food Sci. Technol. 2000, 32, 949–958. [Google Scholar]
- Jo, N.-Y.; Kim, K.-T. Antioxidant Activity and Anti-inflammatory Effect of Extracts from Paulownia tomentosa in LPS-stimulated RAW264.7 macrophage cells. J. Korean Med. 2019, 40, 72–83. [Google Scholar] [CrossRef][Green Version]
- Jiang, T.F.; Du, X.; Shi, Y.P. Determination of Flavonoids from Paulownia tomentosa (Thunb) Steud by Micellar Electrokinetic Capillary Electrophoresis. Chromatographia 2004, 59, 255–258. [Google Scholar] [CrossRef]
- Oprea, E.; Radulescu, V.; Chiliment, S. The Analysis of the Volatile and Semi-volatile Compounds of the Paulownia tomentosa Flowers by Gas Chromatography Coupled with Mass Spectrometry. Rev. Chim. 2004, 55, 410–412. [Google Scholar]
- Schneiderová, K.; Šmejkal, K. Phytochemical profile of Paulownia tomentosa (Thunb). Steud. Phytochem. Rev. 2015, 14, 799–833. [Google Scholar] [CrossRef]
- Litwińczuk, W.; Bochnia, E. Development of royal Paulownia (Paulownia tomentosa Steud.) in vitro shoot cultures Under the influence of different saccharides. Acta Sci. Pol. Hortorum Cultus 2012, 11, 3–13. [Google Scholar]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Biju, J.; Reddy, V.R.K.; Suleiman, C.T. Total Phenolics and Flavonoids in Selected Justicia Species. J. Pharmacol. Pharmacogn. 2015, 2, 51–52. [Google Scholar]
- NCCLS. Performance Standards for Antimicrobial Disc Suspectibility Tests; Approved Standard NCCLS Publication M2-A5: Villanova, PA, USA, 1993. [Google Scholar]
- Borycka, K.; Grabek-Lejko, D.; Kasprzyk, I. Antioxidant and antibacterial properties of commercial bee pollen products. J. Apicult. Res. 2015, 54, 491–502. [Google Scholar] [CrossRef]
- Wang, Y. Application of fourier transform infrared microspectroscopy (FTIR) and thermogravimetric analysis (TGA) for quick identification of Chinese herb Solanum lyratum. Plant Omics 2012, 5, 508–513. [Google Scholar]
- Durak, T.; Depciuch, J. Effect of plant sample preparation and measuring methods on ATR-FTIR spectra results. Environ. Exp. Bot. 2020, 169, 103915. [Google Scholar] [CrossRef]
- Pontaza-Licona, Y.S.; Ramos-Jacques, A.; Cervantes-Chavez, J.; López-Miranda, J.L.; de Jesús Ruíz-Baltazar, Á.; Maya-Cornejo, J.; Rodríguez-Morales, A.L.; Esparza, R.; Estevez, M.; Pérez, R.; et al. Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Results Phys. 2019, 12, 1670–1679. [Google Scholar] [CrossRef]
- Hawrył, A.; Hawrył, M.; Litwińczuk, W.; Bogucka-Kocka, A. Thin-layer chromatographic fingerprint of selected Paulownia species with chemometrics and antioxidant activity. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 367–374. [Google Scholar] [CrossRef]
- Schilling, G.; Hügel, M.; Mayer, W. Verbascosid und Isoverbascosid aus Paulownia tomentosa Steud. Zeitschrift fur Naturforsch. -Sect. B J. Chem. Sci. 1982, 37, 1633–1635. [Google Scholar] [CrossRef]
- Adach, W.; Zuchowski, J.; Moniuszko-Szajwaj, B.; Szumacher-Strabel, M.; Stochmal, A.; Olas, B.; Cieslak, A. Comparative phytochemical, antioxidant, and hemostatic studies of extract and four fractions from paulownia clone in vitro 112 leaves in human plasma. Molecules 2020, 25, 4371. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside-A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-L.; Jia, X.-H.; Xiao, C.-M.; Tang, W.-Z. Paulownia C-geranylated flavonoids: Their structural variety, biological activity and application prospects. Phytochem. Rev. 2019, 18, 549–570. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Knaś, M.; Długosz, E.; Krüzselyi, D.; Kowalska, T.; Sajewicz, M. Antibacterial potential of the phenolics extracted from the Paulownia tomentosa L. leaves as studied with use of high-performance thin-layer chromatography combined with direct bioautography. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 282–289. [Google Scholar] [CrossRef]
- Takó, M.; Beáta Kerekes, E.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef]
- Ghaemi, E.O.; Khorshidi, D.; Moradi, A.; Seifi, A.; Mazendrani, M.; Bazouri, M.; Mansourian, A.R. The efficacy of ethanolic extract of Lemon verbena on the skin infection due to Staphylococcus aureus in an animal model. Pak. J. Biol. Sci. 2007, 10, 4132–4135. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Khameneh, B.; Zahedian Ostad, M.R.; Hosseinzadeh, H. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J. Phytomed. 2018, 8, 246–253. [Google Scholar]
- Jacek, B.; Litwińczuk, W. The selected biomass properties of Paulownia tomentosa strains cultivated for energy purposes in the first two years of vegetation. Ann. Wars. Univ. Life Sci. SGGW. Agric. (Agric. For. Eng.) 2016, 68, 61–66. [Google Scholar]

| No | Code | Species | Origin |
|---|---|---|---|
| 1 | OX 3 | P. elongata × P. fortunei | ‘Oxytree’ (in vitro clone 112) |
| 2 | COT 1 | P. elongata × P. fortunei | ‘Cotevisa 2’ (‘Cotte Visa 2’) |
| 3 | 9501 UR | P. tomentosa × P. fortunei | UR * clone obtained from fast growing seedling of Chinese seed strain ‘9501’ in vitro |
| 4 | 9503 UR | P. tomentosa × P. fortunei | UR clone obtained from fast growing seedling of Chinese seed strain ‘9503’ in vitro |
| 5 | 9503 II/7 UR | P. tomentosa × P. fortunei | UR clone obtained from fast growing seedling of Chinese seed strain ‘9503’ in vitro; deep dark-leaf variant of 9503 |
| 6 | SH 6 UR | P. tomentosa × P. fortunei | UR clone obtained from fast growing seedling of Chinese seed strain ‘Shan Tong’ in vitro |
| 7 | SH 7 UR | P. tomentosa × P. fortunei | UR clone obtained from fast growing seedling of Chinese seed strain ‘Shan Tong’ in vitro |
| 8 | US 1 | P. tomentosa | UR clone obtained from fast growing seedling of US seed strain |
| 9 | SLO NN1 | P. tomentosa | UR clone obtained from fast growing seedling of Slovakian seed strain |
| 10 | WEG 9 PEG | P. tomentosa | UR clone obtained from fast growing PEG-tolerant seedling of Hungarian seed strain |
| 11 | 4C S9 | P. tomentosa | Tetraploid mutant obtained from ‘WEG 9 PEG’ |
| 12 | ZAL A1 | P. tomentosa | UR clone obtained in vitro from Polish seed strain ‘LuD’ through undirect morphogenesis |
| 13 | A4 ST A2 | P. tomentosa | UR clone obtained in vitro from Polish seed strain ‘LuD’ through undirect morphogenesis |
| 14 | LuP 3–7 | P. tomentosa | UR clone obtained from specimen of fastest growing Polish seed strain ‘LuP’ in the field |
| 15 | LuP 1–12 | P. tomentosa | UR clone obtained from specimen ‘1–12’ of fastest growing Polish seed strain ‘LuP’ in the field |
| 16 | LuP Hg 12 | P. tomentosa | UR clone obtained in vitro from Hg-tolerant seedling of Polish seed strain ‘LuP’ |
| 17 | LuP 4/20 A | P. tomentosa | UR clone obtained in vitro from low pH and Al-tolerant seedling A of Polish seed strain ‘LuP’ |
| 18 | LuP 4/20 B | P. tomentosa | UR clone obtained in vitro from low pH and Al-tolerant seedling B of Polish seed strain ‘LuP’ |
| No. | Clone | Antioxidant Activity | TPC [mg GAE/g s.m.] | TFC [mg QE/g s.m.] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FRAP [μmol TE/g s.m.] | DPPH [μmol TE/g s.m.] | ABTS [μmol TE/g s.m.] | |||||||||
| Blades | Petioles | Blades | Petioles | Blades | Petioles | Blades | Petioles | Blades | Petioles | ||
| 1 | OX 3 | 194.80 ± 2.51 | 26.69 ± 0.35 | 162.99 ± 5.40 | 18.13 ± 0.80 | 278.61 ± 14.07 | 140.96 ± 13.29 | 39.40 ± 2.53 | 5.89 ± 0.06 | 27.82 ± 0.16 | 2.13 ± 0.00 |
| 2 | COT 1 | 230.72 ± 3.07 | 27.83 ± 4.19 | 180.97 ± 4.13 | 23.33 ± 0.32 | 401.34 ± 95.38 | 132.12 ± 13.29 | 45.87 ± 0.16 | 6.38 ± 0.13 | 28.92 ± 0.97 | 2.44 ± 0.21 |
| 3 | 9501 UR | 504.08 ± 24.00 | 101.50 ± 6.91 | 335.19 ± 1.59 | 82.66 ± 0.00 | 641.81 ± 16.42 | 490.34 ± 33.62 | 87.67 ± 2.76 | 22.01 ± 0.51 | 75.64 ± 1.75 | 6.82 ± 0.16 |
| 4 | 9503 UR | 479.21 ± 4.47 | 78.90 ± 3.14 | 334.07 ± 1.91 | 63.84 ± 1.84 | 583.21 ± 10.16 | 370.38 ± 6.25 | 80.47 ± 2.84 | 15.22 ± 0.19 | 51.63 ± 1.31 | 4.72 ± 0.19 |
| 5 | 9503 II/7 UR | 425.13 ± 7.26 | 65.43 ± 14.51 | 296.75 ± 37.52 | 63.84 ± 1.20 | 512.45 ± 8.60 | 311.23 ± 10.16 | 78.18 ± 1.50 | 14.00 ± 0.98 | 62.86 ± 1.26 | 4.06 ± 0.21 |
| 6 | SH 6 UR | 329.21 ± 17.86 | 51.76 ± 3.28 | 271.35 ± 14.31 | 41.70 ± 2.00 | 535.12 ± 18.76 | 194.04 ± 10.16 | 65.74 ± 1.58 | 10.31 ± 0.06 | 54.14 ± 4.08 | 3.35 ± 0.11 |
| 7 | SH 7 UR | 362.37 ± 10.61 | 70.95 ± 4.88 | 299.00 ± 8.90 | 81.87 ± 1.60 | 542.86 ± 0.00 | 331.13 ± 7.03 | 76.12 ± 0.79 | 14.60 ± 0.06 | 59.50 ± 1.17 | 4.65 ± 0.16 |
| 8 | US 1 | 360.69 ± 11.86 | 58.12 ± 0.70 | 180.23 ± 26.88 | 31.38 ± 5.99 | 680.44 ± 27.71 | 118.01 ± 6.36 | 76.67 ± 2.05 | 13.53 ± 0.35 | 18.49 ± 0.49 | 1.66 ± 0.11 |
| 9 | SLO NN1 | 205.07 ± 3.63 | 78.45 ± 0.14 | 129.27 ± 0.32 | 61.19 ± 2.40 | 285.80 ± 35.18 | 328.40 ± 18.76 | 36.33 ± 1.50 | 15.02 ± 0.54 | 31.98 ± 1.12 | 4.42 ± 0.19 |
| 10 | WEG 9 PEG | 280.26 ± 0.56 | 73.37 ± 1.19 | 190.42 ± 0.95 | 58.93 ± 0.32 | 407.97 ± 59.42 | 328.37 ± 4.69 | 48.49 ± 1.03 | 13.95 ± 0.09 | 41.32 ± 0.83 | 7.31 ± 1.23 |
| 11 | 4C S9 | 206.64 ± 4.75 | 138.85 ± 2.09 | 162.76 ± 16.53 | 83.95 ± 0.24 | 316.21 ± 7.82 | 587.08 ± 20.32 | 42.52 ± 1.26 | 24.84 ± 0.09 | 31.36 ± 0.53 | 8.05 ± 0.11 |
| 12 | ZAL A1 | 342.83 ± 6.98 | 88.57 ± 7.61 | 221.89 ± 7.95 | 74.69 ± 1.36 | 481.50 ± 89.91 | 393.60 ± 21.89 | 60.94 ± 1.58 | 17.39 ± 0.09 | 43.56 ± 1.07 | 5.81 ± 0.50 |
| 13 | A4 ST A2 | 273.55 ± 12.84 | 66.22 ± 2.79 | 220.32 ± 22.89 | 56.22 ± 0.16 | 356.56 ± 2.35 | 276.96 ± 5.47 | 48.77 ± 0.95 | 13.26 ± 0.51 | 35.73 ± 0.68 | 4.96 ± 0.08 |
| 14 | LuP 3–7 | 277.11 ± 7.82 | 70.26 ± 1.40 | 212.45 ± 13.67 | 57.23 ± 1.28 | 343.30 ± 3.91 | 333.34 ± 11.73 | 47.21 ± 7.58 | 11.70 ± 2.34 | 32.63 ± 0.87 | 3.69 ± 0.10 |
| 15 | LuP 1–12 | 278.29 ± 45.36 | 80.67 ± 13.19 | 167.72 ± 24.84 | 46.25 ± 1.43 | 620.61 ± 24.45 | 221.16 ± 6.96 | 67.86 ± 0.32 | 21.25 ± 0.44 | 16.17 ± 0.28 | 2.48 ± 0.08 |
| 16 | Lu Hg 12 | 344.01 ± 14.79 | 30.59 ± 6.70 | 261.23 ± 6.99 | 24.18 ± 2.00 | 468.78 ± 9.38 | 153.13 ± 0.78 | 64.96 ± 1.58 | 6.72 ± 0.16 | 40.47 ± 2.23 | 2.90 ± 0.11 |
| 17 | LuP 4/20 A | 401.64 ± 58.89 | 113.78 ± 3.07 | 319.46 ± 4.13 | 81.64 ± 0.00 | 669.45 ± 13.29 | 436.17 ± 75.83 | 79.80 ± 1.58 | 20.58 ± 0.57 | 46.44 ± 1.75 | 4.80 ± 0.15 |
| 18 | LuP 4/20 B | 374.80 ± 8.65 | 60.35 ± 4.95 | 292.71 ± 15.26 | 51.24 ± 1.44 | 504.16 ± 12.51 | 267.01 ± 0.78 | 67.13 ± 1.50 | 10.65 ± 1.29 | 47.03 ± 1.51 | 3.89 ± 0.06 |
| Clone | Origin | FRAP [mmol TE/g] | DPPH [mmol TE/g] | ABTS [mmol TE/g] | TPC [mg GAE/g] | TFC [mg QE/g] | |
|---|---|---|---|---|---|---|---|
| High activity | 9503 UR | P. tomentosa × P. fortunei | 1.66 ± 0.02 a | 0.73 ± 0.02 a | 2.84 ± 0.13 a | 349.70 ± 6.31 a | 164.12 ± 0.00 a |
| 9501 UR | P. tomentosa × P. fortunei | 1.78 ± 0.03 b | 0.76 ± 0.02 a | 3.12 ± 0.06 b | 375.74 ± 3.16 b | 233.59 ± 10.80 e | |
| SH 7 UR | P. tomentosa × P. fortunei | 1.56 ± 0.01 c | 0.70 ± 0.00 a | 2.68 ± 0.05 a | 331.10 ± 1.05 c | 166.03 ± 7.01 a | |
| LuP 4/20A | P. tomentosa | 1.31 ± 0.01 d | 0.57 ± 0.00 b | 2.23 ± 0.05 c | 290.17 ± 0.00 d | 118.32 ± 0.00 b c | |
| Low activity | OX 3 | P. elongata × P. fortunei | 1.09 ± 0.01 e | 0.53 ± 0.06 b c | 2.21 ± 0.08 c | 248.51 ± 0.00 e | 147.71 ± 4.86 f |
| COT 1 | P. elongata × P. fortunei | 0.99 ± 0.01 f | 0.45 ± 0.01 c | 2.22 ± 0.05 c | 215.77 ± 4.21 f | 123.66 ± 4.32 b | |
| 4C S9 | P. tomentosa | 1.09 ± 0.02 e | 0.54 ± 0.06 b c | 1.86 ± 0.05 d | 226.93 ± 1.05 g | 110.69 ± 2.16 c | |
| WEG 9 PEG | P. tomentosa | 1.26 ± 0.01 g | 0.53 ± 0.02 b c | 1.94 ± 0.11 d | 267.11 ± 3.15 h | 162.60 ± 6.48 a | |
| Clone | S. aureus | B. cereus | Y. enterocolitica | S. enterica | E. coli |
|---|---|---|---|---|---|
| Diameter of growth inhibition zone with standard deviation (mm) | |||||
| 9503 UR | 17.00 ± 1.00 ab | 9.50 ± 0.71 a | - | - | - |
| 9501 UR | 21.20 ± 1.92 bd | 11.00 ± 1.22 ab | 14.50 ± 1.29 ab | - | - |
| SH 7 UR | 17.67 ± 0.58 b | 11.00 ± 1.00 ab | 11.67 ± 1.52 a | - | - |
| LuP 4/20A | 21.75 ± 1.50 bd | 12.33 ± 2.08 ab | 12.67 ± 0.58 ab | - | - |
| OX 3 | 11.50 ± 3.54 ac | - | - | - | - |
| COT 1 | 10.67 ± 1.53 c | - | - | - | - |
| 4CS9 | 19.33 ± 2.52 b | 11.00 ± 1.73 ab | 16.67 ± 1.53 b | - | - |
| WEG 9 PEG | 16.80 ± 1.48 ab | 14.33 ± 2.08 b | 14.5 ± 2.3 ab | - | - |
| Gentamycin 30 μg | 23.00 ± 1.41 d | 23.50 ± 0.71 c | 22.50 ± 0.71 c | 24.50 ± 0.71 | 22.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżugan, M.; Miłek, M.; Grabek-Lejko, D.; Hęclik, J.; Jacek, B.; Litwińczuk, W. Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones. Agronomy 2021, 11, 2001. https://doi.org/10.3390/agronomy11102001
Dżugan M, Miłek M, Grabek-Lejko D, Hęclik J, Jacek B, Litwińczuk W. Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones. Agronomy. 2021; 11(10):2001. https://doi.org/10.3390/agronomy11102001
Chicago/Turabian StyleDżugan, Małgorzata, Michał Miłek, Dorota Grabek-Lejko, Joanna Hęclik, Beata Jacek, and Wojciech Litwińczuk. 2021. "Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones" Agronomy 11, no. 10: 2001. https://doi.org/10.3390/agronomy11102001
APA StyleDżugan, M., Miłek, M., Grabek-Lejko, D., Hęclik, J., Jacek, B., & Litwińczuk, W. (2021). Antioxidant Activity, Polyphenolic Profiles and Antibacterial Properties of Leaf Extract of Various Paulownia spp. Clones. Agronomy, 11(10), 2001. https://doi.org/10.3390/agronomy11102001