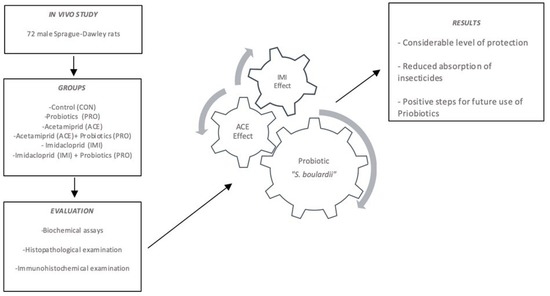
Investigation of the Effects of Probiotics on Sub-Chronic Neonicotinoid Toxicity in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ethics
2.3. Animals
2.4. Biochemical Assays
2.5. Liquid Chromatography–Mass Spectrometry Analysis
2.5.1. Tissue Sample Preparation Procedures
2.5.2. Instrumental Conditions
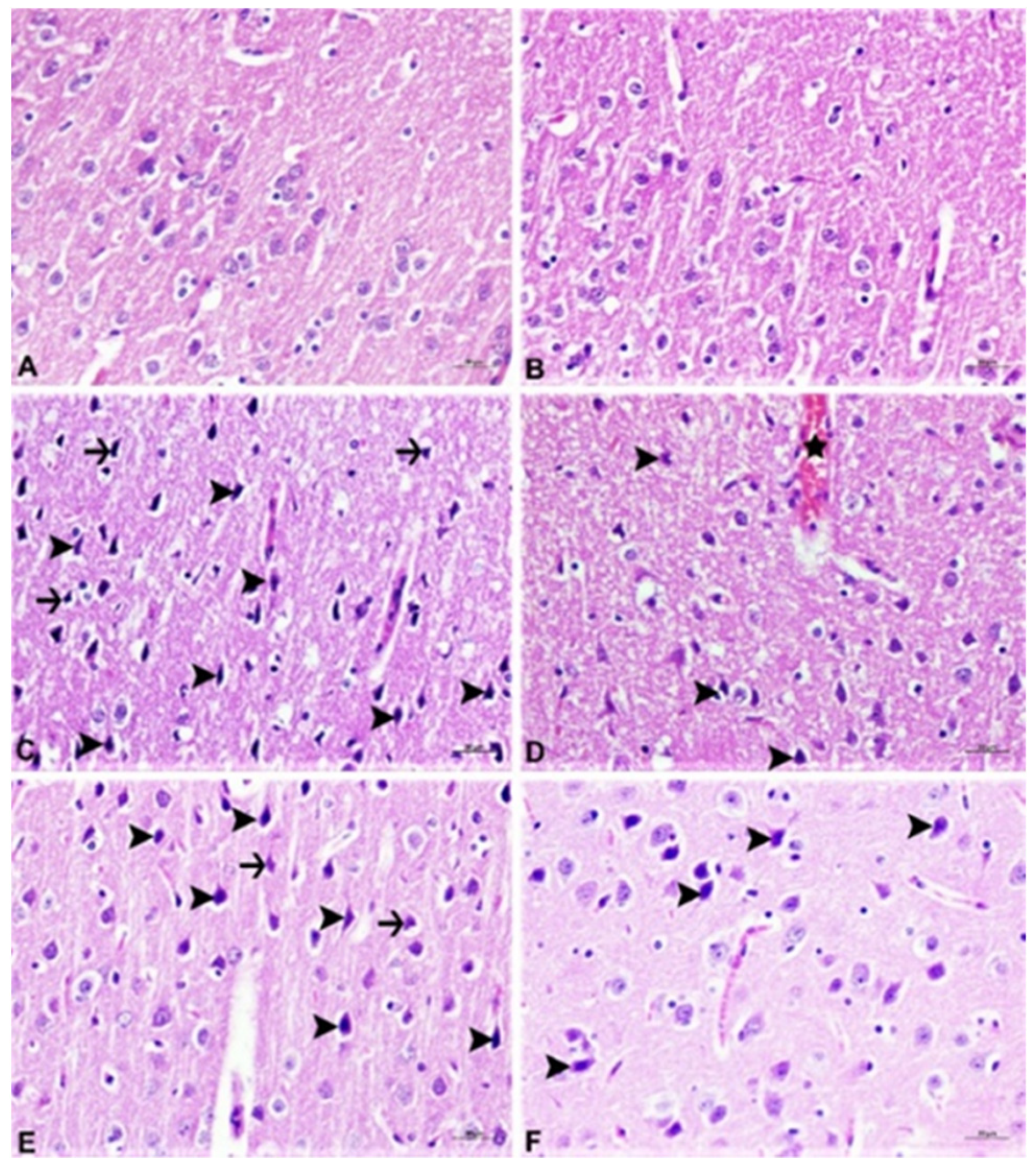
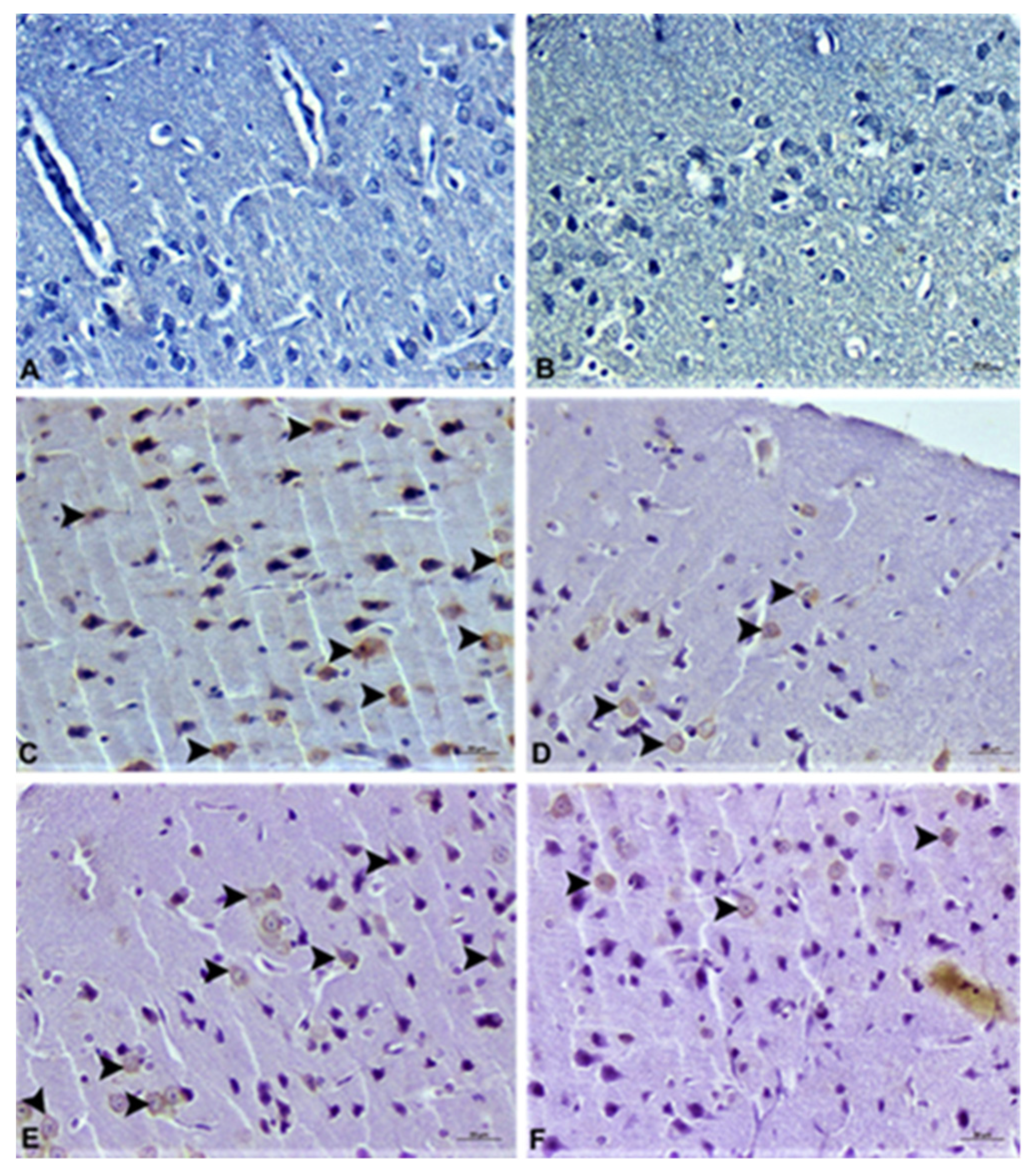
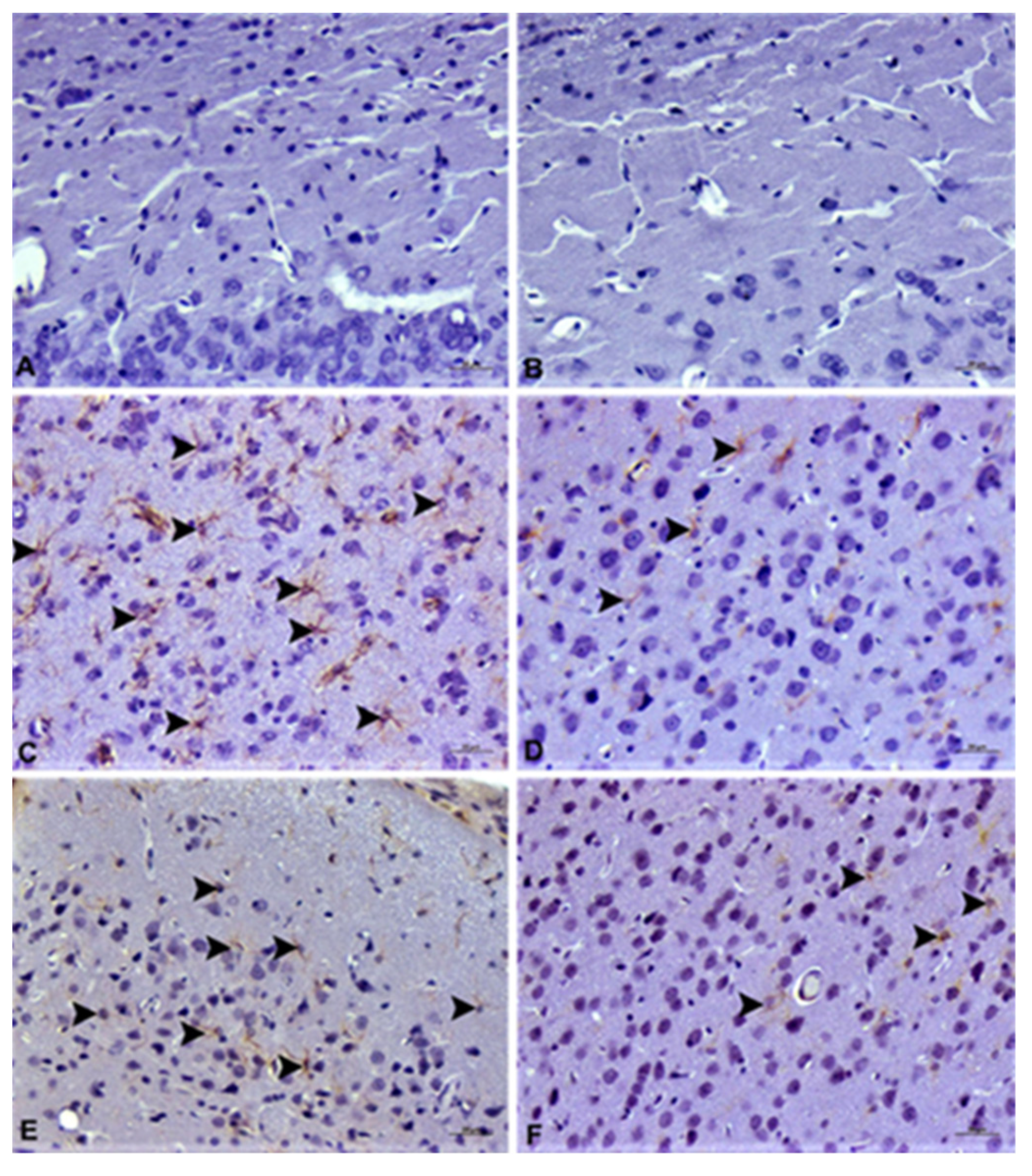
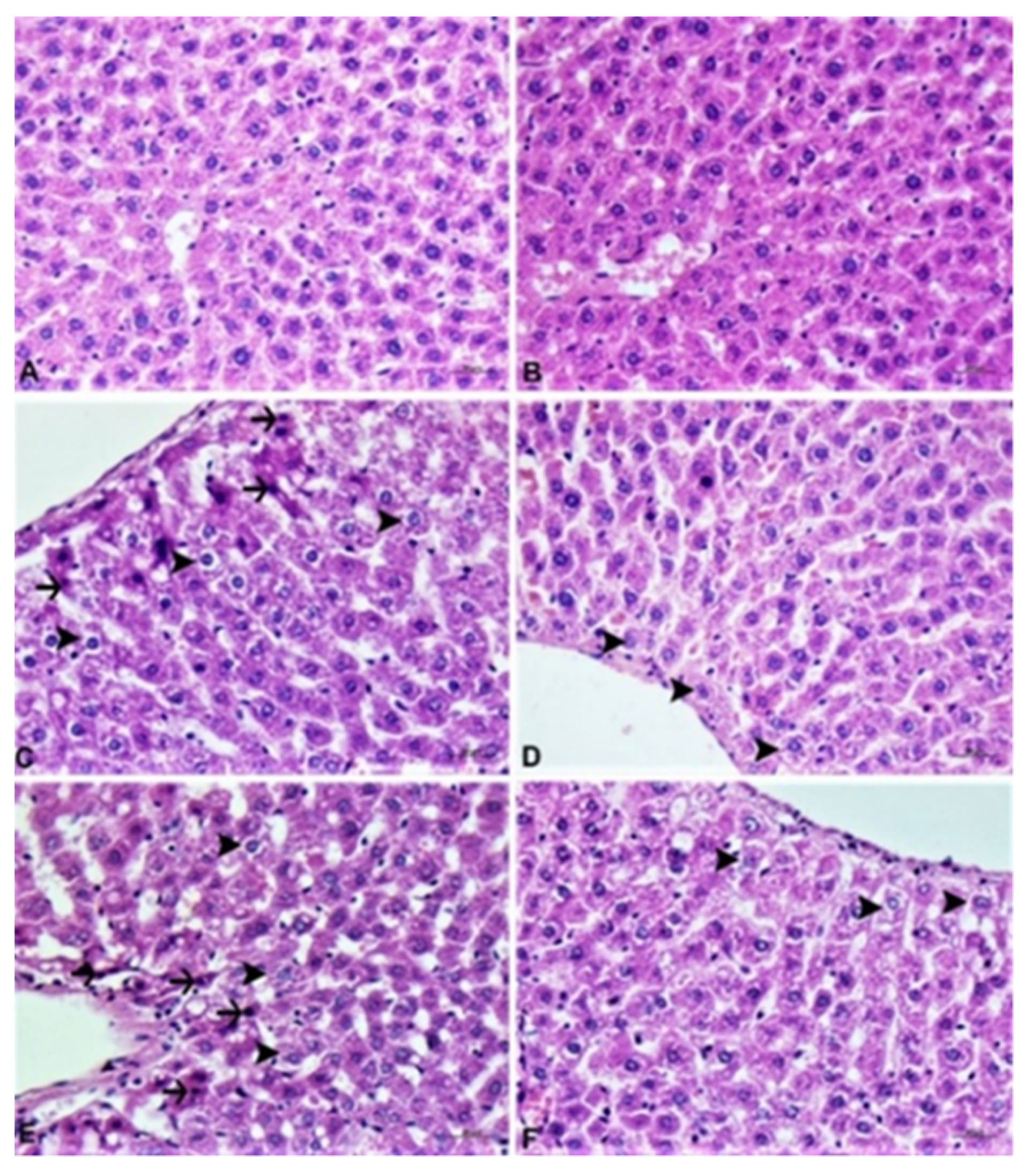
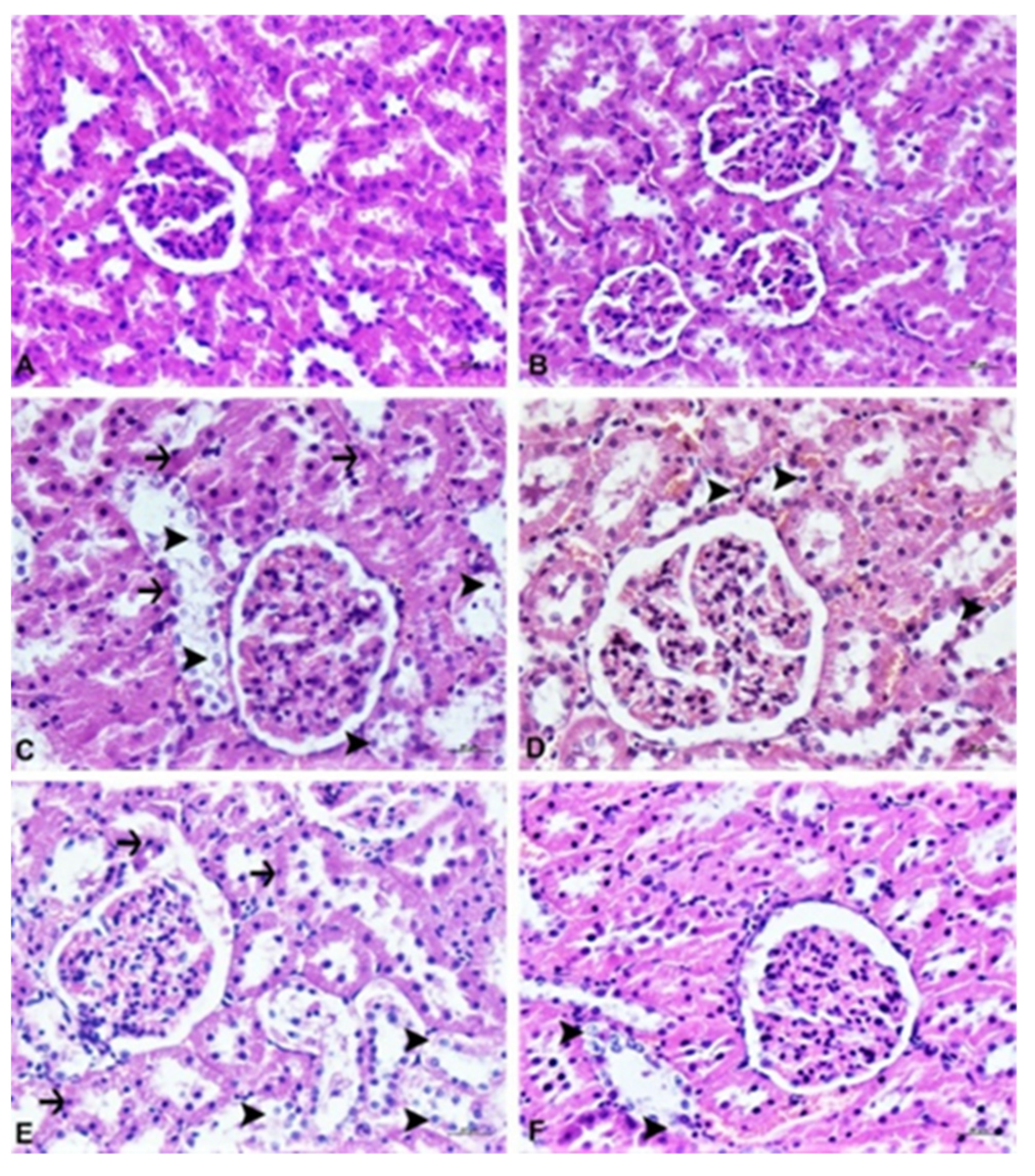
2.6. Pathological Analysis
2.6.1. Histopathological Examination
2.6.2. Immunohistochemical Examination
2.7. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Biochemical Analyses
3.3. Liquid Chromatography–Mass Spectrometry
3.4. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yaqoob, P. Ageing, immunity and influenza: A role for probiotics? Proc. Nutr. Soc. 2014, 73, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini-Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S. Probiotics and prebiotics: Fabulous nutritional supplements. Int. J. Sci. Eng. Res. 2013, 4, 318–333. [Google Scholar]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Gül, Z.; Demircan, C.; Bagdas, D.; Buyukuysal, R.L. Protective Effects of Chlorogenic Acid and its Metabolites on Hydrogen Peroxide-Induced Alterations in Rat Brain Slices: A Comparative Study with Resveratrol. Neurochem. Res. 2016, 41, 2075–2085. [Google Scholar] [CrossRef]
- Trinder, M.; Bisanz, J.; Burton, J.; Reid, G. Probiotic lactobacilli: A potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef. Microbes 2015, 6, 841–847. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Crossthwaite, A.J.; Rendine, S.; Stenta, M.; Slater, R. Target-site resistance to neonicotinoids. J. Chem. Biol. 2014, 7, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, E.B.; Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 2015, 49, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Bal, R.; Türk, G.; Tuzcu, M.; Yilmaz, O.; Kuloglu, T.; Gundogdu, R.; Gür, S.; Agca, A.; Ulas, M.; Çambay, Z.; et al. Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. J. Environ. Sci. Health Part B 2012, 47, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Yardimci, M.; Sevgiler, Y.; Rencuzogullari, E.; Arslan, M.; Buyukleyla, M.; Yilmaz, M. Sex-, tissue-, and exposure duration-dependent effects of imidacloprid modulated by piperonyl butoxide and menadione in rats. Part I: Oxidative and neurotoxic potentials. Arch. Ind. Hyg. Toxicol. 2014, 65, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Chakroun, S.; Ezzi, L.; Grissa, I.; Kerkeni, E.; Neffati, F.; Bhouri, R.; Sallem, A.; Najjar, M.F.; Hassine, M.; Mehdi, M.; et al. Hematological, biochemical, and toxicopathic effects of subchronic acetamiprid toxicity in Wistar rats. Environ. Sci. Pollut. Res. 2016, 23, 25191–25199. [Google Scholar] [CrossRef]
- Devan, R.K.S.; Prabu, P.C.; Panchapakesan, S. Immunotoxicity assessment of sub-chronic oral administration of acetamiprid in Wistar rats. Drug Chem. Toxicol. 2014, 38, 328–336. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Jain, S.; Singh, A.; Punia, J.; Gupta, R.; Chandratre, G.A. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ. Toxicol. Pharmacol. 2013, 35, 408–418. [Google Scholar] [CrossRef]
- Costas-Ferreira, C.; Faro, L. Neurotoxic Effects of Neonicotinoids on Mammals: What Is There beyond the Activation of Nicotinic Acetylcholine Receptors?—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8413. [Google Scholar] [CrossRef]
- Kagawa, N.; Nagao, T. Neurodevelopmental toxicity in the mouse neocortex following prenatal exposure to acetamiprid. J. Appl. Toxicol. 2018, 38, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Duzguner, V.; Erdogan, S. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver & central nervous system of rats. Pestic. Biochem. Physiol. 2012, 104, 58–64. [Google Scholar] [CrossRef]
- EFSA Scientific Committee Guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed. EFSA J. 2011, 9, 2438. [CrossRef]
- Imidacloprid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Imidacloprid (accessed on 16 May 2021).
- Acetamiprid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetamiprid (accessed on 16 May 2021).
- National Research Council (US). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK54050/ (accessed on 16 May 2021). [CrossRef]
- Vardavas, A.I.; Ozcagli, E.; Fragkiadaki, P.; Stivaktakis, P.D.; Tzatzarakis, M.N.; Alegakis, A.K.; Vasilaki, F.; Kaloudis, K.; Tsiaoussis, J.; Kouretas, D.; et al. The metabolism of imidacloprid by aldehyde oxidase contributes to its clastogenic effect in New Zealand rabbits. Mutat. Res. Toxicol. Environ. Mutagen. 2018, 829–830, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kavvalakis, M.P.; Tzatzarakis, M.N.; Theodoropoulou, E.P.; Barbounis, E.G.; Tsakalof, A.K.; Tsatsakis, A.M. Development and application of LC–APCI–MS method for biomonitoring of animal and human exposure to imidacloprid. Chemosphere 2013, 93, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Varmazyari, A.; Taghizadehghalehjoughi, A.; Sevim, C.; Baris, O.; Eser, G.; Yildirim, S.; Hacimuftuoglu, A.; Buha, A.; Wallace, D.; Tsatsakis, A.; et al. Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies. Toxicol. Rep. 2020, 7, 637–648. [Google Scholar] [CrossRef]
- Sevim, Ç.; Çomaklı, S.; Taghizadehghalehjoughi, A.; Özkaraca, M.; Mesnage, R.; Kovatsi, L.; Burykina, T.I.; Kalogeraki, A.; Antoniou, M.N.; Tsatsakis, A. An imazamox-based herbicide causes apoptotic changes in rat liver and pancreas. Toxicol. Rep. 2018, 6, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.G.; Soujanya, S.; Lakshman, M.; Kumar, A.A. Evaluation of the protective role of vitamin C in imidacloprid-induced hepatotoxicity in male Albino rats. J. Nat. Sci. Biol. Med. 2013, 4, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammon, A.M.; Biar, R.S.; Banga, H.S.; Sodhi, S. Patho-biochemical studies on hepatotoxicity and nephrotoxicity on exposure to chlorpyrifos and imidacloprid in layer chickens. Vet. arhiv 2010, 80, 663–672. [Google Scholar]
- El-Deeb, A.E.A.; Abd El-Aleem, I.M.; Sherin, S.G. Harmful effect of some insecticides on vital parameters of albino rats. J. Egypt. Soc. Toxicol. 2007, 36, 53–60. [Google Scholar]
- Emam, H.; Ahmed, E.; Abdel-Daim, M. Antioxidant capacity of omega-3-fatty acids and vitamin E against imidacloprid-induced hepatotoxicity in Japanese quails. Environ. Sci. Pollut. Res. 2018, 25, 11694–11702. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Kouretas, D.; Tzatzarakis, M.; Stivaktakis, P.; Tsarouhas, K.; Golokhvast, K.; Rakitskii, V.; Tutelyan, V.; Hernandez, A.; Rezaee, R.; et al. Simulating real-life exposures to uncover possible risks to human health: A proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2016, 36, 554–564. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid Induces Neurobehavioral Deficits and Increases Expression of Glial Fibrillary Acidic Protein in the Motor Cortex and Hippocampus in Offspring Rats Following in Utero Exposure. J. Toxicol. Environ. Health Part A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Nishito, Y.; Yanagisawa, H.; Kuroda, Y.; Komuta, Y.; Kawano, H.; Hayashi, M. Neonicotinoid Insecticides Alter the Gene Expression Profile of Neuron-Enriched Cultures from Neonatal Rat Cerebellum. Int. J. Environ. Res. Public Health 2016, 13, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, W.; Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Oxidative Stress and DNA Damage Induced by Imidacloprid in Zebrafish (Danio rerio). J. Agric. Food Chem. 2015, 63, 1856–1862. [Google Scholar] [CrossRef]
- Topal, A.; Alak, G.; Ozkaraca, M.; Yeltekin, A.C.; Comaklı, S.; Acıl, G.; Kokturk, M.; Atamanalp, M. Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 2017, 175, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Anadón, A.; Wu, Q.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 471–507. [Google Scholar] [CrossRef]
- de Souza-Pinto, N.C.; Eide, L.; Hogue, B.A.; Thybo, T.; Stevnsner, T.; Seeberg, E.; Klungland, A.; Bohr, V.A. Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res. 2001, 61, 5378–5381. [Google Scholar] [PubMed]
- Thompson, H.J.; Heimendinger, J.; Haegele, A.; Sedlacek, S.M.; Gillette, C.; O’Neill, C.; Wolfe, P.; Conry, C. Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis 1999, 20, 2261–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Pan, Z.; Jin, C.; Ni, Y.; Fu, Z.; Jin, Y. Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere 2019, 227, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrede, J.-P.; Del Rio, J.-C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.L.; Kolf-Clauw, M.; Oswald, I. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, J.; Padfield, P.J.; Burt, J.P.H.; O’Neill, C.A. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am. J. Physiol. Physiol. 2004, 287, C1412–C1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurulain, S.M.; Adeghate, E.; Sheikh, A.; Yasin, J.; Kamal, M.; Sharma, C.; Adem, A.; Ojha, S. Sub-Chronic Exposure of Non-Observable Adverse Effect Dose of Terbufos Sulfone: Neuroinflammation in Diabetic and Non-Diabetic Rats. CNS Neurol. Disord.-Drug Targets 2014, 13, 1397–1405. [Google Scholar] [CrossRef]
- Taghavian, F.; Vaezi, G.; Abdollahi, M.; Malekirad, A.A. Comparative Toxicological Study between Exposed and Non-Exposed Farmers to Organophosphorus Pesticides. Cell J. 2016, 18, 89–96. [Google Scholar] [PubMed]


| Weeks | Control | IMI | ACE | PRO | IMI + PRO | ACE + PRO |
|---|---|---|---|---|---|---|
| 1 | 190.63 ± 63.93 | 192.61 ± 4.67 | 198.45 ± 28.34 | 195.25 ± 32.11 | 195.83 ± 3.63 | 191.18 ± 6.66 |
| 2 | 250 ± 65.48 | 254.53 ± 5.91 | 247.72 ± 35.56 | 255.91 ± 37.73 | 254.83 ± 10.62 | 250.18 ± 7.64 |
| 3 | 283.45 ± 64.24 | 290.69 ± 7.19 | 280.54 ± 36.80 | 282.50 ± 32.52 | 282.16 ± 20.94 | 287.72 ± 9.15 |
| 4 | 313.81 ± 67.97 | 319.76± 8.85 | 302 ± 48.53 | 318.08 ± 38.18 | 302.75 ± 24.33 | 307.27 ± 11.67 |
| 5 | 330.72 ± 64.44 | 340.07± 10.04 | 332 ± 36.62 | 339.91 ± 36.67 | 319.41 ± 25.64 | 328.90 ± 12.13 |
| 6 | 360.45 ± 69.04 | 362.84 ± 10.69 | 357.36 ± 33.67 | 366.08 ± 39.76 | 344.33 ± 30.07 | 351.72 ± 12.43 |
| 7 | 378.27 ± 69.66 | 376.07 ± 11.65 | 357.36 ± 33.67 | 380.66 ± 40.50 | 359.83 ± 34.10 | 370.18 ± 11.47 |
| 8 | 379.36 ± 64.81 | 395.69 ± 11.84 | 396.63 ± 38.15 | 391.75 ± 32.65 | 379.33 ± 37.88 | 392.63 ± 11.22 |
| 9 | 400.81 ± 67.56 | 417.30 ± 11.96 | 416.18 ± 41.80 | 422.83 ± 41.25 | 397.66 ± 40.34 | 405.09 ± 16.37 |
| 10 | 402 ± 64.84 | 420.84 ± 12.24 | 421.36 ± 41.75 | 428.33 ± 41.57 | 398.83 ± 40.56 | 416.45 ± 12.70 |
| 11 | 415.63 ± 58.11 | 428.84 ± 12.46 | 422.09 ± 42.70 | 429.58 ± 37.04 | 417.58 ± 46.04 | 429.09 ± 11.67 |
| 12 | 400.81 ± 67.56 | 417.38 ± 11.94 | 416.18 ± 41.80 | 422.83 ± 41.25 | 397.66 ± 40.34 | 414.18 ± 12.12 |
| 13 | 431.18 ± 59.56 | 450.76 ± 12.88 | 449.45 ± 45.31 | 453.75 ± 53.46 | 439.25 ± 48.19 | 448.72 ± 11.88 |
| Control | IMI | ACE | PRO | IMI + PRO | ACE + PRO | |
|---|---|---|---|---|---|---|
| ALT (IU/L) | 62.6 ± 12.87 | 64.5 ± 8.93 | 70.1 ± 11.68 | 76± 10.95 | 71.1± 8.93 | 68.3± 10.59 |
| AST (IU/L) | 189.1 ± 36.17 | 196.5 ± 23.27 | 194.3 ± 14.67 | 250 ± 61.84 | 205.6 ± 50.01 | 192.8 ± 2.22 |
| ALP (IU/L) | 112.8 ± 20.49 | 186.2 ± 19.09 | 172.2 ± 20.39 | 179.1 ± 20.40 | 185.4 ± 22.33 | 166.8 ± 14.02 |
| LDH (IU/L) | 1758.8 ± 227.41 | 2423.5 ± 216.54 | 2350.7 ± 191.46 | 2577.8 ± 171.15 | 2031.4 ± 276.98 | 1432.4 ± 127.06 |
| UREA(mg/dL) | 40.7 ± 2.50 | 42.7 ± 2.54 | 38.6 ± 2.45 | 39.1 ± 2.21 | 40.6 ± 1.44 | 40.7 ± 4.40 |
| Control | IMI | ACE | PRO | IMI + PRO | ACE + PRO | |
|---|---|---|---|---|---|---|
| WBC (103/µL) | 6.28 ± 1.24 | 7.43 ± 2.88 | 12.05 ± 7.51 | 5.91 ± 4.62 | 6.23 ± 1.07 | 13.82 ± 9.90 |
| Nötrofil | 1.79 ± 0.49 | 1.24 ± 0.54 | 1.73 ± 1.20 | 1.12 ± 0.69 | 1.07 ± 0.05 | 1.51 ± 0.39 |
| Lenfosit | 4.24 ± 0.92 | 5.92 ± 2.36 | 9.73 ± 5.96 | 4.43 ± 3.61 | 4.74 ± 1.00 | 11.87 ± 9.46 |
| Monosit | 0.09 ± 0.06 | 0.11 ± 0.04 | 0.07 ± 0.00 | 0.09 ± 0.09 | 0.08 ± 0.05 | 0.14 ± 0.07 |
| Eozinofil | 0.13 ± 0.09 | 0.15 ± 0.06 | 0.50 ± 0.35 | 0.25 ± 0.34 | 0.32 ± 0.18 | 0.27 ± 0.08 |
| Bazofil | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| RBC (106/µL) | 8.18 ± 0.48 | 8.92 ± 1.03 | 9.23 ± 0.34 | 9.05 ± 0.30 | 9.21 ± 0.52 | 8.71 ± 0.62 |
| HGB (gm/dl) | 14.63 ± 1.04 | 15.47 ± 1.71 | 16.30 ± 0.42 | 16.17 ± 0.47 | 16.50 ± 0.98 | 15.45 ± 0.99 |
| HCT (%) | 47.68 ± 3.22 | 50.67 ± 5.62 | 51.45 ± 0.63 | 51.77 ± 2.19 | 52.52 ± 3.16 | 49.40 ± 2.51 |
| PLT (103/µL) | 732.50 ± 93.22 | 543.37 ± 321.53 | 740.50 ± 17.67 | 866.75 ± 49.77 | 702.50 ± 140.14 | 761 ± 83.76 |
| MCV (fL) | 58.21 ± 0.95 | 56.83 ± 0.95 | 55.70 ± 1.41 | 57.30 ± 4.36 | 56.97 ± 0.33 | 56.85 ± 2.59 |
| MCH (pg) | 17.83 ± 0.28 | 17.36 ± 0.42 | 17.65 ± 0.21 | 17.87 ± 0.47 | 17.90 ± 0.37 | 17.72 ± 0.36 |
| MCHC (g/dL) | 30.66 ± 0.33 | 30.53 ± 0.46 | 31.70 ± 0.42 | 31.32 ± 2.03 | 31.42 ± 0.58 | 31.25 ± 0.96 |
| IG (g/L) | 0.17 ± 0.40 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.03 ± 0.02 |
| Control | IMI | ACE | PRO | IMI + PRO | ACE + PRO | ||
|---|---|---|---|---|---|---|---|
| Imidacloprid (µg/g) | Mean ± SD | - | 0.06 ± 0.01 | - | - | 0.20 ± 0.02 | - |
| Minimum | - | 0.02 | - | - | 0.15 | - | |
| Maximum | - | 0.11 | - | - | 0.25 | - | |
| p = 0.315 | |||||||
| Acetamiprid (µg/g) | Mean ± SD | - | - | 0.51 ± 0.06 | - | - | 0.20 ± 0.02 |
| Minimum | - | - | 0.37 | - | - | 0.15 | |
| Maximum | - | - | 0.66 | - | - | 0.25 | |
| p = 0.003 | |||||||
| Control | IMI | ACE | PRO | IMI + PRO | ACE + PRO | ||
|---|---|---|---|---|---|---|---|
| Imidacloprid (µg/g) | Mean ± SD | - | 0.37 ± 0.30 | - | - | 0.16 ± 0.03 | - |
| Minimum | - | 0.04 | - | - | 0.06 | - | |
| Maximum | - | 1.28 | - | - | 0.22 | - | |
| p = 0.506 | |||||||
| Acetamiprid (µg/g) | Mean ± SD | - | - | 0.88 ± 0.07 | - | - | 0.18 ± 0.05 |
| Minimum | - | - | 0.72 | - | - | 0.06 | |
| Maximum | - | - | 1.08 | - | - | 0.31 | |
| p < 0.001 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevim, C.; Akpinar, E.; Tsatsakis, A.; Yildirim, S.; Tzatzarakis, M.; Vardavas, A.I.; Vardavas, C.I.; Kara, M.; Gul, Z. Investigation of the Effects of Probiotics on Sub-Chronic Neonicotinoid Toxicity in Rats. Agronomy 2021, 11, 2003. https://doi.org/10.3390/agronomy11102003
Sevim C, Akpinar E, Tsatsakis A, Yildirim S, Tzatzarakis M, Vardavas AI, Vardavas CI, Kara M, Gul Z. Investigation of the Effects of Probiotics on Sub-Chronic Neonicotinoid Toxicity in Rats. Agronomy. 2021; 11(10):2003. https://doi.org/10.3390/agronomy11102003
Chicago/Turabian StyleSevim, Cigdem, Erol Akpinar, Aristides Tsatsakis, Serkan Yildirim, Manolis Tzatzarakis, Alexander I. Vardavas, Constantine I. Vardavas, Mehtap Kara, and Zulfiye Gul. 2021. "Investigation of the Effects of Probiotics on Sub-Chronic Neonicotinoid Toxicity in Rats" Agronomy 11, no. 10: 2003. https://doi.org/10.3390/agronomy11102003
APA StyleSevim, C., Akpinar, E., Tsatsakis, A., Yildirim, S., Tzatzarakis, M., Vardavas, A. I., Vardavas, C. I., Kara, M., & Gul, Z. (2021). Investigation of the Effects of Probiotics on Sub-Chronic Neonicotinoid Toxicity in Rats. Agronomy, 11(10), 2003. https://doi.org/10.3390/agronomy11102003