Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Place and Time
2.2. Experimental Design
2.3. Preparation of Plant Extract and Seed Source of A. spinosus
2.4. Fractionation
2.5. Pot Culture
2.6. GC-MS Analysis
2.7. Data Analysis
3. Results and Discussion
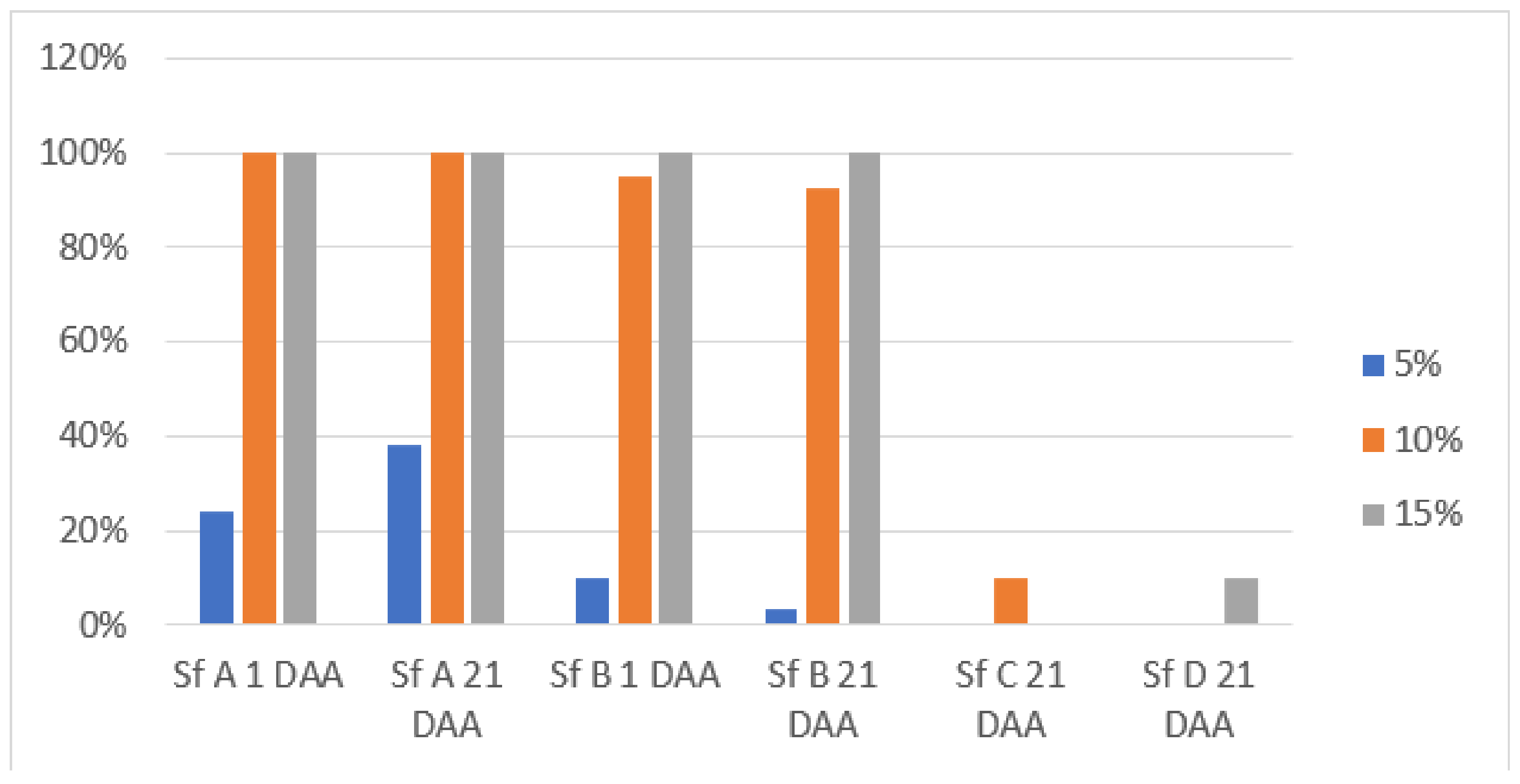
3.1. Weed Control
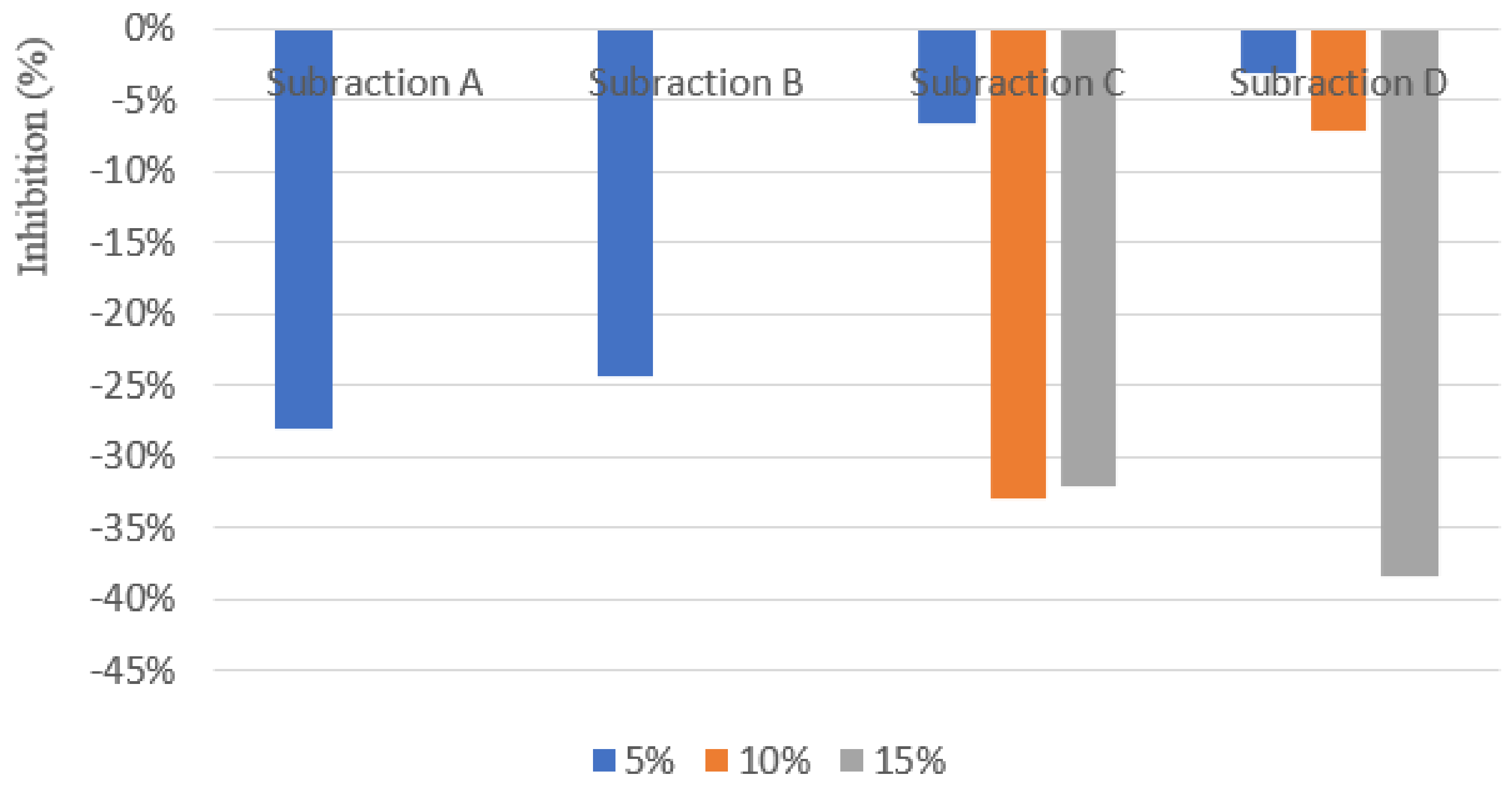
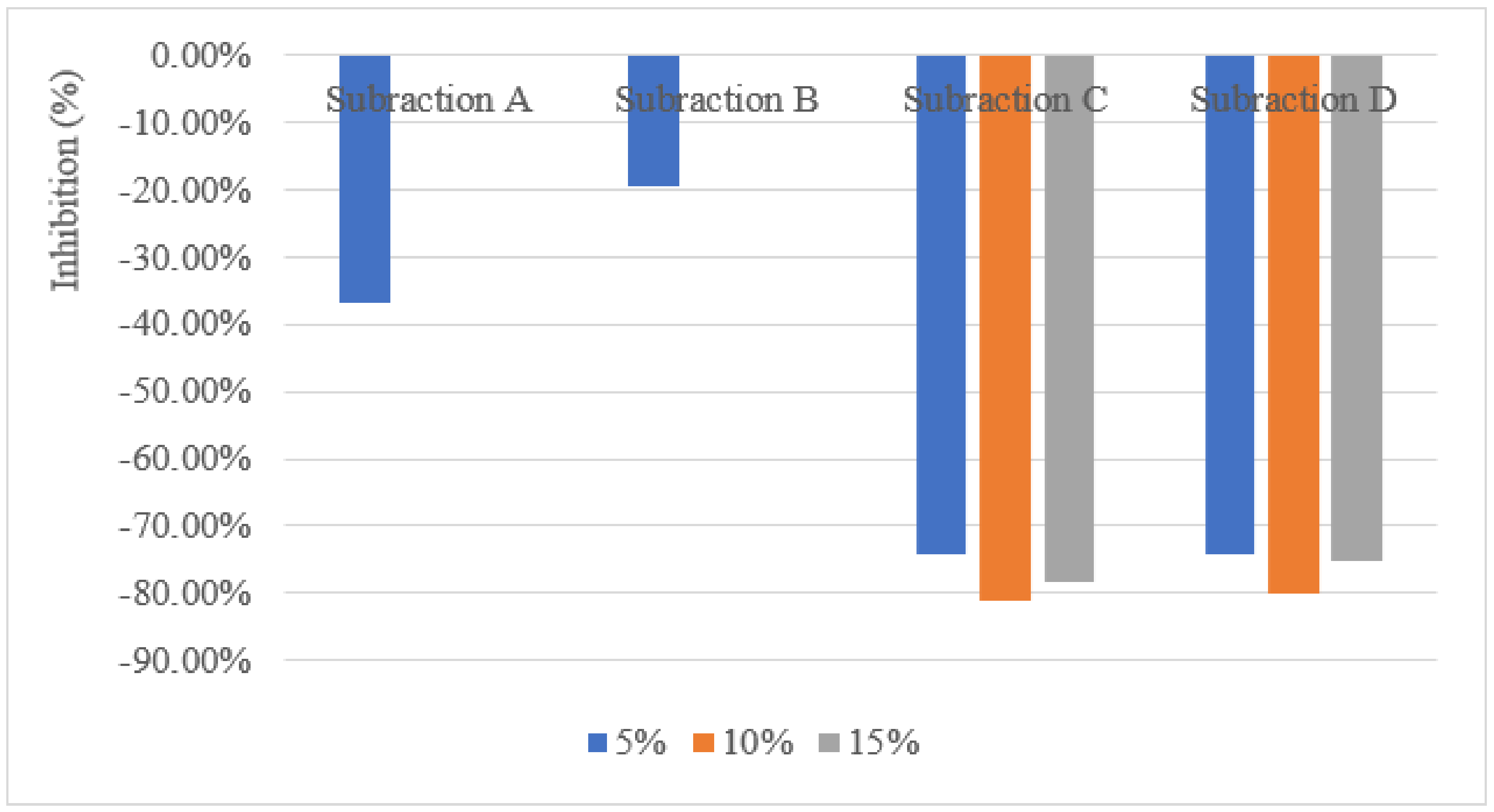
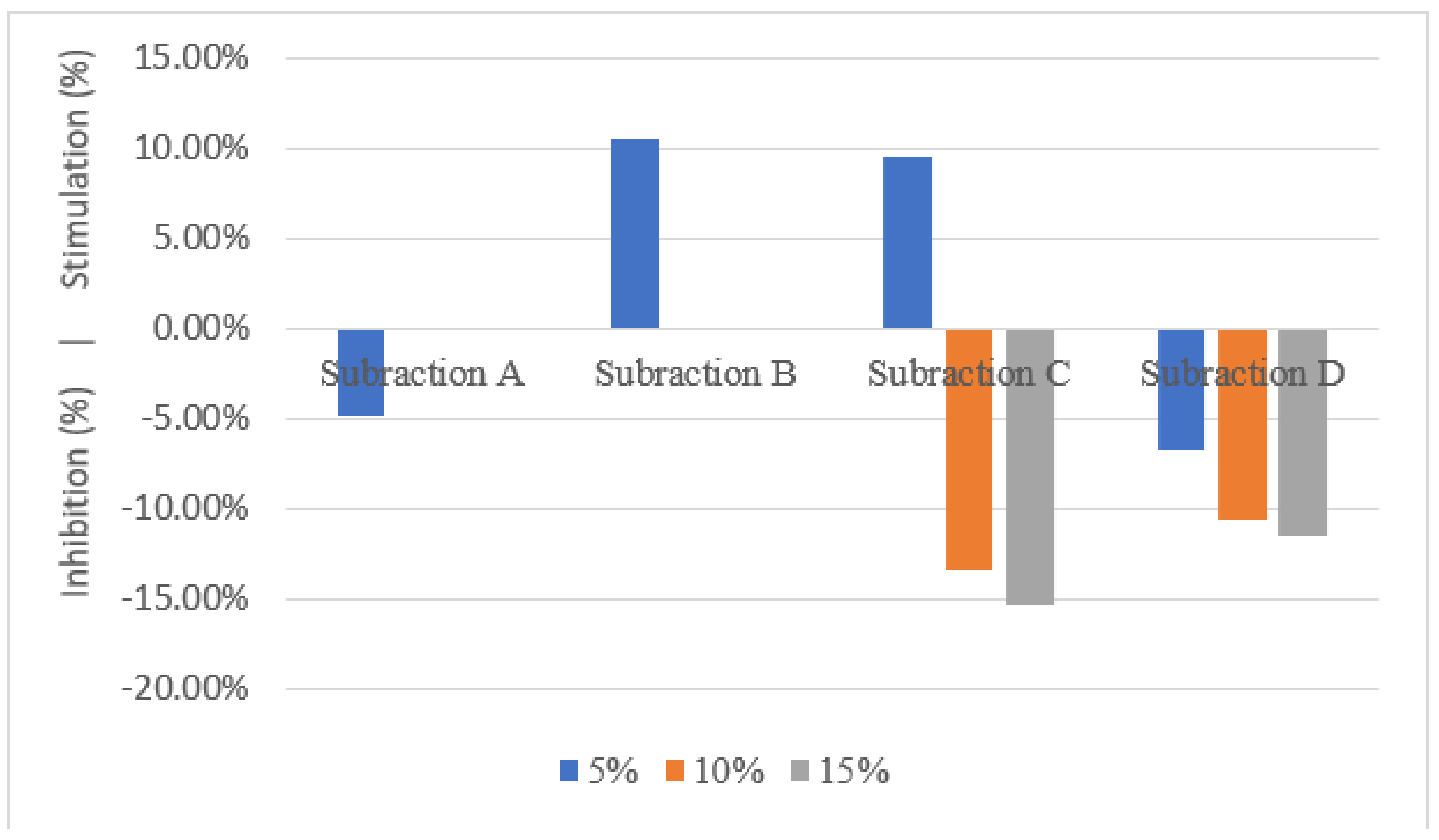
3.2. Shoot Dry Weight, Root Dry Weight and Root Length
3.3. GC-MS Analyses of the Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Inderjit; Wardle, D.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- De-Martino, L.; Mancini, E.; de Almeida, R.; De Feo, V. The anti-germinative activity of twenty seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [Green Version]
- Baeshen, A.A. Morphological and elements constituent effects of allelopathic Activity of some medicinal plants extracts on Zea mays. Int. J. Curr. Res. Acad. Rev. 2014, 2, 135–143. [Google Scholar]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Cahyanti, L.D.; Sumarni, T.; Widaryanto, E. Potential allelopathy of pine leaf (Pinus spp.) as bioherbicide on pigweed (Portulaca oleracea). IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 48–53. [Google Scholar] [CrossRef]
- Casimiro, G.S.; Mansur, E.; Pacheco, G.; Garcia, R.; Leal, I.C.R.; Simas, N.K. Allelopathic Activity of Extracts from Different Brazilian Peanut (Arachis hypogaea L.) Cultivars on Lettuce (Lactuca sativa) and Weed Plants. Sci. World J. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; p. 424. [Google Scholar]
- Erida, G.; Saidi, N.; Hasanuddin; Syafruddin. Allelopathic Screening of Several Weed Species as Potential Bioherbicides. IOP Conf. Ser. Earth Environ. Sci. 2019, 334, 012034. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin; Syafruddin. Herbicidal Potential of Methanolic Extracts of Pinus merkusii Jungh. et de Vriese, Acacia Mangium Willd., Jatropha Curcas L., Tectona Grandis L.f. and Terminalia Catappa L. on Amaranthus Spinosus L. Allelopath. J. 2020, 49, 201–216. [Google Scholar]
- Kong, C.; Hu, F.; Xu, X.; Liang, W.; Zhang, C. Allelopathic plants. Ageratum conyzoides L. Allelopath. J. 2004, 14, 1–12. [Google Scholar]
- Kohli, R.K.; Batish, D.R.; Singh, H.P.; Dogra, K.S. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions 2006, 8, 1501–1510. [Google Scholar] [CrossRef]
- Grainge, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties; John Wiley & Sons Limited: Chichester, UK, 1988; 470p. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kohli, R. Allelopathic Interactions and Allelochemicals: New Possibilities for Sustainable Weed Management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Nature of interference potential of leaf debris of Ageratum conyzoides. Plant Growth Regul. 2008, 57, 137–144. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, T.; Lu, Y. Allelopathic Potential and Chemical Constituents of Volatile Oil from Ageratum conyzoides. J. Chem. Ecol. 1999, 25, 2347–2356. [Google Scholar] [CrossRef]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Dayan, F.; Duke, S. Clues in the Search for New Herbicides. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M., Pedrol, N., Gonzalez, L., Eds.; Springer: Amsterdam, the Netherlands, 2006; pp. 63–83. [Google Scholar]
- Tigre, R.; Silva, N.; Santos, M.; Honda, N.; Falcão, E.; Pereira, E. Allelopathic and bioherbicidal potential of Cladonia verticillaris on the germination and growth of Lactuca sativa. Ecotoxicol. Environ. Saf. 2012, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Adler, M.J.; Chase, C.A. Comparison of the Allelopathic Potential of Leguminous Summer Cover Crops: Cowpea, Sunn Hemp, and Velvetbean. HortScience 2007, 42, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Nice, G.; Johnson, B.; Jordan, T. Weed management in pastures: Spiny pigweed. Purdue Ext. 2011, 4, 1–4. [Google Scholar]
- Fransfried, R.E.; Talbert, R.E. Design of Field Experiments and the Measurement and Analysis of Plant Responses. In Research Methods in Weed Science; Truelove, B., Ed.; Southern Weed Science Society: AL, USA, 1977; p. 19. [Google Scholar]
- Hasan, M.; Ahmad-Hamdani, M.; Rosli, A.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kaab, S.; Rebey, I.; Hanafi, M.; Hammi, K.; Smaoui, A.; Fauconnier, M.; De Clerck, C.; Jijakli, M.; Ksouri, R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot. 2019, 128, 67–76. [Google Scholar] [CrossRef]
- Tesio, F.; Vidotto, F.; Ferrero, A. Allelopathic persistence of Helianthus tuberosus L. residues in the soil. Sci. Hortic. 2012, 135, 98–105. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop. Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Sikolia, S.F.; Ayuma, E. Allelopathic effects of Eucalyptus saligna on germination growth and development of Vigna unguiculata L. Walp. IOSR J. Environ. Sci. Toxicol. Food Technol. 2018, 12, 15–24. [Google Scholar]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Alam Cheema, S.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth Regul. 2018, 38, 343–356. [Google Scholar] [CrossRef]
- Dmitrović, S.; Perišić, M.; Stojić, A.; Živković, S.; Boljević, J.; Nestorović Živković, J. Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Chuang, P.-H.; Lee, C.-W.; Chou, J.-Y.; Murugan, M.; Shieh, B.-J.; Chen, H.-M. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef]
- Sotero, V.; Suarez, P.; Vela, J.E.; Sotero, D.G.; Fujii, Y. Allelochemicals of three Amazon plants identified by GC-MS. Int. J. Eng. Appl. Sci. (IJEAS) 2016, 3, 1–7. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kohli, R.; Saxena, D.B.; Arora, V. Effect of Parthenin—A Sesquiterpene Lactone from Parthenium hysterophorus—On Early Growth and Physiology of Ageratum conyzoides. J. Chem. Ecol. 2002, 28, 2169–2179. [Google Scholar] [CrossRef] [PubMed]

| Effects | Rating | Effects Description |
|---|---|---|
| No effect | 0 | No weed control No crop reduction or injury |
| Slight | 10 | Very poor weed control Slight crop discolouration or stunting Poor weed control |
| 20 | Some crop discolouration. Stunting. or stand loss Poor to deficient weed control | |
| 30 | Crop injury more pronounced. but not lasting | |
| Moderate | 40 | Deficient weed control Moderate injury. crop usually recovers |
| 50 | Deficient to moderate weed control Crop injury more lasting. recovery doubtful | |
| 60 | Moderate weed control Lasting crop injury no recovery | |
| Severe | 70 | Weed control somewhat less than satisfactory Heavy crop injury and stand loss |
| 80 | Satisfactory to good weed control Crop nearly destroyed. A few surviving plants | |
| 90 | Very good to excellent weed control Only occasional live crop plants left | |
| Complete effect | 100 | Complete weed destruction Complete crop destruction |
| NO | Retention Time | Compound Name | Compound Content (%) | Similarity Quality (%) | |||
|---|---|---|---|---|---|---|---|
| Subfraction A | Subfraction B | Subfraction C | Subfraction D | ||||
| 1 | 6.12 | 1,8-cineole | 15.75 | 95 | |||
| 2 | 24.02 | precocene II | 9.39 | 92 | |||
| 3 | 26.06 | allobarbital | 8.53 | 65 | |||
| 4 | 27.18 | 1-pentadecene | 3.83 | 95 | |||
| 5 | 28.12 | octadecanal | 30.52 | 89 | |||
| 6 | 28.23 | octadecanal | 12.69 | 89 | |||
| 7 | 28.29 | 1-octadecyne | 38.57 | 89 | |||
| 8 | 28.36 | octadecanal | 8.23 | 90 | |||
| 9 | 28.98 | phytol | 8.26 | 89 | |||
| 10 | 29.08 | phytol | 11.24 | 90 | |||
| 11 | 31.30 | 1-hexadecine | 4.08 | 96 | |||
| 12 | 33.25 | 9,12,15-octadecatrienoic, methyl ester | 7.32 | 94 | |||
| 13 | 35.00 | tetradecanoic acid, ethyl ester | 10.26 | 89 | |||
| 14 | 35.16 | 10-heneicosene (c,t) | 5.19 | 93 | |||
| 15 | 35.53 | 2-pentadecanone | 3.68 | 84 | |||
| 16 | 36.79 | trans-dodec-5enal | 12.28 | 86 | |||
| 17 | 45.08 | 2,6,10,15,19,23-hexamethyl- (squalene) | 3.63 | 95 | |||
| 18 | 53.36 | bannamurpanin | 24.06 | 64 | |||
| 19 | 54.17 | bannamurpanin | 26.01 | 54 | |||
| 20 | 55.29 | neophytadiene | 5.09 | 91 | |||
| 21 | 55.42 | di-tert-butylphosphine-d | (5.17%) | 75 | |||
| Total area | 52.79 | 62.89 | 47.23 | 90.87 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erida, G.; Saidi, N.; Hasanuddin, H.; Syafruddin, S. Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth. Agronomy 2021, 11, 1991. https://doi.org/10.3390/agronomy11101991
Erida G, Saidi N, Hasanuddin H, Syafruddin S. Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth. Agronomy. 2021; 11(10):1991. https://doi.org/10.3390/agronomy11101991
Chicago/Turabian StyleErida, Gina, Nurdin Saidi, Hasanuddin Hasanuddin, and Syafruddin Syafruddin. 2021. "Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth" Agronomy 11, no. 10: 1991. https://doi.org/10.3390/agronomy11101991
APA StyleErida, G., Saidi, N., Hasanuddin, H., & Syafruddin, S. (2021). Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth. Agronomy, 11(10), 1991. https://doi.org/10.3390/agronomy11101991





