Abstract
The soybean cyst nematode (SCN; Heterodera glycines Ichinohe) is a major soybean-yield-limiting soil-borne pathogen, especially in the Midwestern US. Weed management is recommended for SCN integrated management, since some weed species have been reported to be hosts for SCN. The increase in the occurrence of resistance to herbicides complicates weed management and may further direct ecological–evolutionary (eco–evo) feedbacks in plant–pathogen complexes, including interactions between host plants and SCN. In this review, we summarize weed species reported to be hosts of SCN in the US and outline potential weed–SCN management interactions. Plants from 23 families have been reported to host SCN, with Fabaceae including most host species. Out of 116 weeds hosts, 14 species have known herbicide-resistant biotypes to 8 herbicide sites of action. Factors influencing the ability of weeds to host SCN are environmental and edaphic conditions, SCN initial inoculum, weed population levels, and variations in susceptibility of weed biotypes to SCN within a population. The association of SCN on weeds with relatively little fitness cost incurred by the latter may decrease the competitive ability of the crop and increase weed reproduction when SCN is present, feeding back into the probability of selecting for herbicide-resistant weed biotypes. Therefore, proper management of weed hosts of SCN should be a focus of integrated pest management (IPM) strategies to prevent further eco–evo feedbacks in the cropping system.
1. Introduction
Ecology and evolution may interact resulting in environmental changes, which then feeds back into ecological relationships and species evolution to alter community assembly and ecosystem function [1]. In fact, the plant–pathogen–herbicide complex may be a prime example of eco–evolutionary (eco–evo) feedback in agricultural systems. Herbicides can be agents of eco–evo dynamics, causing shifts in weed community composition and influencing weed–pathogen co-evolution [2]. Thus, animals and microbes which rely on plants may be directly impacted by herbicides and will be driven to evolve in concert with their plant hosts in response to management [3]. Direct and indirect effects impact species evolution and eco–evo feedbacks within the environment [4,5]. The concept that herbicides may influence eco–evo feedbacks in agricultural systems, and specifically in the plant–pathogen–herbicide complex, is relatively novel, and there is definitely a need to study such multi-trophic interactions [2,6].
Herbicides are strong anthropogenic selection agents affecting weed evolution in agricultural systems, especially with the increase in herbicide use often associated with the adoption of genetically modified herbicide-resistant (GMHR) crops [7]. In corn, cotton, and soybean, 89, 95, and 94% of the hectares in the US are planted with GMHR crops, respectively [8], and herbicides are applied post crop emergence. The augmented herbicide selection pressure in GMHR systems is associated with a greater frequency of establishment of herbicide-resistant (HR) weed biotypes. Currently, there is a limited understanding of the dynamics of eco–evo (e.g., weed–pathogen) interactions and the effect of widespread agents of selection (e.g., herbicide use) in many agroecosystems where GMHR crops are used [2,9,10]. In some instances, plant pathogens (e.g., nematodes) may disproportionately impact select crop hosts and may have evolved to have a relatively minimal impact on other plant hosts including weeds. In the case of nematodes, this may be especially true since their range of movement is limited with passive dispersal, thus requiring a host to survive and reproduce [11,12]. Additionally, nematodes, such as the soybean cyst nematode (SCN; Heterodera glycines I.), are known to decrease the soybean canopy in susceptible cultivars, which leads to increased weed densities and weed seed production at harvest [13]. This increased potential for weed germination and establishment in the subsequent season feeds back into the probability of selecting for HR weed biotypes with herbicide applications (Figure 1).
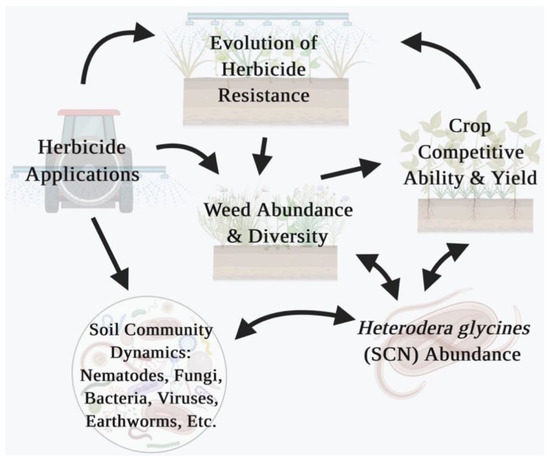
Figure 1.
Example of potential eco–evo feedbacks in the crop-weed–SCN-management interaction in agroecosystems with reliance on herbicide for weed management. Created with BioRender® (BioRender.com).
2. The SCN–Weed Host Relationship
The SCN is the main yield loss causing agent in US soybean, and it is widely distributed across all major soybean production areas [14,15]. In a survey conducted from 2010 to 2014, losses caused by SCN in the US were estimated to be double that of other diseases nationwide [16]. SCN can cause up to 60% of yield losses when susceptible cultivars are planted [17], and up to 30% in loss without showing aboveground symptoms [18]. Hence the emphasis for the need to conduct proper testing and field scouting [18,19,20] to properly assess SCN incidence in production fields.
To decrease yield losses caused by SCN, a set of integrated pest management (IPM) practices are suggested, including using resistant soybean cultivars, crop rotation with non-hosts, weed management, seed-applied nematicides, and the use of biological control products [14,19,21,22,23,24,25,26]. In fact, a single year in a weed-free non-host crop may reduce SCN populations by up to 55% [27]. Currently, most soybean commercial cultivars (>90%) share a common source of resistance (PI 88788), and the heavy reliance on this source of resistance led to the selection of SCN populations that can reproduce on these cultivars, thus limiting management options available to farmers [28]. As a matter of fact, in a survey conducted in major soybean producing areas in the US, and Ontario, Canada, an alarmingly large percentage of SCN field populations—MO (100%), MI (94%), TN (93%), IL (88%), KY (60%), WI (67%), IN (56%), OH (54%), SD (25%), MN (17%) and Ontario (33%)—were reported to be able to reproduce on PI 88788 [29]. To help with this issue, cultivars have been released with new sources of resistance, including Peking (PI 548402) and PI 89772 [30].
The presence of a suitable host is the most important factor affecting plant nematode populations [31]. Weeds may serve as alternative hosts for insects, pathogens, and plant-parasitic nematodes in the absence of a major crop [32]. Although weed communities are not optimal hosts for plant-parasitic nematodes, they often consist of a diverse group of plants, helping to maintain nematode diversity in fields [12]. Early studies were conducted in the 1960s to assess the ability of SCN to reproduce in weed populations, following the first report of SCN in the US in 1954 [33,34,35,36]. Currently, research is still being conducted in modern cropping systems to evaluate the impact of weed management practices on SCN populations [9]. In fact, when rotating crops, knowledge of species host status is key to managing SCN as this nematode can parasitize a broad range of plants including nearly 150 genera of legumes (Fabaceae) and non-legumes [37,38].
3. Weeds Species Hosting SCN
Winter annual weeds are relatively easy to manage using herbicides and tillage, but since their interference with summer annual crops is minimal compared to summer annuals, these weeds are often left to reproduce in the spring [39]. As some of these weeds are hosts of SCN, they serve as an overwintering option, aggravating the problem in a scenario where winter weeds have become common in no-till production fields (Figure 2) [40]. Vetches (Vicia spp.), clovers (Trifolium spp.), senna (Senna spp.) and lupines (Lupinus spp.) included in the family Fabaceae with soybean are examples of SCN–weed hosts. Other plant families may also include species hosting SCN, including Asteraceae, Brassicaceae, Lamiaceae, Plantaginaceae, and others (Table 1; Figure 3). In the Midwest, numerous research reports indicate common broadleaf weeds as potential hosts of SCN, including purple deadnettle (Lamium purpureum L.), henbit (Lamium amplexicaule L.), field pennycress (Thlaspi arvense L.), shepherd’s-purse (Capsella bursa-pastoris (L.) Medik), common chickweed (Stellaria media (L.) Vill.), smallflowered bittercress (Cardamine parviflora L.), common mallow (Malva neglecta Wallr.), white clover (Trifolium repens L.), Canada thistle (Cirsium arvense (L.) Scop.), common cocklebur (Xanthium strumarium L.), and others (Table 1) [24,37,39,41,42,43].
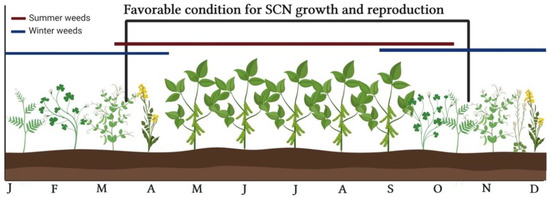
Figure 2.
Dynamics of SCN and winter annual weeds in soybean cropping systems. Adapted from [37]. Created with BioRender® (BioRender.com).

Table 1.
Weed species 1 reported as hosts of SCN, with respective botanic classifications, life cycle (LC), growth habit (GRH), herbicide resistance cases, HG (Heterodera glycines) types 7, location, and female index.
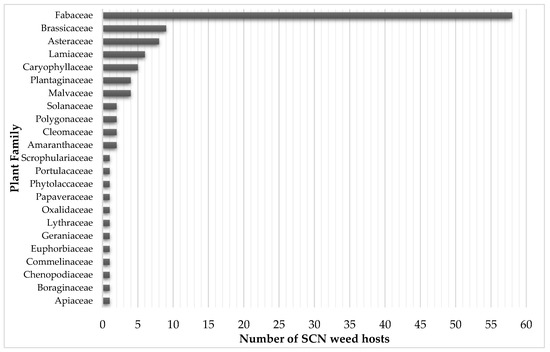
Figure 3.
Number of weed species hosting SCN per plant family from a total of 116 species listed in Table 1.
For instance, SCN cysts containing eggs were detected in roots of most purple deadnettle plants sampled in southern Indiana, confirming greenhouse screening results, and indicating possible advantageous implications regarding SCN management when these weeds are managed [55]. Therefore, this review presents an updated summary of weeds reported as potential hosts of SCN in field and greenhouse conditions and discusses implications in SCN management. Most weed hosts were documented from the literature in the US, but some reports from Brazil were also included especially when SCN was parasitizing weeds also present in the US. The most predictive time point for measuring nematode populations for assessing yield losses is at planting [31], since initial conditions are of critical importance in the crop-nematode relationship, winter annuals and early season weed management of the species in this review should be a focus of IPM strategies to prevent further eco–evo feedbacks in soybean cropping systems.
4. Weed Management Challenges and Influence on SCN Populations
Some weed hosts of SCN have HR biotypes reported in the US, which may limit chemical control options in fields with incidence of resistant weed populations. Herbicide resistance was first reported in the US after the introduction of selective, systemic herbicides in the late 1940s [61], and has increased over the decades with the introduction of new herbicide chemistries, expansion of herbicide use, and the introduction of GMHR crops in the mid- to late-1990s. Lack of proper herbicide stewardship, including the lack of rotation of herbicide sites of action (SOA), led to the selection of more than 500 unique cases (species x SOA) of herbicide resistance worldwide, including 23 of the 26 known herbicide SOAs and more than 150 weed species, in 92 crops and 70 countries [60]. HR weeds raise concerns not only due to their ability to compete with crops for resources, but also since they can maintain populations of plant-parasitic nematodes in the absence of a major susceptible crop host such as soybean. Additionally, the presence of HR weeds that serve as potential hosts of SCN in production fields may further drive evolutionary processes governing the interaction between SCN and HR weeds.
Of the 116 weed species reported to have documented associations with SCN in the literature, 14 (12%) also have documented HR biotypes with resistance to eight herbicide SOAs (Table 1). Four species (Amaranthus tuberculatus (Moq.) Sauer, Conyza canadensis (L.) Cronquist, Portulaca oloracea L., and Xanthium strumarium L.) have documented resistance to more than one SOA, and A. tuberculatus has several populations with multiple resistance to up to five SOAs in a single population [60]. Conyza canadensis and X. strumarium have populations with multiple resistance to two SOA groups. Of the 14 weed species with documented resistances to herbicides, nine species display resistance to SOA group 2 (acetolactate synthesis inhibitors), six species to SOA group 5 (photosystem II inhibitors), two species to SOA group 9 (enolpyruvylshikimate-3-phosphate inhibitors), and five species each have resistances to SOA groups 7 (photosystem II inhibitors—ureas and amides), 14 (protoporphorynogen oxidase inhibitors), 15 (long-chain fatty acid inhibitors), 17 (nucleic acid inhibitors), or 22 (photosystem I electron diverter). In fact, all 14 of the weed species with documented herbicide resistance, except for Camelina microcarpa Andrz. ex. DC, were included in one or more of the lists of most common or troublesome weeds, as determined by the studies listed in Table 2 [62,63,64,65]. Also, in that table, “common weeds” refers those species frequently observed throughout the listed cropping systems, while “troublesome weeds” indicate those that are difficult to manage but may be relatively uncommon [62].

Table 2.
Weeds known to host SCN that are also found on the “Most Common” and “Most Troublesome” weed lists 1 for soybean and rotational crops.
Some of the weed species documented to have SCN associations pose known management challenges, while others may be relatively easily managed. Thirty out of the 116 species with SCN association (26%) are included in the most “common” or “troublesome weeds” categories (Table 2). The incidence of these species (“common” or “troublesome”) in cropping systems is likely a result of population and community shifts ensuing from the interaction between management selection pressure, ecological associations, and edaphic factors. Although selective herbicides have often been documented to induce shifts in weed species [66,67], additional management practices known to induce such shifts include crop rotation, tillage, fertility applications, and harvest practices [68]. The impact of the combined effect of the diverse agricultural management practices on weed selection and species shifts can be used advantageously in devising Integrated Weed Management (IWM) strategies.
IWM tactics may help slow the evolution of herbicide resistance, and potentially modulate the evolution of weed-crop pathogen relationships. Examples of IWM tactics are tillage, the use of cover crops, crop rotation, preventing weeds from germinating, and using crop cultivars with fast canopy closure. These IWM tactics may affect SCN incidence in a multitude of ways. As no-till systems became more popular with the emergence of GMHR crops in the mid-1990s, they facilitated to some extent the emergence of SCN–weed hosts. These weeds included wind-dispersed species such as C. canadensis, small-seeded broadleaf species (A. tuberculatus), and perennials species (Cirsium arvense (L.) Scop., Sonchus arvensis L.) (Table 1) [68]. Tillage as a weed management tool may interact with nematode populations; for example, nematode species diversity has been shown to be greater in systems with little disturbance [69]. Moreover, in row crops, similar to weed propagules, nematodes are often shown to be distributed in patterns that follow the direction of tillage [31].
Cover crops have been extensively studied as tools to suppress weeds [70]. Cover crop selection and management also impact the total abundance, biomass, and metabolic footprints of nematode communities [71,72,73]. Nevertheless, results may diverge across studies as these effects are often site sensitive, relying mainly on crop susceptibility to target nematode groups and on field conditions, including the levels of nematode infestation [74]. Some cover crops not only suppress plant-parasitic nematodes (PPN) directly, but also promote beneficial organisms in the soil, enhance the abundance of higher trophic level organisms, limit availability of resources [73], and increase the resilience of soil microbial communities [75]. Shifts in nematode communities may not occur instantly by disturbances caused by cropping practices [76], as these changes may be driven by a cascade effect in the soil. The suppression of nematode populations by cover crops results from the combined effect of releasing pre-existing nematocidal compounds by the cover crop, producing nematocidal compounds during residue decomposition, and introducing and/or increasing antagonistic microorganisms in the soil. Cover crops may also increase plant tolerance and resistance via the induction of systemic defense pathways (ISR), and result in soil modifications unsuitable for nematode reproduction [77].
Crop rotation, enhancing the competitive ability of crops against weeds, and prevention of the establishment of weed populations, are cited as focal objectives of IWM [78]. However, weeds that host SCN may negatively impact the crop competitive ability, thus augmenting potential yield losses. Weeds may be persistent across rotational cropping systems since some weed species can be common or troublesome in multiple crops (Table 2). One of the benefits of rotating crops vis-à-vis weed management, is the subsequent ability to use diverse herbicide SOAs. However, some weed species with resistance to multiple SOAs, such as A. tuberculatus, are becoming difficult to manage with herbicides regardless of the adopted rotational crop. Tactics to prevent the introduction of weed propagules may be positively associated with SCN management; the same factors that disperse weed propagules within and between fields, by farm equipment and human activities, animals, rodents, birds, and water and wind movement, may also be responsible for long-distance dispersal of nematodes [31].
5. Implications on SCN Management
Over 116 weeds species can host SCN (Table 1). Most of these weeds (58 species or 50%) belong to the Fabaceae family, which include soybean (Figure 2). Although some species support a full SCN life cycle, others may only host juveniles without any ensuing increase in the SCN population. In fact, it is notable that the reproduction potential of SCN in most of those weed hosts does not surpass levels observed in soybean cultivars resistant to SCN [79]. As with how different soybean cultivars vary in their susceptibility to SCN, susceptibility to SCN may also vary across weed biotypes and populations. Differences in susceptibility may explain in some instances discrepancies in studies investigating the host range of SCN among weed species. Moreover, differences in SCN population levels, HG types, dormancy, environmental, and edaphic conditions, may also influence the ability of these weed species to host SCN [53]. HG type reflects the ability of SCN populations to reproduce on a set of soybean indicator lines, providing insights pertaining to SCN management, as field populations can be better managed with cultivars resistant to that specific HG type in a production field. One fact to be noted is that HG type definitions apply to field SCN populations and not individual nematodes [80].
Although weeds may not be optimal SCN hosts, the presence of weed hosts reduces the efficacy of SCN management practices, and therefore, increases the pressure and potential yield losses caused by SCN [12]. If allowed to persist through incomplete or failed management, weeds may drive the selection of SCN populations able to reproduce in both soybean and weed species. More importantly, the association between weeds and PPN goes beyond those weeds only serving as an overwintering option for nematodes. These alternative hosts may also protect nematodes from pesticides and other unfavorable environmental factors [12]. SCN populations are continually exposed to selection pressure for reproduction in SCN-resistant crop cultivars. Therefore, even a small reduction in the initial SCN populations levels through effective weed management would result in long-term benefits for farmers by reducing potentially harmful eco–evo feedbacks into production systems (Figure 2).
Temperature and moisture are generally considered to be the most important abiotic factors that manage nematode populations [31]. Even though previous research indicates that some weed species are hosts of SCN in greenhouse screenings, Creech and Johnson [81] note that, based on a survey conducted in Indiana, the presence of winter annual weeds in fields does not appear to correlate with SCN counts. These authors suggest that the lack of SCN reproduction when temperatures fall below 10°C reduces the ability of SCN to infect winter annual weeds. Temperature is known to be an important factor affecting SCN growth, development, and reproduction rates, even though SCN can occur in a fairly wide range of temperatures [82,83]. Although temperatures in winter field conditions may not be optimal to complete a full life cycle, having a susceptible weed host in late fall and early spring, when soybean is not present in the field, could increase initial populations of SCN in these fields (Figure 2). In fact, Creech, et al. [83] demonstrated that after hatching, SCN juveniles can start their development and survive inside purple deadnettle roots in a dormant stage under cold temperatures. Juveniles were also noted to continue development as temperatures increase again in spring, giving an advantage to the pathogen compared to a field with optimal weed management. As a matter of fact, in Table 1, twenty-seven out of the 116 species documented as SCN hosts (23%) can complete their life cycles as winter annual plants.
Creech, et al. [41] investigated the occurrence of SCN in henbit and purple deadnettle in seven production fields in Indiana, Illinois, and Ohio. They reported that eggs, cysts, and juveniles can be associated with these weeds following fall and spring samplings. Nevertheless, greater levels of cyst and egg production were detected in fall compared to spring. Therefore, weed management in the fall would be best at minimizing the potential for SCN reproduction on winter weeds, but additional weed management before planting in the spring would also have a positive impact on SCN management. In contrast, Webb [84] documented purple deadnettle as a poor SCN host under field conditions, and there were no subsequent changes in nematode population density when that weed was removed. However, in yet a different study, the presence of purple deadnettle in the fall was reported to be the main factor leading to increases in SCN egg populations [85]. These apparent contradictions in the literature highlight the influence of environmental and edaphic factors on the ability of SCN to parasitize and reproduce in populations of these weeds. Nevertheless, research has been conducted to assess the efficacy of weed management to reduce SCN pressure in soybean fields. Nelson et al. [86] evaluated the impact of herbicide applications (chlorimuron + sulfentrazone) on SCN (race 4) population densities. Results demonstrated a stable SCN population in plots under fall-applied herbicides, while the nontreated control had increased SCN counts in year 1 compared to treatments. In the second year, they observed a reduction in SCN populations with spring-applied herbicides compared to the control. Moreover, glyphosate, when used as an early management method for henbit, was reported to reduce SCN reproduction potential in SCN infested fields [87].
6. Indirect Impacts of Weed Management on SCN and Feedbacks Affecting Soil Microbial and Nematode Communities
Weed management practices (tillage, herbicides) may have direct [69] and indirect impacts on SCN populations, which then feed back into the ecological system, affecting other aspects of soil communities, microbial and non-microbial (Figure 2) [2]. Populations of PPN may be relatively tolerant to some applications of pesticides, while other types of nematodes, such as fungivores and bacterivores, may be negatively impacted by those applications [88,89]. PPN generally compose a larger percentage of the nematode community in agricultural habitats compared to natural habitats [90]. In fact, it has long been known that PPN interact with other organisms (fungi, bacteria, viruses, earthworms, mites, insects, rodents, and other nematodes) to impact plant growth [31,91]. For example, root-feeding nematodes influence the surrounding microbial community; increased organic matter has been shown to increase carbon flow from plant roots to microbes [69]. Herbicide use, tillage, and crop rotations may have an impact on soil communities, disrupting interactions such as the weed–SCN relationship. The consequences of these disruptions may extend beyond the impacts on crop yield and have indirect effects on fundamental processes such as soil decomposition and N mineralization [2,31].
7. Conclusions
Eco–evo feedbacks in the crop-weed–SCN-management agroecosystem are not straightforward and impacts of environmental and temporal heterogeneity likely amplify stochastic responses. Additionally, there may be emergent properties that result from species evolution which further impact the system; for example, in theory, HR plants may be more or less desirable host species for SCN or other plant pathogens due to metabolic or other changes [2]. However, an understanding of species ecology and evolution in agroecosystems is an important step in the ability to create sustainably managed cropping systems.
With regards to practical management implications, research indicates that crop rotational sequence, and the use of cover crops and resistant crop cultivars have a stronger impact on SCN field populations compared to weed management, especially in fields with low SCN pressure [40,92]. However, in a scenario where cropping systems intensify the production of staple crops to supply increasing demand for food, feed, and fuel [93,94], targeting weed hosts in fields with high SCN populations, may reduce initial SCN pressure. Extension programs should keep informing growers about the ability of SCN to reproduce on weeds [95], and how proper weed management practices could reduce SCN population densities in soybean fields.
Author Contributions
Conceptualization, L.F.R. and K.L.G.; Literature research, L.F.R.; writing and original draft preparation, L.F.R. and K.L.G.; review and editing, L.F.R., K.L.G., M.F.P., A.M.F. and J.P.B.; Design of figures, L.F.R.; Final formatting and submission, L.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Post, D.M.; Palkovacs, E.P. Eco-evolutionary feedbacks in community and ecosystem ecology: Interactions between the ecological theatre and the evolutionary play. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Iriart, V.; Baucom, R.S.; Ashman, T.L. Herbicides as anthropogenic drivers of eco-evo feedbacks in plant communities at the agro-ecological interface. Mol. Ecol. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Prosser, R.S.; Anderson, J.C.; Hanson, M.L.; Solomon, K.R.; Sibley, P.K. Indirect effects of herbicides on biota in terrestrial edge-of-field habitats: A critical review of the literature. Agric. Ecosyst. Environ. 2016, 232, 59–72. [Google Scholar] [CrossRef]
- terHorst, C.P. Evolution in response to direct and indirect ecological effects in pitcher plant inquiline communities. Am. Nat. 2010, 176, 675–685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Meester, L.; Brans, K.I.; Govaert, L.; Souffreau, C.; Mukherjee, S.; Vanvelk, H.; Korzeniowski, K.; Kilsdonk, L.; Decaestecker, E.; Stoks, R. Analysing eco-evolutionary dynamics—The challenging complexity of the real world. Funct. Ecol. 2019, 33, 43–59. [Google Scholar] [CrossRef]
- Neve, P.; Busi, R.; Renton, M.; Vila-Aiub, M.M. Expanding the eco-evolutionary context of herbicide resistance research. Pest Manag. Sci. 2014, 70, 1385–1393. [Google Scholar] [CrossRef]
- Mortensen, D.A.; Egan, J.F.; Maxwell, B.D.; Ryan, M.R.; Smith, R.G. Navigating a critical juncture for sustainable weed management. Bioscience 2012, 62, 75–84. [Google Scholar] [CrossRef]
- USDA-ERS. Adoption of Genetically Engineered Crops in the U.S.; United States Department of Agriculture: Washington, DC, USA, 2019. Available online: https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us (accessed on 28 November 2020).
- Palkovacs, E.P.; Hendry, A.P. Eco-evolutionary dynamics: Intertwining ecological and evolutionary processes in contemporary time. F1000 Biol. Rep. 2010, 2. [Google Scholar] [CrossRef]
- Hart, S.P.; Turcotte, M.M.; Levine, J.M. Effects of rapid evolution on species coexistence. Proc. Natl. Acad. Sci. USA 2019, 116, 2112–2117. [Google Scholar] [CrossRef]
- Schroeder, J.; Thomas, S.H.; Murray, L.W. Impacts of crop pests on weeds and weed–crop interactions. Weed Sci. 2005, 53, 918–922. [Google Scholar] [CrossRef]
- Thomas, S.H.; Schroeder, J.; Murray, L.W. The role of weeds in nematode management. Weed Sci. 2005, 53, 923–928. [Google Scholar] [CrossRef]
- Alston, D.G.; Bradley, J., Jr.; Schmitt, D.; Coble, H. Response of Helicoverpa zea (Lepidoptera: Noctuidae) populations to canopy development in soybean as influenced by Heterodera glycines (Nematoda: Heteroderidae) and annual weed population densities. J. Econ. Entomol. 1991, 84, 267–276. [Google Scholar] [CrossRef]
- Niblack, T.L.; Tylka, G.L. Soybean Cyst Nematode Management Guide, 5th ed.; North Central Soybean Research Program: Urbandale, IA, USA, 2008; pp. 1–16. [Google Scholar]
- Bandara, A.Y.; Weerasooriya, D.K.; Bradley, C.A.; Allen, T.W.; Esker, P.D. Dissecting the economic impact of soybean diseases in the United States over two decades. PLoS ONE 2019, 15, e0231141. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Corker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Nicholas, S.; et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada from 2010 to 2014. Plant Health Progr. 2017, 18, 19–27. [Google Scholar] [CrossRef]
- Hershman, D.E. 2015 Soybean Cyst Nematode (SCN) Management Recommendations for Kentucky; University of Kentucky Extension: Lexington, KY, USA, 2014; p. 4. [Google Scholar]
- Niblack, T.L. Soybean Cyst Nematode Management Reconsidered. Plant Dis. 2005, 89, 1020–1026. [Google Scholar] [CrossRef]
- Mueller, D.; Wise, K.; Sisson, A.; Smith, D.; Sikora, E.; Bradley, C.; Robinson, A. A Farmer’s Guide to Soybean Diseases; APS Press: St. Paul, MN, USA, 2016; p. 155. [Google Scholar]
- Tylka, G.L.; Marett, C.C. Known distribution of the soybean cyst nematode, Heterodera glycines, in the United States and Canada, 1954 to 2017. Plant Health Progr. 2017, 18, 167–168. [Google Scholar] [CrossRef]
- Niblack, T. Nematodes. In Illinois Agronomy Handbook, 24th ed.; Extension, U.O.I., Ed.; University of Illinois Cooperative Extension Service: Urbana, IL, USA, 2009; pp. 27–36. [Google Scholar]
- Gavassoni, W.L.; Tylka, G.L.; Munkvold, G.P. Effects of Tillage Practices on Dissemination and Spatial Patterns of Heterodera glycines and Soybean Yield. Plant Dis. 2007, 91, 973–978. [Google Scholar] [CrossRef]
- Wight, J.P.; Allen, F.L.; Donald, P.A.; Tyler, D.D.; Saxton, A.M. Impact of Crop Rotation and Bio-covers on Soybean Cyst Nematode. Plant Health Progr. 2011, 12, 1–9. [Google Scholar] [CrossRef]
- Werle, R.; Giesler, L.J.; Bernards, M.L.; Lindquist, J.L. Likelihood of soybean cyst nematode (Heterodera glycines) reproduction on henbit (Lamium amplexicaule) roots in Nebraska. Weed Technol. 2015, 29, 35–41. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; Canullo, G.H. Cropping systems for the management of phytonematodes. Phytoparasitica 1992, 20, 211–224. [Google Scholar] [CrossRef]
- Riga, E.; Topp, E.; Potter, J.; Welacky, T.; Anderson, T.; Tenuta, A. The impact of plant residues on the soybean cyst nematode, Heterodera glycines. Can. J. Plant Pathol. 2001, 23, 168–173. [Google Scholar] [CrossRef]
- Faghihi, J.; Ferris, R. Soybean Cyst Nematode (E-210-W); Purdue University Extension: West Lafayette, IN, USA, 2012; p. 4. [Google Scholar]
- Niblack, T.; Colgrove, A.; Colgrove, K.; Bond, J. Shift in virulence of soybean cyst nematode is associated with use of resistance from PI 88788. Plant Health Progr. 2008, 9, 1–7. [Google Scholar] [CrossRef]
- The SCN Coalition. How the SCN Problem Evolved; The SCN Coalition: Online. 2018, p. 1. Available online: https://www.thescncoalition.com/application/files/6715/4266/6783/SCN_Problem_Evolved_Infographic_NOV_2018.pdf (accessed on 15 September 2020).
- Winsor, S. Soybean Cyst Nematode Management in the Western Corn Belt. Crops Soils 2020, 53, 4–12. [Google Scholar] [CrossRef]
- Norton, D.; Niblack, T.L. Biology and Ecology of Nematodes; M. Dekker: New York, NY, USA, 1991; pp. 47–72. [Google Scholar]
- Bendixen, L.E. Major Weed Hosts of Nematodes in Crop Production (Special Circular 119); Ohio Agricultural Research and Development Center: Wooster, OH, USA, 1988; p. 26. [Google Scholar]
- Riggs, R.; Hamblen, M. Soybean-cyst nematode host studies in the family Leguminosae. Rep. Ark. Agric. Expt. Stat. 1962, 50, 1–18. [Google Scholar]
- Riggs, R.; Hamblen, M. Additional weed hosts of Heterodera glycines. Plant Dis. 1966, 50, 15. [Google Scholar]
- Smart, G.C. Physiological strains and one additional host of the soybean cyst nematode Heterodera glycines. Plant Dis. 1964, 48, 542–543. [Google Scholar]
- Smart, G.C. Additional hosts of the soybean cyst nematode, Heterodera glycines, including hosts in two additional plant families. Plant Dis. 1964, 48, 388–390. [Google Scholar]
- Johnson, W.G.; Earl Creech, J.; Mock, V.A. Role of winter annual weeds as alternative hosts for soybean cyst nematode. Crop Manag. 2008, 7, 1–9. [Google Scholar] [CrossRef]
- Riggs, R.D. Nonhost root penetration by soybean cyst nematode. J. Nematol. 1987, 19, 251–254. [Google Scholar]
- Venkatesh, R.; Harrison, S.K.; Riedel, R.M. Weed Hosts of Soybean Cyst Nematode (Heterodera glycines) in Ohio. Weed Technol. 2000, 14, 156–160. [Google Scholar] [CrossRef]
- Creech, J.E.; Westphal, A.; Ferris, V.R.; Faghihi, J.; Vyn, T.J.; Santini, J.B.; Johnson, W.G. Influence of winter annual weed management and crop rotation on soybean cyst nematode (Heterodera glycines) and winter annual weeds. Weed Sci. 2008, 56, 103–111. [Google Scholar] [CrossRef]
- Creech, J.E.; Webb, J.S.; Young, B.G.; Bond, J.P.; Harrison, S.K.; Ferris, V.R.; Faghihi, J.; Westphal, A.; Johnson, W.G. Development of soybean cyst nematode on henbit (Lamium amplexicaule) and purple deadnettle (Lamium purpureum). Weed Technol. 2007, 21, 1064–1070. [Google Scholar] [CrossRef]
- Poromarto, S.H.; Gramig, G.G.; Nelson, B.D., Jr.; Jain, S. Evaluation of weed species from the Northern Great Plains as hosts of soybean cyst nematode. Plant Health Progr. 2015, 16, 23–28. [Google Scholar] [CrossRef]
- Basnet, P.; Clay, S.A.; Byamukama, E. Determination of weed hosts of soybean cyst nematode in South Dakota. Weed Technol. 2020, 34, 377–382. [Google Scholar] [CrossRef]
- Riggs, R.; Hamblen, M. Further Studies on the Host Range of the Soybean-Cyst Nematode. Bullet. Ark. Agric. Expt. Stat. 1966, 718, 1–20. [Google Scholar]
- Rajan, L.A. Phytosanitary importance of Heterodera glycines for South-east Asian countries. EPPO Bull. 2005, 35, 531–536. [Google Scholar] [CrossRef]
- Dias, W.P.; Ferraz, S.; Silva, A.A.; Lima, R.D.; Valle, L.A.C. Host suitability of some weed species to the soybean cyst nematode (Heterodera glycines Ichinohe). Nematropica 1995, 25, 85. [Google Scholar]
- Rodriguez-Kabana, R.; King, P.; Robertson, D.; Weaver, C.; Carden, E. New crops with potential for management of soybean nematodes. Nematropica 1988, 8, 45–52. [Google Scholar]
- Poromarto, S.H.; Nelson, B.D. Evaluation of northern-grown crops as hosts of soybean cyst nematode. Plant Health Progr. 2010, 11, 1–9. [Google Scholar] [CrossRef]
- Mendes, M.L. O Nematoide de Cisto da Soja (Heterodera Glycines Ichinohe, 1952); Potafos: Piracicaba, Brasil, 1993. [Google Scholar]
- Moore, W.F.; Bost, S.C.; Brewer, F.L.; Dunn, R.A.; Endo, B.Y.; Grau, C.R.; Hardman, L.L.; Jacobsen, B.J.; Leffel, R.; Newman, M.A.; et al. Soybean Cyst Nematode; Soybean Industry Committee: Washington, DC, USA, 1984; p. 23. [Google Scholar]
- Carnielli, A. Response of crops used in rotation to Heterodera glycines. Nematropica 1995, 25, 82. [Google Scholar]
- Valle, S.P.; Dias, W.P. Host suitability of some plant species to the Heterodera glycines. Nematropica 1995, 25, 107. [Google Scholar]
- Donald, P.; Hayes, R.; Walker, E. Potential for soybean cyst nematode reproduction on winter weeds and cover crops in Tennessee. Plant Health Progr. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Epps, J.; Chambers, A. Comparative rates of reproduction of Heterodera glycines on 12 host plants. Plant Dis. 1966, 50, 608–610. [Google Scholar]
- Creech, J.E.; Johnson, W.G.; Faghihi, J.; Ferris, V.R.; Westphal, A. First report of soybean cyst nematode reproduction on purple deadnettle under field conditions. Crop Manag. 2005, 4, 1–2. [Google Scholar] [CrossRef]
- Ward, N.; Rershman, D.; Dunwell, W. Soybean Cyst Nematode: A Potential Problem for Nurseries; University of Kentucky Cooperative Extension Service: Lexington, KY, USA, 2011; p. 4. [Google Scholar]
- Epps, J.M.; Chambers, A. New host records for Heterodera glycines; including one host in the Labiatae. Plant Dis. 1958, 42, 194. [Google Scholar]
- University of Illinois Extension. The Soybean Cyst Nematode Problem; Report on Plant Disease, RPD 501; Department of Crop Science, University of Illinois Plant Clinic: Urbana-Champaign, IL, USA, 2014; Available online: http://ipm.illinois.edu/diseases/rpds/501.pdf (accessed on 7 October 2020).
- Goodey, J.B.; Franklin, M.T.; Hooper, D.J. The Nematode Parasites of Plants Cataloged under their Hosts, 3rd ed.; Commonwealth Agricultural Bureaux: St. Albans, UK, 1965; p. 214. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: http://www.weedscience.org (accessed on 10 September 2020).
- Shaner, D.L. Lessons learned from the history of herbicide resistance. Weed Sci. 2014, 62, 427–431. [Google Scholar] [CrossRef]
- Van Wychen, L. 2016 Survey of the Most Common and Troublesome Weeds in Broadleaf Crops, Fruits & Vegetables in the United States and Canada; WSSA. 2016. Available online: http://wssa.net/wp-content/uploads/2016-Weed-Survey_Broadleaf-crops.xlsx (accessed on 10 September 2020).
- Van Wychen, L. 2015 Baseline Survey of the Most Common and Troublesome Weeds in the United States and Canada; WSSA. 2015. Available online: http://wssa.net/wp-content/uploads/2015-Weed-Survey_Baseline.xls (accessed on 10 September 2020).
- Van Wychen, L. 2017 Survey of the Most Common and Troublesome Weeds in Grass Crops, Pasture and Turf in the United States and Canada; WSSA. 2017. Available online: http://wssa.net/wp-content/uploads/2017-Weed-Survey_Grass-crops.xlsx (accessed on 10 September 2020).
- Van Wychen, L. 2019 Survey of the Most Common and Troublesome Weeds in Broadleaf Crops, Fruits & Vegetables in the United States and Canada; WSSA. 2019. Available online: http://wssa.net/wp-content/uploads/2019-Weed-Survey_broadleaf-crops.xlsx (accessed on 10 September 2020).
- Owen, M.D. Weed species shifts in glyphosate-resistant crops. Pest Manag. Sci. 2008, 64, 377–387. [Google Scholar] [CrossRef]
- Johnson, W.G.; Davis, V.M.; Kruger, G.R.; Weller, S.C. Influence of glyphosate-resistant cropping systems on weed species shifts and glyphosate-resistant weed populations. Eur. J. Agron. 2009, 31, 162–172. [Google Scholar] [CrossRef]
- Murphy, C.E.; Lemerle, D. Continuous cropping systems and weed selection. Euphytica 2006, 148, 61–73. [Google Scholar] [CrossRef]
- Yeates, G. Effects of plants on nematode community structure. Annu. Rev. Phytopathol. 1999, 37, 127–149. [Google Scholar] [CrossRef]
- Teasdale, J.R. Contribution of cover crops to weed management in sustainable agricultural systems. J. Prod. Agric. 1996, 9, 475–479. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Djigal, D.; Chabrier, C.; Duyck, P.-F.; Achard, R.; Quénéhervé, P.; Tixier, P. Cover crops alter the soil nematode food web in banana agroecosystems. Soil Biol. Biochem. 2012, 48, 142–150. [Google Scholar] [CrossRef]
- McSorley, R. Assessment of rotation crops and cover crops for management of root-knot nematodes (Meloidogyne spp.) in the southeastern United States. Nematropica 2011, 41, 200–214. [Google Scholar]
- Sun, F.; Pan, K.; Li, Z.; Wang, S.; Tariq, A.; Olatunji, O.A.; Sun, X.; Zhang, L.; Shi, W.; Wu, X. Soybean supplementation increases the resilience of microbial and nematode communities in soil to extreme rainfall in an agroforestry system. Sci. Total Environ. 2018, 626, 776–784. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Gage, K.L.; Schwartz-Lazaro, L.M. Shifting the paradigm: An ecological systems approach to weed management. Agriculture 2019, 9, 179. [Google Scholar] [CrossRef]
- Ferraz, S.; LAC, V.; Dias, C. Utilização de plantas antagônicas no controle do nematóide de cistos da soja (Heterodera glycines Ichinohe). In O Nematóide do Cisto da Soja: A Experiência Brasileira; Silva, J., Ed.; Sociedade Brasileira de Nematologia: Jaboticabal, Brasil, 1999; pp. 52–53. [Google Scholar]
- Tylka, G.L. Understanding soybean cyst nematode HG types and races. Plant Health Progr. 2016, 17, 149–151. [Google Scholar] [CrossRef]
- Creech, J.E.; Johnson, W.G. Survey of broadleaf winter weeds in Indiana production fields infested with soybean cyst nematode (Heterodera glycines). Weed Technol. 2006, 20, 1066–1075. [Google Scholar] [CrossRef]
- Mock, V.A.; Creech, J.E.; Ferris, V.R.; Faghihi, J.; Westphal, A.; Santini, J.B.; Johnson, W.G. Influence of winter annual weed management and crop rotation on soybean cyst nematode (Heterodera glycines) and winter annual weeds: Years four and five. Weed Sci. 2012, 60, 634–640. [Google Scholar] [CrossRef]
- Creech, J.E.; Santini, J.B.; Conley, S.P.; Westphal, A.; Johnson, W.G. Purple deadnettle (Lamium purpureum) and soybean cyst nematode response to cold temperature regimes. Weed Sci. 2007, 55, 592–598. [Google Scholar] [CrossRef]
- Webb, J.S. The Influence of Winter Annual Weed Control on Soybean Cyst Nematode and Summer Annual Weed Growth and Management; Southern Illinois University: Carbondale, IL, USA, 2007. [Google Scholar]
- Harrison, S.K.; Venkatesh, R.; Riedel, R.M. Purple deadnettle (Lamium purpureum) emergence and removal time effects on soybean cyst nematode (Heterodera glycines). Weed Sci. 2008, 56, 327–335. [Google Scholar] [CrossRef]
- Nelson, K.A.; Johnson, W.G.; Wait, J.D.; Smoot, R.L. Winter-annual weed management in corn (Zea mays) and soybean (Glycine max) and the impact on soybean cyst nematode (Heterodera glycines) egg population densities. Weed Technol. 2006, 20, 965–970. [Google Scholar] [CrossRef]
- Werle, R.; Bernards, M.L.; Giesler, L.J.; Lindquist, J.L. Influence of two herbicides on soybean cyst nematode (Heterodera glycines) reproduction on henbit (Lamium amplexicaule) roots. Weed Technol. 2013, 27, 41–46. [Google Scholar] [CrossRef]
- Yardirn, E.N.; Edwards, C.A. The effects of chemical pest, disease and weed management practices on the trophic structure of nematode populations in tomato agroecosystems. Appl. Soil Ecol. 1998, 7, 137–147. [Google Scholar] [CrossRef]
- Zhao, J.; Neher, D.A.; Fu, S.; Li, Z.; Wang, K. Non-target effects of herbicides on soil nematode assemblages. Pest Manag. Sci. 2013, 69, 679–684. [Google Scholar] [CrossRef]
- Niblack, T.L.; Bernard, E.C. Plant-parasitic nematode communities in dogwood, maple, and peach nurseries in Tennessee. J. Nematol. 1985, 17, 132–139. [Google Scholar]
- Powell, N. Interactions between nematodes and fungi in disease complexes. Annu. Rev. Phytopathol. 1971, 9, 253–274. [Google Scholar] [CrossRef]
- Mock, V.A. Interaction of Soybean Cyst Nematode with Cropping Practices and Winter Annual Weeds. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2009. [Google Scholar]
- Edgerton, M.D. Increasing crop productivity to meet global needs for feed, food, and fuel. Plant Physiol. 2009, 149, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Creech, J.E.; Johnson, W.G.; Faghihi, J.; Ferris, V.R. Survey of Indiana producers and crop advisors: A perspective on winter annual weeds and soybean cyst nematode (Heterodera glycines). Weed Technol. 2007, 21, 532–536. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).