Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut)
Abstract
1. Introduction
2. Materials and Methods
2.1. Peanut Cultivar and Microbial Strains Used
2.2. Viability of Inoculated Seeds In Vitro
2.3. Greenhouse Studies to Assess the Microorganism Effect on Growth and Development
2.4. Data Analysis
- Germination percentage (%).
- Seedling and plant heights (cm).
- Chlorophyll concentration using SPAD-502 Plus chlorophyll meter manufactured by Konica Minolta Inc., Japan (SPAD Units).
- Dry biomass of the harvested plant parts (g).
- Number of matured pods per pot.
- Number of harvested plants per pot.
- Seed biomass per treatment and 100-seed weight per treatment.
3. Results
3.1. In Vitro Study of Germination of Seeds Inoculated with Bradyrhizobium and Trichoderma Assay 10 Days after Inoculation (DAI)
3.2. Greenhouse Studies
3.2.1. Germination and Seedling Vigor
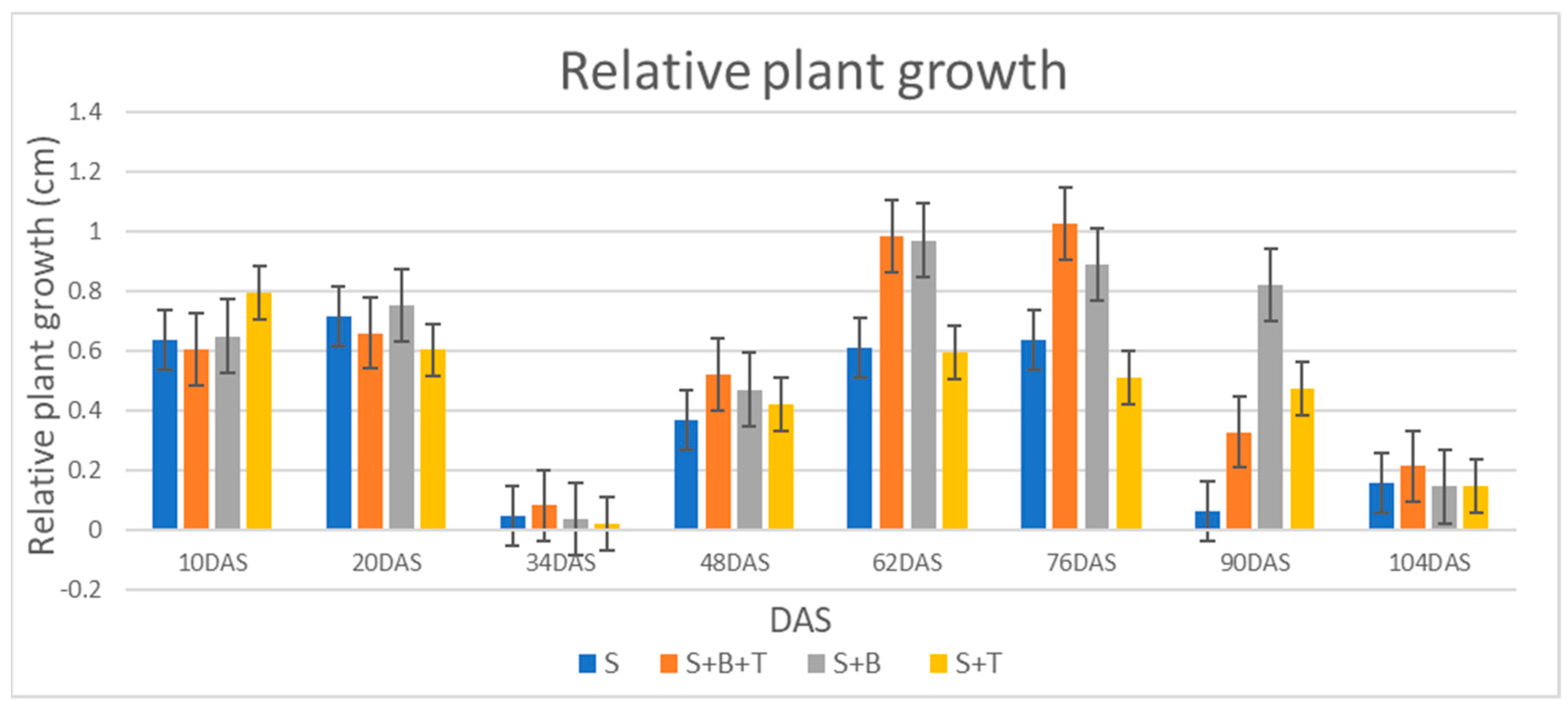
3.2.2. Relative Growth Rate (RGR)
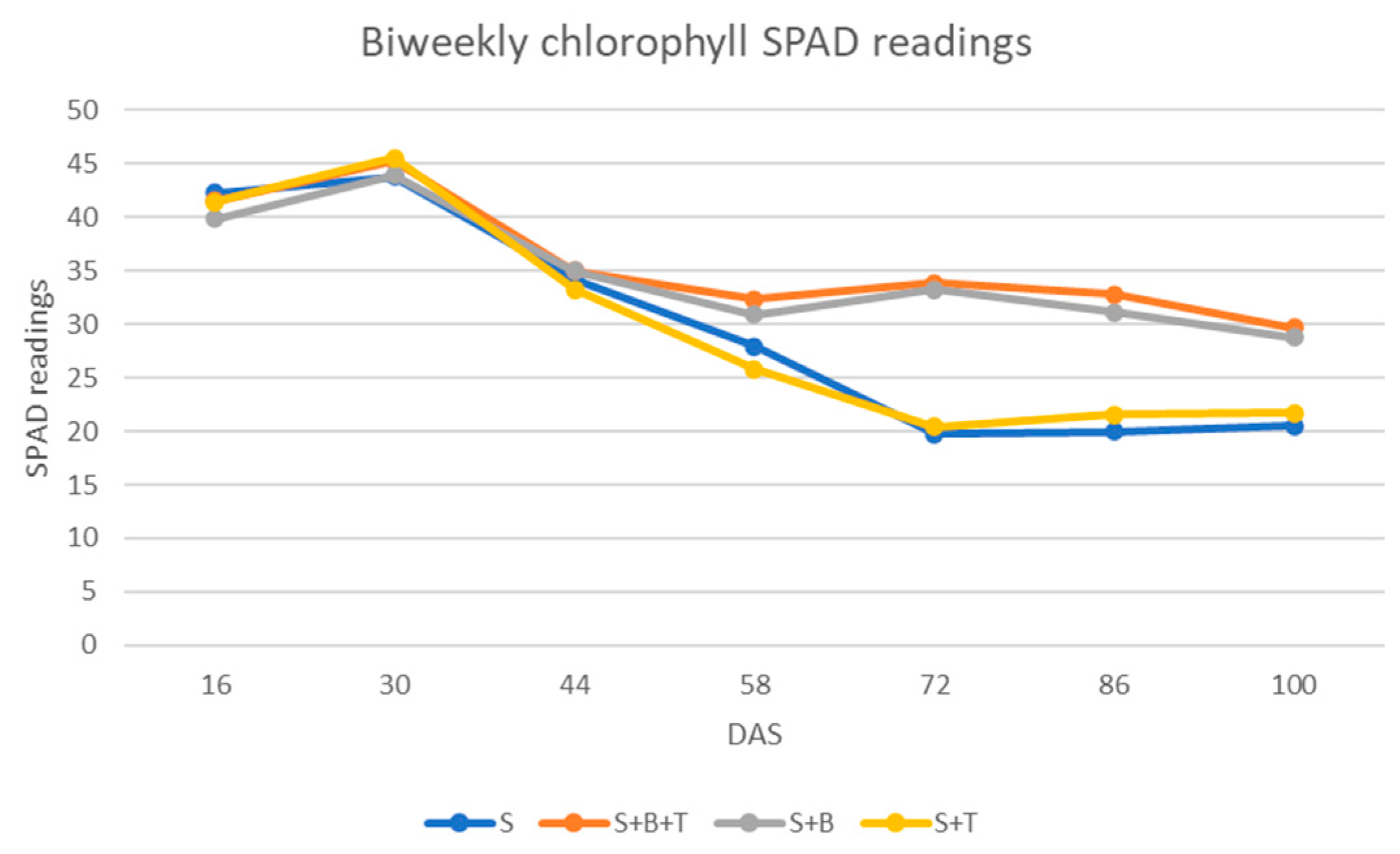
3.2.3. Chlorophyll Content
3.2.4. Effect of Co-Inoculated Microorganisms on Dry Matter and Yield of Peanut Crop
3.2.5. Other Yield Traits: 100 Seed Weight, Shelling Percentage, Seed and Pod Harvest Index (HI)
3.2.6. Interaction of Bradyrhizobium and Trichoderma in Peanut Growth, Development, and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giller, K.E.; Wilson, K.J. Nitrogen Fixation in Tropical Cropping Systems; CAB International: Wallingford, UK, 1991; pp. 167–237. [Google Scholar]
- Graham, P.H.; Vance, C.P. Nitrogen Fixation in Perspective: An Overview of Research and Extension Needs. Field Crops Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- Vance, C.P. Enhanced Agricultural Sustainability through Biological Nitrogen Fixation. In Biological Fixation of Nitrogen for Ecology and Sustainable Agriculture; Legocki, A., Bothe, H., Puhler, A., Eds.; Springer: Berlin, Germany, 1997; pp. 179–186. [Google Scholar]
- Howieson, J.G.; O’Hara, G.W.; Carr, S.J. Changing Roles for Legumes in Mediterranean Agriculture: Developments from an Australian Perspective. Field Crops Res. 2000, 65, 107–122. [Google Scholar] [CrossRef]
- Li, P.D.; Dai, C.C.; Wang, X.X.; Zhang, T.L.; Chen, Y. Variation of Soil Enzyme Activities and Microbial Community Structure in Peanut Monocropping System in Subtropical China. African J. Agric. Res. 2012, 7, 1870–1879. [Google Scholar]
- Castro, S.; Permigiani, M.; Vinocur, M.G.; Fabra, A. Nodulation in peanut (Arachis hypogaea L.) roots in the presence of native and inoculated rhizobia strains. Appl. Soil Ecology. 1999, 13, 39–44. [Google Scholar] [CrossRef]
- Walker, M.E.; Minton, N.A.; Dowler, C.C. Effects of herbicides, a nematicide and Rhizobium inoculant on yield, chemical composition, and nodulation of Starr peanuts (Arachis hypogaea L.). Peanut Sci. 1976, 3, 49–51. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.Q.; Han, X.R.; Yang, J.F.; Liang, C.H.; Zhan, X.M. Autotoxicity of peanut and identification of phytotoxic substances in rhizosphere soil. Allelopath. J. 2013, 31, 297–308. [Google Scholar]
- Daimon, H.; Hori, K.; Shimizu, A.; Nakagawa, M. Nitrate-Induced Inhibition of Root Nodule Formation and Nitrogenase Activity in the Peanut (Arachis hypogaea L.). Plant Prod. Sci. 1999, 2, 81–86. [Google Scholar] [CrossRef][Green Version]
- Daimon, H.; Yoshioka, M. Responses of root nodule formation and nitrogen fixation activity to nitrate in a spit-root system in Peanut (Arachis hypogaea L.). J. Agron. Crop. Sci. 2001, 187, 89–95. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.; Lin, Y.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L. The role of root exudates and allelochemicals in the rhizosphere. Plant. Soil. 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant. Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Isawa, T.; Sameshima, R.; Mitsui, H.; Minamisawa, K. IS1631 occurrence in Bradyrhizobium japonicum highly reiterated sequence-possessing strains with high copy numbers of repeated sequences RSα and RSβ. Appl. Environ. Microbiol. 1999, 65, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Hardarson, G. Methods for enhancing symbiotic nitrogen fixation. Plant. Soil. 1993, 152, 1–17. [Google Scholar] [CrossRef]
- Herridge, D.F.; Marcellos, H.; Felton, W.L.; Turner, G.L.; Peoples, M.B. Legume N2 Fixation an Efficient Source of N for Cereal Production, Nuclear Methods in Soil-Plant Aspects of Sustainable Agriculture (Proc. Sem. Colombo, 1993); IAEA: Vienna, Austria, 1993. [Google Scholar]
- Glazer, N.; Nikaido, H. Fundamentals of applied microbiology. In Microbial Biotechnology, 2nd ed.; Barton, L., Hamilton, A., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 29. [Google Scholar]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Manjula, K.; Singh, S.D.; Kishore, G.K. Role of endophytic bacteria in biological control of plant diseases. Annu. Rev. Plant. Pathol. 2002, 1, 231–252. [Google Scholar]
- Podile, A.R.; Kishore, G.K. Biological control of peanut disease. In Biological Control of Crop Diseases; Gnanamanickam, S.S., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 131–160. [Google Scholar]
- Kubicek, C.P.; Mach, R.L.; Peterbauer, C.K.; Lorito, M. Trichoderma: From genes to biocontrol. J. Plant. Pathol. 2001, 83, 11–23. [Google Scholar]
- Emma, W.G.; Simeon, O.K. The Use of Trichoderma harzianum and T. viride as Potential Biocontrol Agents against Peanut Microflora and Their Effectiveness in Reducing Aflatoxin Contamination of Infected Kernels. Biotechnology 2008, 7, 439–447. [Google Scholar]
- Kishore, G.K.; Pande, S.; Rao, J.N.; Podile, A.R. Biological Control of Crown Rot of Groundnut by Trichoderma Harzianum and T. viride. Int. Arachis Newslett. 2001, 21, 39–40. [Google Scholar]
- Eald, Y.; Barak, R.; Chet, I. Parasitism of sclerotia of Sclerotium rolfsii by Trichoderma harzianum. Soil Biol. Biochem. 1984, 16, 381–386. [Google Scholar] [CrossRef]
- Federico, G.; Rojo, M.M.; Reynoso, M.F.; Sofía, N.; Chulze, A.M.T. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 2007, 26, 549–555. [Google Scholar]
- Neumann, B.; Laing, M. Trichoderma: An ally in the quest for soil system sustainability. In Biological Approaches to Sustainable Soil Systems; Uphoff, N., Ball, A.S., Fernandes, E., Herren, H., Husson, O., Laing, M., Palm, C., Pretty, J., Sanchez, P., Sanginga, N., et al., Eds.; CRC Press, Taylor & Farncis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006; pp. 491–500. [Google Scholar]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.; Harman, G.E. Solubilization of phosphate and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295–22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Windham, M.T.; Elad, Y.; Baker, R. A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology 1986, 76, 518–521. [Google Scholar] [CrossRef]
- Baker, R. Improved Trichoderma spp. for promoting crop productivity. Trends Biotechnol. 1989, 7, 34–38. [Google Scholar] [CrossRef]
- Kleifeld, O.; Chet, I. Trichoderma—Plant interaction and its effect on increased growth response. Plant. Soil 1992, 144, 267–272. [Google Scholar] [CrossRef]
- Inbar, J.; Abramsky, M.; Chet, I. Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings under commercial conditions. Eur. J. Plant. Pathol. 1994, 100, 337–346. [Google Scholar] [CrossRef]
- Horace, G.; Cutler, R.H.; Cox, F.G.; Crumley, P.D.C. 6-Pentyl-α-pyrone from Trichoderma harzianum: Its Plant Growth Inhibitory and Antimicrobial Properties. Agric. Biol. Chem. 1986, 50, 2943–2945. [Google Scholar]
- Thimann, K.V. On the Nature of Inhibitions Caused by Auxin. Am. J. Bot. 1937, 7, 407–412. [Google Scholar] [CrossRef]
- Cleland, R. The dosage-response curve for auxin-induced cell elongation: A reevaluation. Planta 1972, 104, 1–9. [Google Scholar] [CrossRef]
- Brenner, M.L. Modern methods for plant growth substance analysis. Ann. Rev. Plant. Physiol. 1981, 32, 511–538. [Google Scholar] [CrossRef]
- Brussaard, L.; Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosys. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Jansson, J.K.; Trevors, J.T. Modern Soil Microbiology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 177–210. [Google Scholar]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Kong, W.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant. Soil. 2010, 330, 423–433. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Shenker, M.; Oliver, I.; Helmann, M.; Hadar, Y.; Chen, Y. Utilization by tomatoes of iron mediated by a siderophore produced by Rhizopus arrhizus. J. Plant. Nutr. 1992, 15, 2173–2182. [Google Scholar] [CrossRef]
- Zamurrad, M.; Tariq, M.; Shah, F.H.; Subhani, A.; Ijaz, M.; Iqbal, M.S.; Koukab, M. Performance based evaluation of groundnut genotypes under medium rainfall conditions of chakwal. J. Agric. Food Appl. Sci. 2013, 1, 9–12. [Google Scholar]
- Jeyaramraja, P.R.; Woldesenbet, F. Characterization of yield components in certain groundnut (Arachis hypogaea L.) varieties of Ethiopia. J. Exp. Biol. Agric. Sci. 2014, 2, 592–596. [Google Scholar]
- Colby, S. Calculating Synergistic and Antagonistic Responses of Herbicide Combinations. Weeds 1967, 15, 20–22. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Themaat, E.V.L.; Ahmadinejad, N.; Assenza, F. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Chet, I. Induction of defense response in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Nageswara, R.R.C.; Talwar, H.S.; Wright, G.C. Rapid assessment of specific leaf area and leaf nitrogen in peanut (Arachis hypogaea L.) using a chlorophyll meter. J. Agron. Crop. Sci. 2001, 186, 175–182. [Google Scholar] [CrossRef]
- Mekhemar, G.A.A.; Shaaban, M.; Ragab, A.A.; Biomy, A.M.M. Response of faba bean to inoculation with Rhizobium leguminosarum bv. Viceae and plant growth promoting rhizobacteria under newly reclaimed soils. J. Appl. Sci. 2005, 20, 126–144. [Google Scholar]
- Badawi, F.S.F.; Biomy, A.M.M.; Desoky, A.H. Peanut plant growth and yield as influenced by co-inoculation with Bradyrhizobium and some rhizo-microorganisms under sandy loam soil conditions. Ann. Agric. Sci. 2011, 56, 17–25. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostas, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental Growth Conditions of Trichoderma spp. Affects Indole Acetic Acid Derivatives, Volatile Organic Compounds, and Plant Growth Promotion. Front. Plant. Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- McFadden, A.G.; Sutton, J.C. Relationships of populations of Trichoderma spp. in soil to disease in maize. Can. J. Plant. Sci. 1975, 55, 579–586. [Google Scholar] [CrossRef]
- Yang, H.; Powell, N.T.; Baker, K.R. The influence of Trichoderma harzianum on root-knot Fusarium wilt complex in cotton. J. Nematol. 1976, 8, 81–86. [Google Scholar]
- Komon-Zelazowska, M.; Bissett, J.; Zafari, D.; Hatvani, L.; Manczinger, L.; Woo, S.; Lorito, M.; Kredics, L.; Kubicek, C.P.; Druzhinina, I.S. Genetically closely related but phenotypically divergent Trichoderma species cause green mold disease in oyster mushroom farms worldwide. Appl. Environ. Microbiol. 2007, 73, 7415–7426. [Google Scholar] [CrossRef]
- Aly, A.A.; Hussein, E.M.; Allam, A.D.A.; Amein, A.M.; El-Samawaty, A.M.A. Use of Trichoderma spp., Aspergillus spp., and Penicillium spp. to suppress damping-off of cotton seedlings. J. Agri. Sci. Mansoura Univ. 2000, 25, 7611–7619. [Google Scholar]
- Zhang, W.; Wang, H.W.; Wang, X.X.; Xie, X.G.; Siddikee, M.A.; Xu, R.S.; Dai, C.C. Enhanced nodulation of peanut when co-inoculated with fungal endophyte Phomopsis liquidambari and bradyrhizobium. Plant Phys. Biochem. 2016, 98, 1–11. [Google Scholar]

| Parameter 1 | Germination Percentage (%) | Fresh Biomass 2 (g) |
|---|---|---|
| S | 45 | 1.24 b |
| S + B + T | 60 | 1.34 b |
| S + B | 65 | 1.49 b |
| S + T | 70 | 2.04 a |
| Parameter 1 | Germination Percentage (%) | Seedling Vigor Index 2 | ||
|---|---|---|---|---|
| 10 DAS | 20 DAS | 10 DAS | 20 DAS | |
| S | 68.82 | 81.17 | 443.1 a | 1104.6 a |
| S + B + T | 60.80 | 87.65 | 379.9 a | 1118.7 a |
| S + B | 67.90 | 86.41 | 442.8 a | 1214.7 a |
| S + T | 78.70 | 91.67 | 627.9 a | 1284.3 a |
| Treatments | Relative Growth Rate (cm/Day) | Chlorophyll (SPAD Value) |
|---|---|---|
| S 1 | 0.25 b 2 | 29.77 b |
| S + B + T | 0.55 a | 35.77 a |
| S + B | 0.48 a | 34.67 a |
| S + T | 0.30 b | 29.77 b |
| Parameter | S 1 | S + B + T | S + B | S + T |
|---|---|---|---|---|
| Plants survived | 2.69 b 2 | 2.87 a | 2.75 ab | 2.74 b |
| Shoot dry wt/pot (g) | 16.42 b | 19.19 a | 18.50 a | 16.82 b |
| Root dry wt/pot (g) | 5.52 b | 6.78 a | 6.39 a | 5.21 b |
| No. of pods/pot | 2.85 b | 3.98 a | 3.59 a | 2.33 c |
| Pod wt/pot (g) | 3.13 b | 4.00 a | 3.86 a | 2.24 c |
| Seed wt/pot (g) | 1.62 b | 2.00 a | 2.02 a | 1.11 c |
| Shell wt/pot (g) | 1.50 b | 1.99 a | 1.84 a | 1.12 c |
| Parameter | S 1 | S + B + T | S + B | S + T |
|---|---|---|---|---|
| 100 seed wt. | 29.17 a 2 | 30.11 a | 31.96 a | 26.35 a |
| Shelling% | 47.09 a | 45.15 a | 46.98 a | 41.68 a |
| Seed HI | 7.49 a | 8.04 a | 5.35 b | 5.23 b |
| Pod HI | 14.31 a | 15.84 a | 16.06 a | 10.41 b |
| Parameter | S 1 | S + B | S + B%S 2 | S + T | S + T%S 2 | S + B + T | S + B + T%S 2 | ER | Effect 3 |
|---|---|---|---|---|---|---|---|---|---|
| Germination (10 DAS) | 0.68 | 0.67 | −1.47 | 0.78 | 14.71 | 0.60 | −11.76 | 13.45 | A |
| Germination (20 DAS) | 0.80 | 0.85 | 6.25 | 0.91 | 13.75 | 0.87 | 8.75 | 19.14 | A |
| Relative Growth Rate (cm/day) | 0.25 | 0.48 | 92.00 | 0.30 | 20.00 | 0.55 | 120.00 | 93.60 | S |
| Chlorophyll content (SPAD Unit) | 29.77 | 34.67 | 16.46 | 29.77 | 0.00 | 35.77 | 20.15 | 16.46 | S |
| Plant survival per pot | 2.69 | 2.75 | 2.23 | 2.74 | 1.86 | 2.87 | 6.69 | 4.05 | S |
| Shoot dry weight per pot (g) | 16.42 | 18.50 | 12.67 | 16.82 | 2.44 | 19.19 | 16.87 | 14.79 | S |
| Root dry weight per pot (g) | 5.52 | 6.39 | 15.76 | 5.21 | −5.62 | 6.78 | 22.83 | 11.03 | S |
| No. of pods per pot | 2.85 | 3.59 | 25.96 | 2.33 | −18.25 | 3.98 | 39.65 | 12.46 | S |
| Pod dry weight per pot (g) | 3.13 | 3.86 | 23.32 | 2.24 | −28.43 | 4.00 | 27.80 | 1.52 | S |
| Seed dry weight per pot (g) | 1.62 | 2.02 | 24.69 | 1.11 | −31.48 | 2.00 | 23.46 | 0.98 | S |
| Shell dry weight per pot (g) | 1.50 | 1.84 | 22.67 | 1.12 | −25.33 | 1.99 | 32.67 | 3.08 | S |
| 100 seed dry weight (g) | 29.17 | 31.96 | 9.56 | 26.35 | −9.67 | 30.11 | 3.22 | 0.82 | S |
| Shelling % | 47.09 | 46.98 | −0.23 | 41.68 | −11.49 | 45.15 | −4.12 | −11.75 | S |
| Seed harvest index | 7.49 | 5.35 | −28.57 | 5.23 | −30.17 | 8.04 | 7.34 | −67.37 | S |
| Pod harvest index | 14.31 | 16.06 | 12.23 | 10.41 | −27.25 | 15.84 | 10.69 | −11.69 | S |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neelipally, R.T.K.R.; Anoruo, A.O.; Nelson, S. Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut). Agronomy 2020, 10, 1415. https://doi.org/10.3390/agronomy10091415
Neelipally RTKR, Anoruo AO, Nelson S. Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut). Agronomy. 2020; 10(9):1415. https://doi.org/10.3390/agronomy10091415
Chicago/Turabian StyleNeelipally, Ravi Teja Kumar Reddy, Ambrose O. Anoruo, and Shad Nelson. 2020. "Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut)" Agronomy 10, no. 9: 1415. https://doi.org/10.3390/agronomy10091415
APA StyleNeelipally, R. T. K. R., Anoruo, A. O., & Nelson, S. (2020). Effect of Co-Inoculation of Bradyrhizobium and Trichoderma on Growth, Development, and Yield of Arachis hypogaea L. (Peanut). Agronomy, 10(9), 1415. https://doi.org/10.3390/agronomy10091415





