Defining Optimal Strength of the Nutrient Solution for Soilless Cultivation of Saffron in the Mediterranean
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Corm Production
3.2. Fertigation and Uptake Parameters
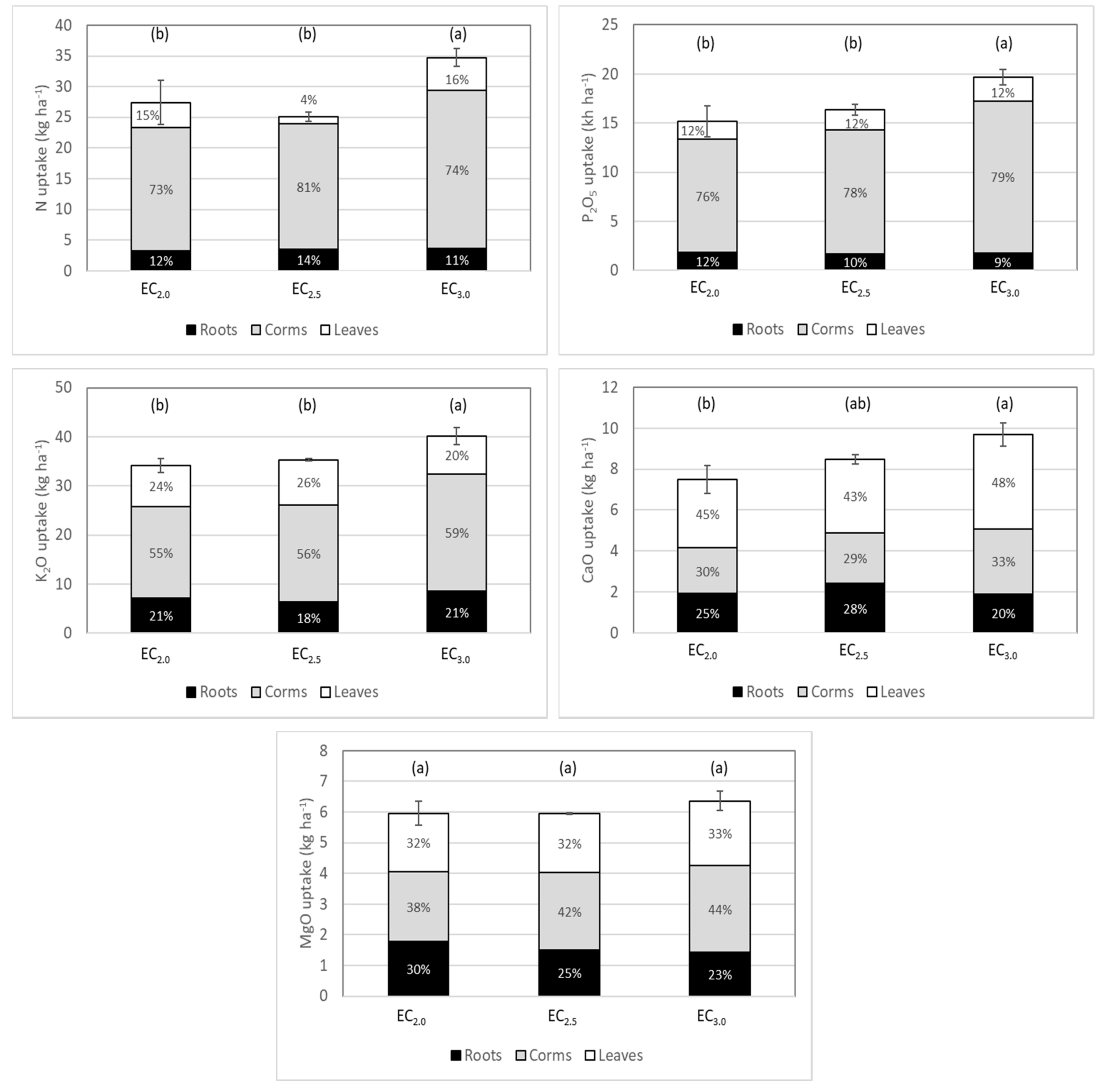
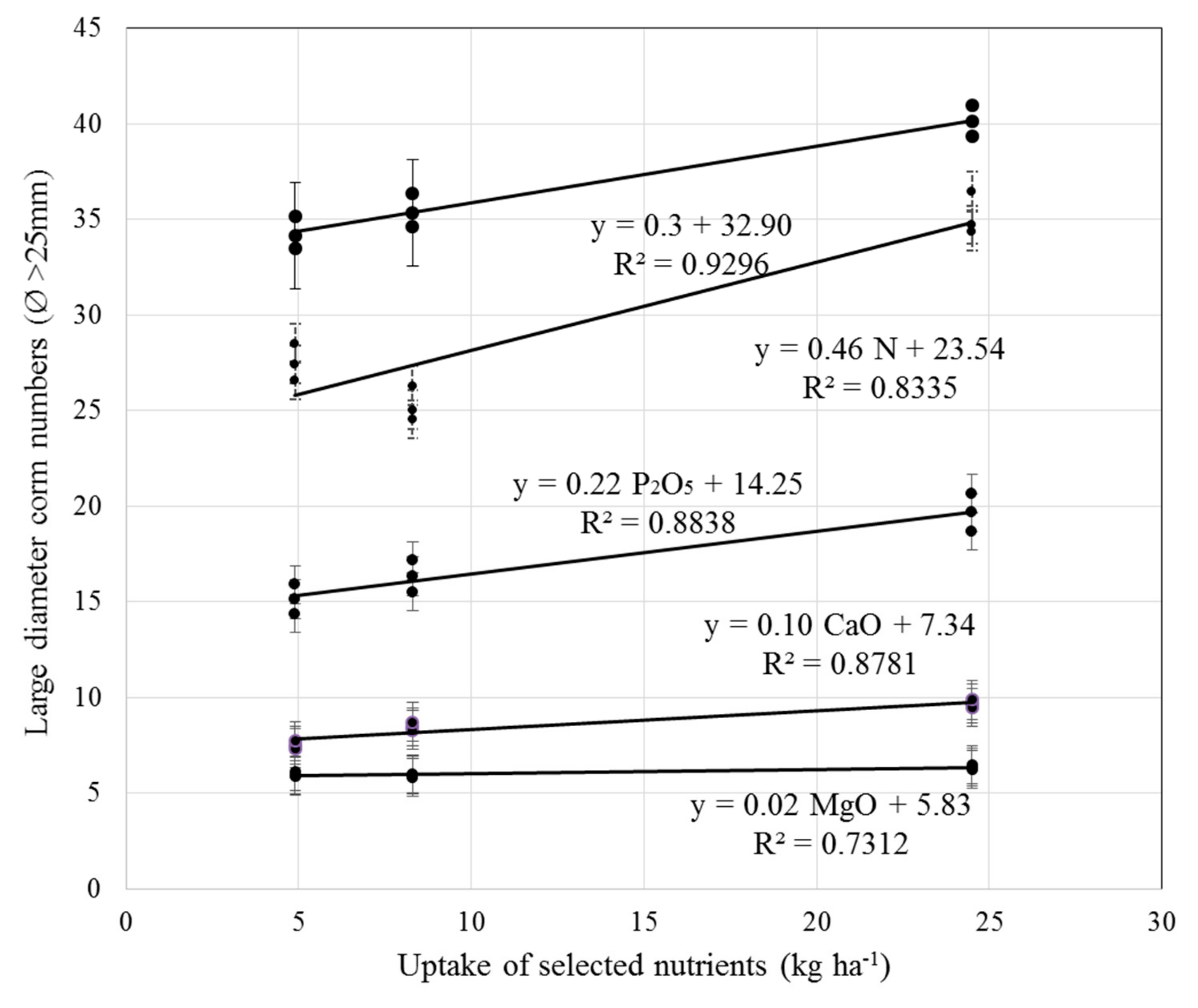
3.3. Plant Analysis and Total Plant Uptake
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gresta, F.; Lombardo, G.M.; Siracusa, L.; Ruberto, G. Saffron, an alternative crop for sustainable agricultural systems. A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef]
- Giupponi, L.; Ceciliani, G.; Leoni, V.; Panseri, S.; Pavlovic, R.; Lingua, G.; Di Filippo, A.; Giorgi, A. Quality traits of saffron produced in Italy: Geographical area effect and good practices. J. Appl. Bot. Food Qual. 2019, 92, 336–342. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An update review of saffron and its active constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar]
- Basker, D.; Negbi, M. Uses of saffron. Econ. Bot. 1983, 37, 228–236. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. phytomedicine 2015, 5, 376–391. [Google Scholar]
- Milajerdi, A.; Djafarian, K.; Hosseini, B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J. Nutr. Intermed. Metab. 2016, 3, 23–32. [Google Scholar] [CrossRef]
- Abdullaev, F.; Espinosa-Aguirre, J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004, 28, 426–432. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.A.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. ORIGINAL ARTICLE: Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, G.M.; Siracusa, L.; Ruberto, G. Effect of mother corm dimension and sowing time on stigma yield, daughter corms and qualitative aspects of saffron (Crocus sativus L.) in a Mediterranean environment. J. Sci. Food Agric. 2008, 88, 1144–1150. [Google Scholar] [CrossRef]
- Alonso, G.L.; Arghittu, A.; Astraka, K.; Betza, T.; Camba, E.; Cañadas Sanchez, W.; Carmona Delgado, M.; Cilloco, M.T.; Corona, J.; Curreli, M.; et al. White Book, Saffron in Europe. In Problems and Strategies for Improving the Quality and Strengthen Competitiveness; ALEXANDROS-LLC: Athens, Greece, 2007. [Google Scholar]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- De Juan, J.A.; Córcoles, H.L.; Muñoz, R.M.; Picornell, M.R. Yield and yield components of saffron under different cropping systems. Ind. Crop. Prod. 2009, 30, 212–219. [Google Scholar] [CrossRef]
- Fernández, J.A. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant Sci. 2004, 2, 127–159. [Google Scholar]
- Poggi, L.M. Problemáticas y nuevas perspectivas tecnológicas para la producción de azafrán. Hort. Argent. 2008, 28, 39–62. (In Spanish) [Google Scholar]
- Molina, R.V.; Valero, M.; Navarro, Y.; Guardiola, J.; García-Luis, A. Temperature effects on flower formation in saffron (Crocus sativus L.). Sci. Hortic. 2005, 103, 361–379. [Google Scholar] [CrossRef]
- Gresta, F.; Avola, G.; Lombardo, G.; Siracusa, L.; Ruberto, G. Analysis of flowering, stigmas yield and qualitative traits of saffron (Crocus sativus L.) as affected by environmental conditions. Sci. Hortic. 2009, 119, 320–324. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.; Ahuja, P. State of Art of Saffron (Crocus sativus L.) Agronomy: A Comprehensive Review. Food Rev. Int. 2008, 25, 44–85. [Google Scholar] [CrossRef]
- Landi, R. Lo zafferano: Tradizione e tipicità; Camera di Commercio Industria, Artigianato e Agricultura di Firenze: Firenze, Italy, 2007. [Google Scholar]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular Mycorrhizal Fungi Modulate the Crop Performance and Metabolic Profile of Saffron in Soilless Cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Gresta, F.; Santonoceto, C.; Avola, G. Crop rotation as an effective strategy for saffron ( Crocus sativus L.) cultivation. Sci. Hortic. 2016, 211, 34–39. [Google Scholar] [CrossRef]
- Molina, R.V.; García-Luis, A.; Valero, M.; Navarro, Y.; Guardiola, J. Extending the harvest period of saffron. Acta Hortic. 2004, 650, 219–225. [Google Scholar] [CrossRef]
- Rubio Terrado, P. El azafrán. Aspectos socioeconómicos y culturales. Stud.: Rev. Humanid. 2007, 13, 199–228. [Google Scholar]
- Perez-Vidal, C.; Gracia, L. Computer based production of Saffron (Crocus sativus L.): From mechanical design to electronic control. Comput. Electron. Agric. 2020, 169. [Google Scholar] [CrossRef]
- Torricelli, R.; Yousefi Javan, I.; Albertini, E.; Venanzoni, R.; Hosseinzadeh, Y.G. Morphological and molecular characterization of Italian, Iranian and Spanish saffron (Crocus sativus L.) accessions. Appl. Ecol. Environ. Res. 2019, 17, 1875–1887. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; De Pascale, S. Soilless cultivation of saffron in Mediterranean environment. Acta Hortic. 2006, 718, 515–522. [Google Scholar]
- Souret, F.F.; Weathers, P.J. The Growth of Saffron (Crocus sativus L.) in Aeroponics and Hydroponics. J. Herbs Spices Med. Plants 2000, 7, 25–35. [Google Scholar] [CrossRef]
- Diaz, J.G.; Salas, M.C.; Guzman, M. Soilless production of saffron (Crocus sativus L). In Proceedings of the VII Congreso Iberico de Agroingenieria y Ciencias Horticolas, Madrid, Spain, 26–29 August 2013; pp. 1400–1405. [Google Scholar]
- Putra, P.A.; Yuliando, H. Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Kamgar-Haghighi, A.A. Saffron irrigation regime. Int. J. Plant Prod. 2009, 3, 1–16. [Google Scholar]
- Dastranj, M.; Sepaskhah, A. Saffron response to irrigation regime, salinity and planting method. Sci. Hortic. 2019, 251, 215–224. [Google Scholar] [CrossRef]
- Avarseji, Z.; Kafi, M.; Teimouri, M.S.; Orooji, K. Investigation of salinity stress and potassium levels on morphophysiological characteristics of saffron. J. Plant Nutr. 2013, 36, 299–310. [Google Scholar] [CrossRef]
- Azafrán: La especia preferida por faraones y reyes que fortalece el corazón. Available online: https://www.abc.es/espana/la-rica-espana/20150331/abci-azafran-castilla-mancha-aragon-201503261046.html (accessed on 9 June 2020).
- Mollafilabi, A.; Koocheki, A.; Moeinerad, H.; Kooshki, L. Effect of plant density and weight of corm on yield and yield components of saffron (Crocus sativus L.) under soil, hydroponic and plastic tunnel cultivation. Acta Hortic. 2013, 997, 51–58. [Google Scholar]
- Ghanbari, J.; Khajoei-Nejad, G.; Van Ruth, S.M. Effect of saffron (Crocus sativus L.) corm provenance on its agro-morphological traits and bioactive compounds. Sci. Hortic. 2019, 256, 256. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient Solutions for Soilless Cultures. In Plant Nutrition of Greenhouse Crops; Springer Science and Business Media LLC: New York, NY, USA, 2009; pp. 257–275. [Google Scholar]
- Yatoo, A.R.; Hasan, B.; Shah, M.H.; Bali, A.S. Growth and productive performance of saffron (Crocus sativus L.) as influenced by corm size, FYM and fertility levels. Proceedings of National symposium on Saffron, Regional Research Laboratory, Jammu, India, 25–26 November 1999. [Google Scholar]
- Kempen, E.; Agenbag, G.A.; Deckers, S. Variations in water and macronutrient uptake of soilless tomato as affected by the nutrient solution composition. S. Afr. J. Plant Soil 2016, 34, 139–148. [Google Scholar] [CrossRef]
- Elgabaly, M.M. On the mechanism of anion uptake by plant roots. Effect of the dominant cation in the root on chloride uptake by excised barley roots. Plant Soil 1962, 16, 157–164. [Google Scholar]
- Koocheki, A.; Seyyedi, S.M. Relationship between nitrogen and phosphorus use efficiency in saffron (Crocus sativus L.) as affected by mother corm size and fertilization. Ind. Crop. Prod. 2015, 71, 128–137. [Google Scholar] [CrossRef]

| Shoot Length | Corm Yield | Corm Number | Corm Number by Diameter Class | ||||
|---|---|---|---|---|---|---|---|
| (cm) | (g m−2) | N m−2 | >25 mm | 20–25 mm | <20 mm | ||
| EC2.0 | 41.9 | 455.3 | 245.1 b | 4.9 b | 39.2 a | 201.0 | |
| EC2.5 | 42.3 | 426.4 | 284.1 a | 8.3 b | 27.6 ab | 248.2 | |
| EC3.0 | 43.9 | 469.3 | 250.0 b | 24.5 a | 17.2 b | 208.3 | |
| ns | ns | * | * | * | ns | ||
| Na+ | NH4+ | K+ | Ca2+ | Mg2+ | Cl− | NO3− | H2PO4− | SO42− | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation (mg·L−1) | EC2.0 | 94 | 19 c | 92 c | 191 c | 40 c | 188 | 647 c | 120 c | 135 c | |
| EC2.5 | 97 | 29 b | 136 b | 262 b | 47 b | 174 | 882 b | 144 b | 155 b | ||
| EC3.0 | 91 | 37 a | 207 a | 346 a | 54 a | 172 | 1217 a | 183 a | 196 a | ||
| ns | * | * | * | * | ns | * | * | * | |||
| Drainage (mg·L−1) | EC2.0 | 133 | 12 c | 115 c | 227 | c | 45 b | 243 | 714 c | 114 c | 110 c |
| EC2.5 | 130 | 22 b | 150 b | 273 | b | 53 a | 254 | 921 b | 145 b | 142 b | |
| EC3.0 | 116 | 28 a | 191 a | 341 | a | 54 a | 275 | 1220 a | 201 a | 169 a | |
| ns | * | * | * | * | ns | * | * | * | |||
| Uptake (mg m2 day−1) | EC2.0 | 71 | 68 | 148 c | 328 | c | 44 b | 130 b | 863 | 257 b | 368 b |
| EC2.5 | 89 | 84 | 326 b | 427 | b | 45 b | 118 b | 826 | 261 b | 335 b | |
| EC3.0 | 111 | 86 | 456 a | 586 | a | 78 a | 186 a | 838 | 460 a | 499 a | |
| ns | ns | * | * | * | * | ns | * | * | |||
| N | P- | K | Ca | Mg | ||
|---|---|---|---|---|---|---|
| Whole plant | EC2.0 | 0.81 | 0.19 a | 0.98 b | 0.23 b | 0.15 |
| EC2.5 | 0.76 | 0.17 b | 0.96 b | 0.27 a | 0.13 | |
| EC3.0 | 0.83 | 0.19 a | 1.06 a | 0.28 a | 0.14 | |
| ns | * | * | * | ns | ||
| EC2.0 | 0.77 | 0.19 a | 1.39 b | 0.32 ab | 0.25 a | |
| Roots | EC2.5 | 0.74 | 0.15 b | 1.12 c | 0.36 a | 0.19 b |
| EC3.0 | 0.81 | 0.17 ab | 1.57 a | 0.30 b | 0.19 b | |
| ns | * | * | * | * | ||
| EC2.0 | 0.88 | 0.22 b | 0.68 | 0.07 | 0.06 | |
| Corms | EC2.5 | 0.81 | 0.22 b | 0.65 | 0.07 | 0.06 |
| EC3.0 | 0.91 | 0.24 a | 0.70 | 0.08 | 0.07 | |
| ns | * | ns | ns | ns | ||
| EC2.0 | 0.78 | 0.15 a | 0.88 b | 0.31 c | 0.15 b | |
| Leaves | EC2.5 | 0.74 | 0.13 b | 1.12 a | 0.38 b | 0.17 ab |
| EC3.0 | 0.77 | 0.15 a | 0.92 b | 0.47 a | 0.18 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas, M.d.C.; Montero, J.L.; Diaz, J.G.; Berti, F.; Quintero, M.F.; Guzmán, M.; Orsini, F. Defining Optimal Strength of the Nutrient Solution for Soilless Cultivation of Saffron in the Mediterranean. Agronomy 2020, 10, 1311. https://doi.org/10.3390/agronomy10091311
Salas MdC, Montero JL, Diaz JG, Berti F, Quintero MF, Guzmán M, Orsini F. Defining Optimal Strength of the Nutrient Solution for Soilless Cultivation of Saffron in the Mediterranean. Agronomy. 2020; 10(9):1311. https://doi.org/10.3390/agronomy10091311
Chicago/Turabian StyleSalas, María del Carmen, José Luis Montero, José Gregorio Diaz, Francesca Berti, María F. Quintero, Miguel Guzmán, and Francesco Orsini. 2020. "Defining Optimal Strength of the Nutrient Solution for Soilless Cultivation of Saffron in the Mediterranean" Agronomy 10, no. 9: 1311. https://doi.org/10.3390/agronomy10091311
APA StyleSalas, M. d. C., Montero, J. L., Diaz, J. G., Berti, F., Quintero, M. F., Guzmán, M., & Orsini, F. (2020). Defining Optimal Strength of the Nutrient Solution for Soilless Cultivation of Saffron in the Mediterranean. Agronomy, 10(9), 1311. https://doi.org/10.3390/agronomy10091311










