Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Archives
2.1.1. ISE Samples for the Comparison of XRF Sample Preparation Methods and Calibrations
2.1.2. Samples for Matching Library Creation
2.1.3. Assessing Impact of Soil Texture—Irish Tillage Soils Archive
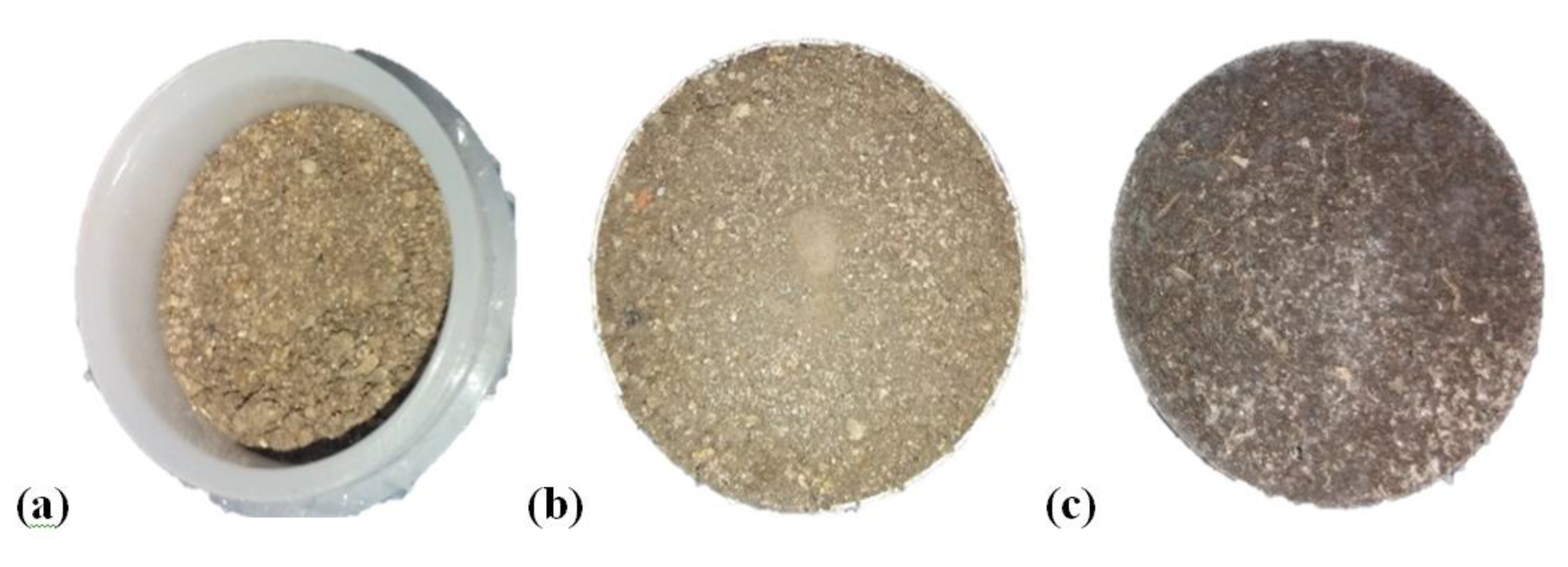
2.2. Sample Preparation for EDXRF Analysis
2.3. EDXRF Analysis
2.4. ICP-OES Analysis
2.5. Data Analysis
3. Results and Discussion
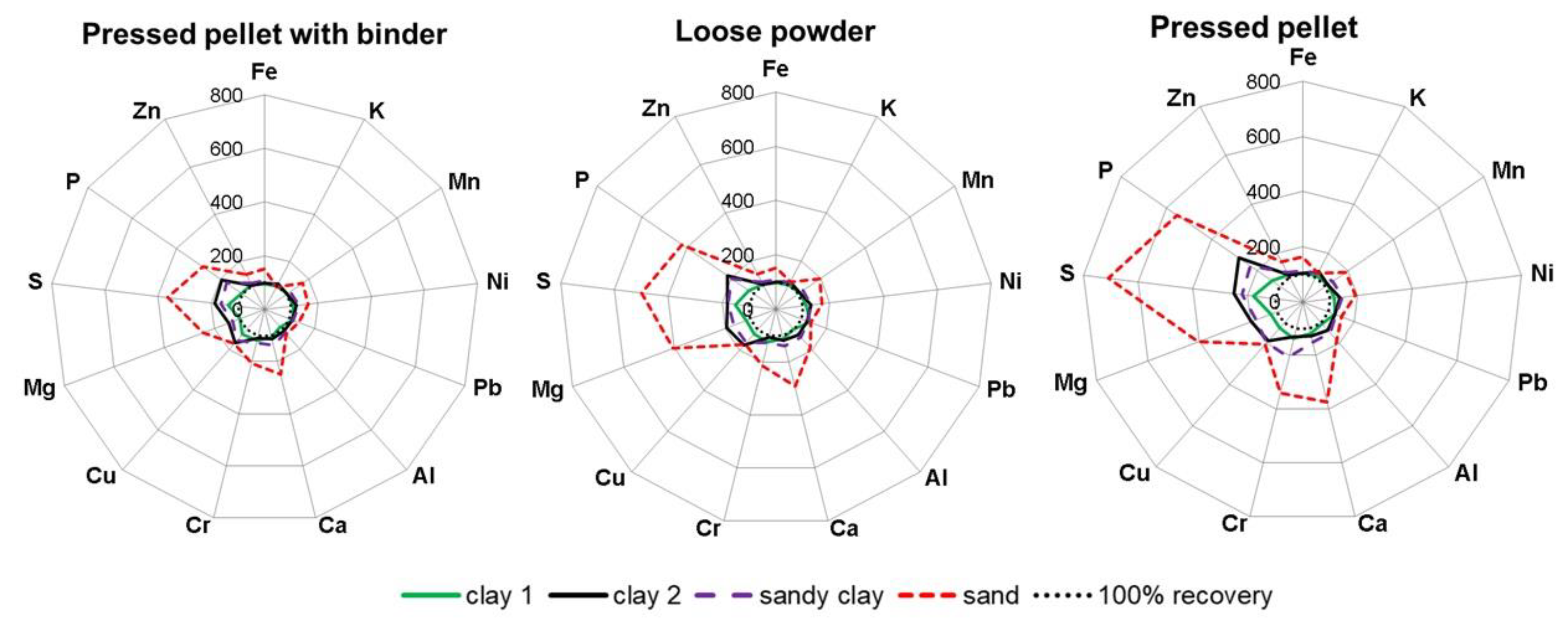
3.1. Effects of Sample Preparation on EDXRF Analytical Results
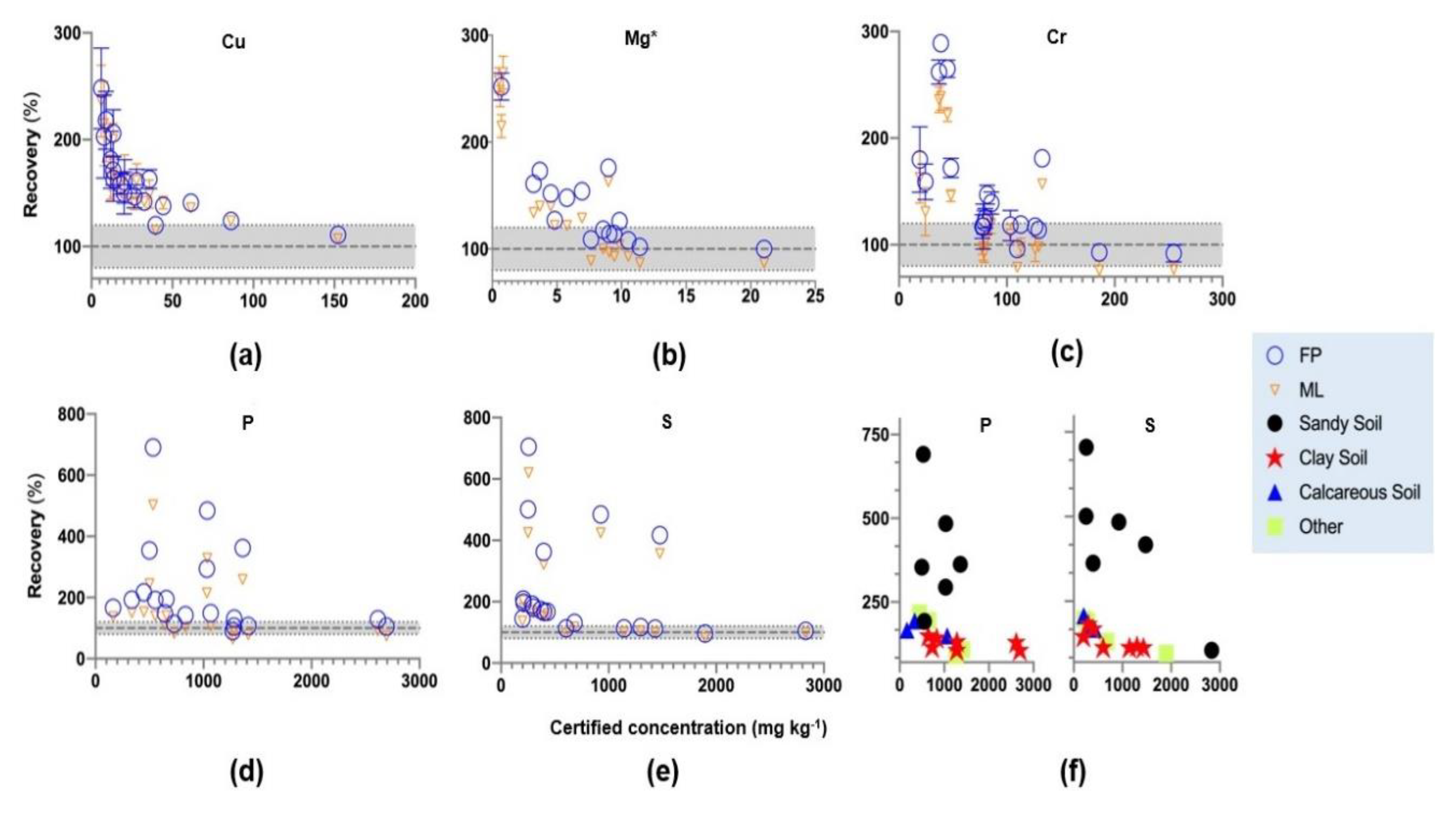
3.2. Influence of Matching Library on EDXRF Results Using ISE Samples
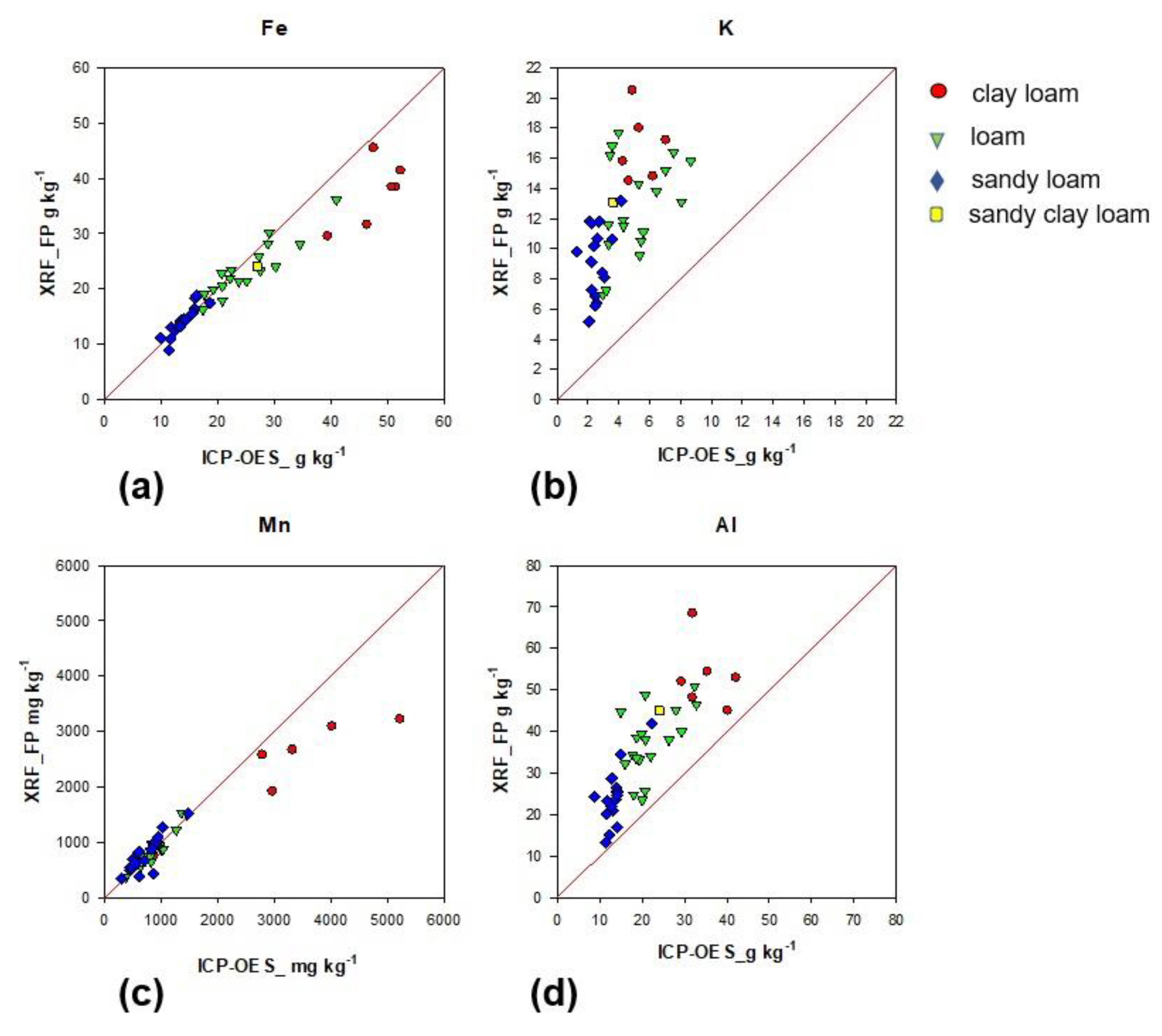
3.3. Particle Size Effects on Fe, K, Mn, and Al Determined Using EDXRF Analysis in Tillage Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Certified Reference Materials | ||||
|---|---|---|---|---|
| Element | GBW07403 | GBW07405 | ISE 921 | BAM-U110 |
| Al | 64,800 | 114,247 | 56,800 ± 1440 | * 50,382 ± 1081 |
| Ca | 9071 | 679 | 43,000 ± 1180 | * 40,638 ± 2105 |
| Cu | 11.4 | 144 | 93.8 ± 5.84 | 263 ± 12 |
| Cr | 32 | 118 | 130 ± 10.6 | 230 ± 13 |
| Fe | 13,989 | 88,269 | 31,900 ± 1130 | * 28,229 ± 663 |
| K | 25,226 | 12,447 | 19,100 ± 640 | * 20,381 ± 4560 |
| Mg | 3498 | 3679 | 11,100 ± 370 | * 8380 ± 735 |
| Mn | 304 | 1360 | 1190 ± 43 | 621 ± 20 |
| Ni | 12 | 40 | 42.4 ± 3.21 | 101 ± 5 |
| P | 320 | 390 | 1400 ± 69 | * 3648 ± 49 |
| Pb | 26 | 552 | 167 ± 7.5 | 197 ± 14 |
| S | 120 | 410 | 702 ± 79.8 | * 10,593 |
| Si | 349,290 | 245,746 | 271,000 ± 4600 | * 258,125 |
| Zn | 31 | 494 | 522 ± 19.9 | 1000 ± 50 |
| Element | Voltage (kv) | Secondary Target | Peak Detected (KeV) | Measurement Time (s) |
|---|---|---|---|---|
| Cu | 50 | Mo | Kα, 8.047 | 100 |
| Cr | 50 | Cu | Kα, 5.414 | 100 |
| Mn | 50 | Mo | Kα, 5.898 | 100 |
| Ni | 50 | Mo | Kα, 7.477 | 100 |
| Zn | 50 | Mo | Kα, 8.638 | 100 |
| Pb | 50 | Mo | Lβ1, 12.611 | 100 |
| Al | 25 | Rx9 | Kα, 1.487 | 100 |
| S | 25 | Rx9 | Kα, 2.308 | 100 |
| P | 25 | Rx9 | Kα, 2.015 | 100 |
| Mg | 25 | Si | Kα,1.254 | 300 |
| Ca | 50 | Cu | Kα, 3.691 | 100 |
| K | 50 | Cu | Kα, 3.313 | 100 |
| Fe | 50 | Mo | Kα, 6.403 | 100 |
| Elements | Concentration Threshold (mg kg−1) | Average Recovery Including Sand (%) | Average Recovery (%) Minus Sand | Calibration Method |
|---|---|---|---|---|
| Al | 40760 | - | 106 ± 10.2 (n = 12) | ML |
| Ca | 3750 | 120 ± 29 (n = 17) | 106 ± 12 (n = 14) | ML |
| Cr | 77.3 | 101 ± 21 (n = 14) | 97 ± 14.9 (n = 12) | ML |
| Cu | 39.66 | 125 ± 14 (n = 5) | 122 ± 14.5 (n = 4) | ML |
| Fe | 6040 | 106 ± 10.4 (n = 17) | 102 ± 4.5 (n = 14) | FP |
| K | 5988 | 108 ± 10.1 (n = 20) | - | FP |
| Mg | 4844 | 107 ± 22.8 (n = 12) | 102 ± 15.3 (n = 11) | ML |
| Mn | 238 | 114 ± 18.3 (n = 15) | 107 ± 4.9 (n = 13) | FP |
| Ni | 21.27 | 117 ± 11.9 (n = 14) | 113 ± 5.8 (n = 12) | ML |
| P | 727 | 130 ± 87.04 (n = 12) | 85 ± 14.17 (n = 9) | ML |
| Pb | 19.16 | 113 ± 6.2 (n = 16) | - | ML |
| S | 603.5 | 164 ± 130 (n = 9) | 100 ± 10 (n = 7) | ML |
| Zn | 56.45 | 109 ± 7.3 (n = 13) | 107 ± 3.6 (n = 12) | FP |

References
- Pyle, S.M.; Nocerino, J.M.; Deming, S.N.; Palasota, J.A.; Palasota, J.M.; Miller, E.L.; Hillman, D.C.; Kuharic, C.A.; Cole, W.H.; Fitzpatrick, P.M.; et al. Comparison of AAS, ICP-AES, PSA, and XRF in determining lead and cadmium in soil. Environ. Sci. Technol. 1996, 30, 204–213. [Google Scholar] [CrossRef]
- Gäbler, H.-E. Mobility of heavy metals as a function of pH of samples from an overbank sediment profile contaminated by mining activities. J. Geochem. Explor. 1997, 58, 185–194. [Google Scholar] [CrossRef]
- Correia, A.G.; Da Silva, R.J.B.; Pedra, F.; Nunes, J. Assessment of the determination of heavy metals in organic soil improvers by ICP-OES. Accredit. Qual. Assur. 2014, 19, 87–97. [Google Scholar] [CrossRef]
- Kilbride, C.; Poole, J.; Hutchings, T. A comparison of Cu, Pb, As, Cd, Zn, Fe, Ni and Mn determined by acid extraction/ICP-OES and ex situ field portable X-ray fluorescence analyses. Environ. Pollut. 2006, 143, 16–23. [Google Scholar] [CrossRef]
- Shuttleworth, E.; Evans, M.; Hutchinson, S.M.; Rothwell, J.J. Assessment of lead contamination in peatlands using field portable XRF. Water Air Soil Pollut. 2014, 225, 1844. [Google Scholar] [CrossRef]
- USEPA. Method 3052 Microwave Assisted Acid Digestion of Sileceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- USEPA. Method 3050b Acid Digestion of Soils, Sediments and Sludges, Revision 2 ed; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- Sastre, J.; Sahuquillo, Á.; Vidal, M.; Rauret, G. Determination of Cd, Cu, Pb and Zn in environmental samples: Microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal. Chim. Acta 2002, 462, 59–72. [Google Scholar] [CrossRef]
- Hassan, N.M.; Rasmussen, P.E.; Dabek-Zlotorzynska, E.; Celo, V.; Chen, H. Analysis of environmental samples using microwave-assisted acid digestion and inductively coupled plasma mass spectrometry: Maximizing total element recoveries. Water Air Soil Pollut. 2007, 178, 323–334. [Google Scholar] [CrossRef]
- Quevauviller, P.; Van Der Sloot, H.; Ure, A.; Muntau, H.; Gómez, A.; Rauret, G. Conclusions of the workshop: Harmonization of leaching/extraction tests for environmental risk assessment. Sci. Total Environ. 1996, 178, 133–139. [Google Scholar] [CrossRef]
- Chen, M.; Ma, L.Q. Comparison of three aqua regia digestion methods for twenty florida soils. Soil Sci. Soc. Am. J. 2001, 65, 491–499. [Google Scholar] [CrossRef]
- Anderson, P.; Davidson, C.M.; Littlejohn, D.; Ure, A.M.; Garden, L.M.; Marshall, J. Comparison of techniques for the analysis of industrial soils by atomic spectrometry. Int. J. Environ. Anal. Chem. 1998, 71, 19–40. [Google Scholar] [CrossRef]
- Kalnicky, D.J.; Singhvi, R. Field portable XRF analysis of environmental samples. J. Hazard. Mater. 2001, 83, 93–122. [Google Scholar] [CrossRef]
- Wu, C.-M.; Tsai, H.-T.; Yang, K.-H.; Wen, J.-C. How reliable is X-Ray fluorescence (XRF) measurement for different metals in soil contamination? Environ. Forensics 2012, 13, 110–121. [Google Scholar] [CrossRef]
- Thomas, C.L.; Acquah, G.E.; Whitmore, A.P.; McGrath, S.P.; Haefele, S.M. The effect of different organic fertilizers on yield and soil and crop nutrient concentrations. Agronomy 2019, 9, 776. [Google Scholar] [CrossRef]
- Melquiades, F.L.; Appoloni, C.R. Application of XRF and field portable XRF for environmental analysis. J. Radioanal. Nucl. Chem. 2004, 262, 533–541. [Google Scholar] [CrossRef]
- Rouillon, M.; Taylor, M.P. Can field portable X-ray fluorescence (pXRF) produce high quality data for application in environmental contamination research? Environ. Pollut. 2016, 214, 255–264. [Google Scholar] [CrossRef]
- Tavares, T.R.; Mouazen, A.M.; Alves, E.E.N.; Dos Santos, F.R.; Melquiades, F.L.; De Carvalho, H.W.P.; Molin, J.P. Assessing soil key fertility attributes using a portable X-ray fluorescence: A simple method to overcome matrix effect. Agronomy 2020, 10, 787. [Google Scholar] [CrossRef]
- Markowicz, A. An overview of quantification methods in energy-dispersive X-ray fluorescence analysis. Pramana 2011, 76, 321–329. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Cadisch, G. Quantification of total element concentrations in soils using total X-ray fluorescence spectroscopy (TXRF). Sci. Total Environ. 2013, 463, 374–388. [Google Scholar] [CrossRef]
- Willis, J.P.; Feather, C.E.; Turner, K. Guidelines for XRF Analysis: Setting up Programmes for WDXRF and EDXRF, 1st ed.; James Willis Consultants cc: Cape Town, South Africa, 2014; Chapter 2–16. [Google Scholar]
- USEPA. Method 6200 Field Portable X-ray Fluorescence Spectrometry for Determination of Elemental Concentrations in Soil and Sediment; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Mejia-Piña, K.; Huerta-Diaz, M.A.; González-Yajimovich, O. Calibration of handheld X-ray fluorescence (XRF) equipment for optimum determination of elemental concentrations in sediment samples. Talanta 2016, 161, 359–367. [Google Scholar] [CrossRef]
- Bernick, M.; Kalnicky, D.; Prince, G.; Singhvi, R. Results of field-portable X-ray fluorescence analysis of metal contaminants in soil and sediment. J. Hazard. Mater. 1995, 43, 101–110. [Google Scholar] [CrossRef]
- Daly, K.; Fenelon, A. A rapid and multi-element method for the analysis of major nutrients in grass (Lolium perenne) using energy-dispersive X-ray fluorescence spectroscopy. Ir. J. Agric. Food Res. 2017, 56, 1–11. [Google Scholar] [CrossRef]
- Rousseau, R.M.; Willis, J.P.; Duncan, A.R. Pratical XRF calibration procedures for major and trace elements. X-ray Spectrom. 1996, 25, 179–189. [Google Scholar] [CrossRef]
- Metzger, K.; Zhang, C.; Ward, M.; Daly, K. Mid-infrared spectroscopy as an alternative to laboratory extraction for the determination of lime requirement in tillage soils. Geoderma 2020, 364, 114171. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size analysis in. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods; Part 2. Chemical and Microbiological Properties; Klute, A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1986. [Google Scholar]
- Shibata, Y.; Suyama, J.; Kitano, M.; Nakamura, T. X-ray fluorescence analysis of Cr, As, Se, Cd, Hg, and Pb in soil using pressed powder pellet and loose powder methods. X-ray Spectrom. 2009, 38, 410–416. [Google Scholar] [CrossRef]
- Takahashi, G. Sample preparation for X-ray fluorescence analysis III. Pressed and loose powder methods. Rigaku J. 2015, 31, 25–30. [Google Scholar]
- Willis, J.P.; Turner, K.; Pritchard, G. XRF in the Workplace: A Guide to Pratical XRF Spectrometry; PANalytical Australia: Chipping Norton, Australia, 2011. [Google Scholar]
- Omote, J.; Kohno, H.; Toda, K. X-Ray fluorescence analysis utilizing the fundamental parameter method for the determination of the elemental composition in plant samples. Anal. Chim. Acta 1995, 307, 117–126. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kawahara, N.; Hara, S.; Yamada, Y.; Matsuo, T.; Mantler, M. Fundamental Parameter Method Using Scattering X-rays in X-ray Fluorescence Analysis; CPDS-International Centre for Diffraction Data: Newtown Township, PA, USA; Denver, CO, USA, 2006; pp. 255–260, ISSN 1097-0002. [Google Scholar]
- Singh, V.; Agrawal, H. Qualitative soil mineral analysis by EDXRF, XRD and AAS probes. Radiat. Phys. Chem. 2012, 81, 1796–1803. [Google Scholar] [CrossRef]
- Maruyama, Y.; Ogawa, K.; Okada, T.; Kato, M. Laboratory experiments of particle size effect in X-ray fluorescence and implications to remote X-ray spectrometry of lunar regolith surface. Earth Planets Space 2008, 60, 293–297. [Google Scholar] [CrossRef]
- Kadachi, A.N.; Al-Eshaikh, M.A. Limits of detection in XRF spectroscopy. X-ray Spectrom. 2012, 41, 350–354. [Google Scholar] [CrossRef]
- Fees, S. (Applied Rigaku Technologies, Austin, TX, USA). Personal communication, 2019.
- ISO. Soil Quality, Extraction of Trace Elements Soluble in Aqua Regia; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hedayat, A.S.; Sinha, B.; Yang, M. Statistical methods in assessing agreement. J. Am. Stat. Assoc. 2002, 97, 257–270. [Google Scholar] [CrossRef]
- Sacristán, D.; Viscarra-Rossel, R.; Recatalá, L. Proximal sensing of Cu in soil and lettuce using portable X-ray fluorescence spectrometry. Geoderma 2016, 265, 6–11. [Google Scholar] [CrossRef]
- Parsons, C.T.; Grabulosa, E.M.; Pili, E.; Floor, G.H.; Román-Ross, G.; Charlet, L. Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: Considerations for sample preparation and measurement conditions. J. Hazard. Mater. 2013, 262, 1213–1222. [Google Scholar] [CrossRef]
- Horta, A.; Malone, B.P.; Stockmann, U.; Minasny, B.; Bishop, T.; McBratney, A.B.; Pallasser, R.; Pozza, L. Potential of integrated field spectroscopy and spatial analysis for enhanced assessment of soil contamination: A prospective review. Geoderma 2015, 241, 180–209. [Google Scholar] [CrossRef]
- Ichikawa, S.; Nakamura, T. Approaches to solid sample preparation based on analytical depth for reliable X-ray fluorescence analysis. X-ray Spectrom. 2016, 45, 302–307. [Google Scholar] [CrossRef]
- Potts, P.J.; Williams-Thorpe, O.; Webb, P.C. The bulk analysis of silicate rocks by portable X-ray fluorescence: Effect of sample mineralogy in relation to the size of the excited volume. Geostand. Newsl. 1997, 21, 29–41. [Google Scholar] [CrossRef]
- McGarry, C. (Dublin Analytical Instruments Ltd., Dublin, Ireland). Personal communication, 2020.
- Rousseau, R.M. Detection limit and estimate of uncertainty of analytical XRF results. Rigaku J. 2001, 18, 33–47. [Google Scholar]
- Saini, N.K.; Mukherjee, P.K.; Rathi, M.S.; Khanna, P.P. Evaluation of energy-dispersive x-ray fluorescence spectrometry in the rapid analysis of silicate rocks using pressed powder pellets. X-ray Spectrom. 2000, 29, 166–172. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Zhang, Q.; Wang, Y. Determination of major/minor and trace elements in seamount phosphorite by XRF spectrometry. Geostand. Geoanalytical Res. 2004, 28, 81–88. [Google Scholar] [CrossRef]
- Matsunami, H.; Matsuda, K.; Yamasaki, S.-I.; Kimura, K.; Ogawa, Y.; Miura, Y.; Yamaji, I.; Tsuchiya, N. Rapid simultaneous multi-element determination of soils and environmental samples with polarizing energy dispersive X-ray fluorescence (EDXRF) spectrometry using pressed powder pellets. Soil Sci. Plant Nutr. 2010, 56, 530–540. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Xu, J.; Pan, Y.; Bo, W.; Liu, B.; Zhang, P.; Bai, J.; Zhang, Q. The technique of high-pressure powder pressing with polyester film covering for XRF of geochemical samples. X-ray Spectrom. 2020, 1–11. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015. [Google Scholar] [CrossRef]
- CEC. Council directive of 12 June 1986 on the protection of the environment and in particular of the soil when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 181, 0006–0012. [Google Scholar]
- Toth, G.L.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hessel, R. Soil Threats in Europe; Publications Office: Luxembourg, 2015. [Google Scholar] [CrossRef]
- Rao, B.K.R.; Bailey, J.; Wingwafi, R.W. Comparison of three digestion methods for total soil potassium estimation in soils of papua new guinea derived from varying parent materials. Commun. Soil Sci. Plant Anal. 2011, 42, 1259–1265. [Google Scholar] [CrossRef]
- Vanhoof, C.; Tirez, K. Energy-dispersive X-ray fluorescence systems as analytical tool for assessment of contaminated soils. J. Environ. Monit. 2004, 6, 344–350. [Google Scholar] [CrossRef]

| ISE Normal Distribution Approximation (NDA) Results | Calculated Limit of Detection (LOD) for the XRF Used for This Study | ||||
|---|---|---|---|---|---|
| Element | NDA Mean | NDA SD | Mean LOD | ||
| Max | Min | Max | Min | mg kg−1 | |
| Al ** | 94.94 | 16.18 | 5.11 | 0.52 | 88.6 |
| Ca ** | 221 | 1.186 | 9 | 0.08 | 56.3 |
| Fe ** | 60.07 | 2.62 | 3.62 | 0.16 | 5.64 |
| K | 21865 | 5987.7 | 1199 | 153 | 3.4 |
| S | 2831 | 197.1 | 524 | 27.7 | 4 |
| P | 2694 | 162.9 | 157 | 12.7 | 10.3 |
| Mg | 21042 | 312 | 1794 | 63.8 | 114.8 |
| Mn | 1479 | 75.09 | 85 | 9.06 | 12.1 |
| Cu | 152.3 | 5.06 | 7.14 | 1.05 | 1.7 |
| Cr | 254.8 | 19.29 | 23.7 | 2.28 | 5.6 |
| Ni | 111.6 | 6.59 | 5.5 | 0.998 | 1.9 |
| Pb | 296.6 | 4.32 | 21.8 | 0.981 | 2.4 |
| Zn | 1037 | 17.7 | 43 | 2.4 | 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croffie, M.E.T.; Williams, P.N.; Fenton, O.; Fenelon, A.; Metzger, K.; Daly, K. Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis. Agronomy 2020, 10, 1309. https://doi.org/10.3390/agronomy10091309
Croffie MET, Williams PN, Fenton O, Fenelon A, Metzger K, Daly K. Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis. Agronomy. 2020; 10(9):1309. https://doi.org/10.3390/agronomy10091309
Chicago/Turabian StyleCroffie, Maame E. T., Paul N. Williams, Owen Fenton, Anna Fenelon, Konrad Metzger, and Karen Daly. 2020. "Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis" Agronomy 10, no. 9: 1309. https://doi.org/10.3390/agronomy10091309
APA StyleCroffie, M. E. T., Williams, P. N., Fenton, O., Fenelon, A., Metzger, K., & Daly, K. (2020). Optimising Sample Preparation and Calibrations in EDXRF for Quantitative Soil Analysis. Agronomy, 10(9), 1309. https://doi.org/10.3390/agronomy10091309





