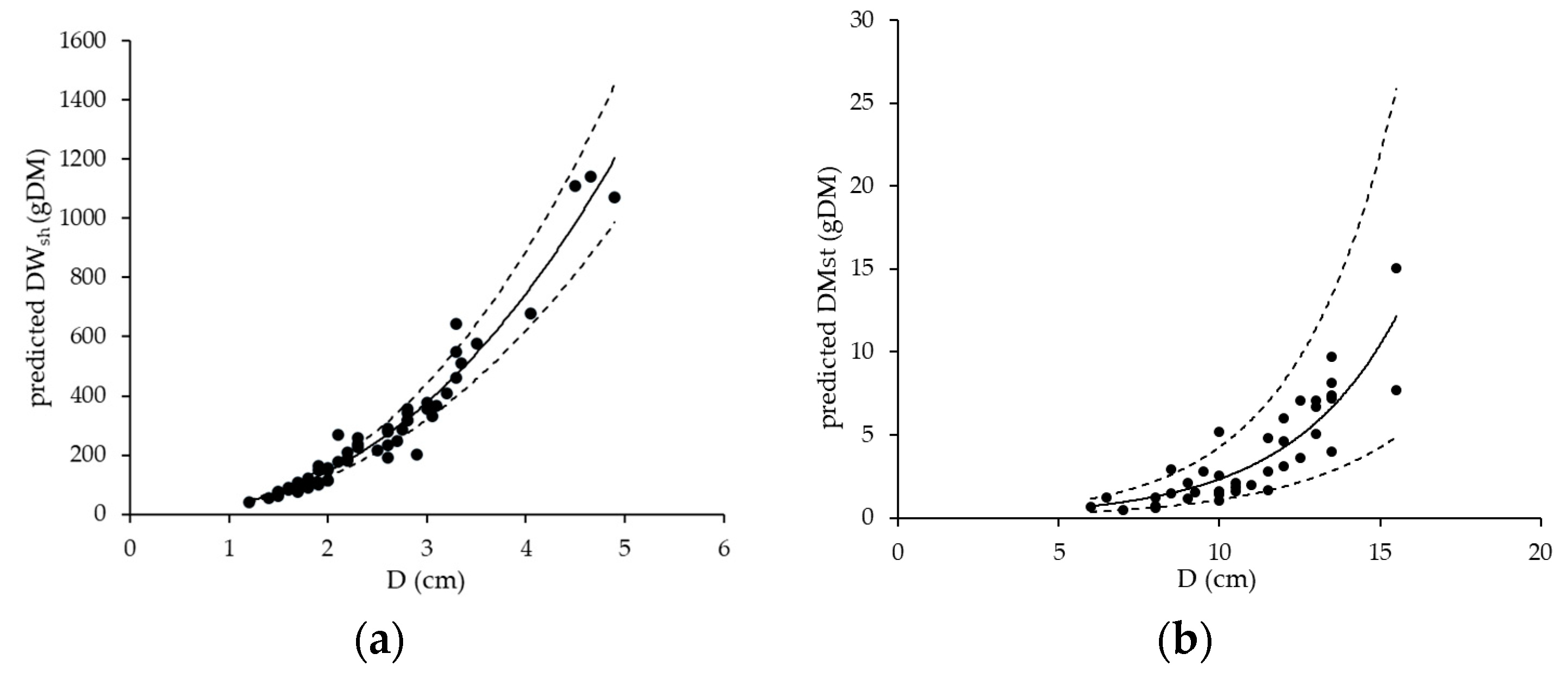
4.1. NBP of the Two Investigated Cropping Systems
Our results showed that, one year after its conversion from an SRC stand to an ACS, the system turns from a carbon sink into a carbon source. In our experiment, SRC had a positive NBP, while the ACS had a negative NBP. Our NBP results for ACS were close to those reported by Kutsch et al. [
55] for five arable crop rotations (winter cereal-based four-year rotations) and two monocultures (barley and rice) over four years in different environments across Europe. Indeed, they estimated an average NBP for the seven sites equal to -95 ± 87 g C m
−2 year
−1. Our results were also consistent with Janssens et al. [
56], who reported a carbon loss of −90 ± 50 g C m
−2 year
−1 on croplands. Kay et al. [
57] reported a value for an ACS based on willow and hazelnut SRC in an Atlantic climate of 36 to 105 g C m
−2 of net carbon sequestration potential. This result is similar to our result for SRC but not for ACS, where we observed a negative NBP. However, Feliciano et al. [
58] reviewed the potential carbon storage in agroforestry systems and found no clear results, reporting from very negative to very positive carbon budgets (−800 g C m
−2 to 800 g C m
−2, respectively). They stated that, in a general framework of a lack of standardized methodologies for carbon budget assessment, climatic conditions, site history and management play a crucial role in determining the actual C storage of such systems. For example, Douglas et al. [
27] suggested that the introduction of poplar and alder conservation trees in a long-term pasture (>30 years) could lead to no soil C stock gain at a depth of 1 m, because the trees are too young (14 to 16 years) to allow for a significant shift to the soil C stock.
Cropland sites from Europe, even if managed according to good practice guidelines [
59], can be both moderate carbon sinks and sources, while other model-based studies (ORCHIDEE-STICS) predicted an almost neutral C balance for European agriculture [
60].
In general, there is a great variability among sites in different environmental conditions and also among carbon budget estimation methods. These two issues make it difficult to obtain reliable data that are representative of the average situation in Europe or in the Mediterranean [
61]. In general, few studies are available on the C balance using a flux approach repeated for more consecutive years after the conversion from a less intensively managed or unmanaged system to a more intensively managed system. We could say that the few examples of studies on the carbon storage of systems undergoing conversion from forest to silvoarable considered only changes in the soil organic carbon stock. Cardinael et al. [
28], in an effort to revisit the IPCC Tier 1 for soil organic and biomass carbon storage in agroforestry systems, reported that, where a conversion took place, there were C losses of −26 g C m
−2 year
−1 from forest to silvoarable in South America and −53 ± 218 g C m
−2 year
−1 from forest to silvopasture in three different tropical regions. Therefore, our hypothesis is that more carbon is lost in the first years after the conversion and that these C losses are mainly linked with organic matter and residue mineralization through respiration processes. This C loss rate, however, could slow down in the long term, so our NBP values for ACS could become closer to the ones cited, showing a tendency to reach a steadier state.
4.2. Contribution of the C Input to the NBP
Regarding the C inputs, in the ACS, the total carbon input (
Table 5) was lower than in the SRC. In particular, the sorghum biomass and annual weeds’ biomass were not sufficient to close the gap, with a much higher poplar stool biomass production in the SRC.
The sorghum aboveground biomass, in a field trial 3 km away from our site, with different soil conditions and different management but with the same sorghum variety, was 52% higher than in the ACS (1153.1 ± 103.5 g DM m
−2 compared to 550.9 ± 95 g DM m
−2 in the ACS) (data not published). Nassi o Di Nasso et al. [
62], after a long-term (12-year) experiment in Pisa with arable crop rotations, including rainfed sorghum, reported an aboveground production of about 1470 g DM m
−2, which is three-fold higher than that in the ACS. The sorghum aboveground biomass measured in the ACS was lower due to the lower crop growth and partly to the reduction of the cropped area (11% less than an open field cultivation). However, it is worth noting that there are no data available for a comparison with our result in the ACS for sorghum aboveground production.
Weeds were more present in the ACS than in the SRC since they were favored by the conversion. It is likely that in the ACS, with the beginning of tillage operations after clearing, weed seed bank germination took place at a fast rate, stimulated by the beginning of the application of fertilizers and seedbed preparation, as well as the higher light availability in the alleys.
In the SRC, the C input was remarkably higher than in the ACS (+22% respect ACS), both for the C accumulated in the poplar stools and the leaf litter.
Nassi o Di Nasso et al. [
3] reported a poplar stool yield of 1380 ± 90 g DM m
−2 in a 12-year-old SRC poplar stand in Pisa, which is higher than our result in the SRC (1073.9 ± 51.7 g DM m
−2). Giannini et al. [
63] reported a value of around 1000 g DM m
−2, which is close to our value in the SRC, in a young SRC poplar stand (3 years) in organic soil in Tuscany. However, Ventura et al. [
64] reported a poplar C biomass of 1100 ± 200 g C m
−2 year
−1 in a young SRC poplar stand (3 years old) in Northern Italy, which is more or less two times higher than our C content value in the SRC (522.7 ± 24.5 g C m
−2 year
−1).
Regarding poplar leaf litter, Ventura et al. [
64] reported a value of 210 ± 20 g C m
−2 year
−1, which is not far from our values in the SRC (182.5 ± 15.5 g C m
−2 year
−1).
Due to the fact that the poplar woody biomass was removed from the system through harvesting, the results also showed that the C output, like the C input, was higher in the SRC than in the ACS. Hence, the net gain in C accumulation for the SRC came from the other biomass sources that were not removed from the system, i.e., weeds, leaf litter, roots and poplar stumps.
In particular, the total C input in the ACS showed a higher proportion of belowground biomass (CBGB), even if it was not significantly different and it also showed a higher proportion of roots than the SRC, along with the presence of poplar stumps. We should note, however, that the CBGB standard error is higher in the ACS than in the SRC. An explanation of the differences in the statistical results for the roots (root biomass not significantly different vs. root C content significantly different) could be the C concentration, which was found to be lower and have a higher variability in the ACS than in the SRC.
The stumps in the ACS were less dense than in the SRC, but competition with contiguous poplar rows was not present, so the poplar stools reached a higher crown diameter and the stumps expanded, resulting in a calculated higher annual stump biomass accumulation (
Table A3). Moreover, these data should be treated carefully, considering that the annual accumulation of biomass in the stumps was assumed to follow a linear trend, according to the age, and that the poplar plants density varied only after 2017.
There are few comparable data on sorghum belowground biomass accumulation in Mediterranean environments. Applying the root-to-shoot ratios studied by Myers [
65] to the average rainfed sorghum long-term aboveground biomass production for the area [
62], we find a value of 294 g DM m
−2. Our result for fine roots in the ACS (441.74 ± 51.2 g DM m
−2) is higher, but we did not separate the relative contribution of sorghum, weeds and poplar.
An important point regarding the C inputs is the high uncertainty related to the quantification and dynamics of the belowground biomass. More than half of the C assimilated by the plant is transported belowground via root growth and turnover, root exudates (of organic substances) and litter deposition. With this process, Fernández-Núñez et al. [
66] estimated, in a silvopasture in Spain, a root C contribution of up to the 33% of the C sequestered in ecosystems. Our results show that the total C content in the belowground biomass (C
BGB) is 47% in the ACS and 33% in the SRC of the total C input (C
input), thus confirming this proportion. The dynamics of the root growth, decay and turnover are some of the least understood aspects of belowground interactions in agroforestry [
67].
In our experiment, we did not know how poplar roots were distributed in the alleys after clearing. Fine roots of both trees and crops have a relatively fast turnover (measured in days to weeks). Lignified coarse roots of trees decompose much more slowly once trees are harvested (and ground, in our case) and may contribute substantially to hidden belowground C inputs, but we could not detect this [
68]. A probable process is a lack of intra-specific competition in the inter-row, with root web destruction in the first 50 cm and inter-specific competition with sorghum at the same time, which we expect to be fierce in the first 60 cm, as described for silvoarable systems with walnut and hybrid poplar in France [
69]. Recently, an attempt at understanding the root dynamics in silvoarable systems has been made by Beuschel et al. [
9]. In their study on soil quality parameters after an SRC conversion to a silvoarable system in Germany, they reported data on root determination, finding no living poplar roots in the cultivated alleys and six-fold more dead poplar roots close to the tree rows compared to those in the cultivated alley, suggesting a reduced root biomass after conversion. However, tree roots develop for longer and at a lower depth than herbaceous annual crops, and in soils under trees, a considerable amount of C, which is very challenging to detect and measure, is stored below the plough layer (50 cm) [
30]. Trees appear to extend roots to deeper layers when there is competition and disturbance in the plough layer in order to exploit other pools of water and nutrients to sustain their growth [
70,
71]. Hence, it becomes difficult to estimate and calculate the relative contribution of belowground biomass to the overall carbon input in the ACS. The lack of root measurements in the soil layers deeper than 30 cm could have diminished the calculated belowground carbon and could have led to a higher NBP in both the systems. However, our hypothesis is that the exclusion of the roots under the plough layer could have been a greater disadvantage for the ACS than the SRC in this experiment.
Regarding fine roots, Chimento and Amaducci [
43] reported a fine root biomass weight at a depth of 0–30 cm for a 6-year-old SRC poplar in Northern Italy equal to 300 g DM m
−2. Our result for the SRC was similar to the one reported (341.4 ± 48.5 g DM m
−2). In this study, the root biomass weight was sampled at a depth of 0–102 cm and the data showed that 71% of the fine roots were located in the first 0–30 cm. According to these data, in our SRC system, about 30% of the fine roots are missing from the NBP calculation; while we do not have data from other experiments on a comparable ACS system, where the percentage of poplar fine roots in the first 30 cm could be lower than 71%.
As for coarse roots and stumps, since poplar is an opportunistic rooter, it does not produce roots in deep soil layers when the water table is sufficiently high [
72], as observed by Berhongaray et al. [
73]. In their experiment, where the average water table was 85 cm, they found almost no coarse roots below 60 cm in a young SRC poplar plantation (4 years old), after the complete excavation of the stumps. The situation in our ACS could be very different because of the competition in the plough layer and the breaking of the coarse roots with stand clearing, which may have forced the poplar to reorganize its root system at deeper layers. Oliveira et al. [
74] made a huge effort to determine SRC poplar roots in Spain, excavating stumps for different clones in different sites to validate a model. They measured the single-stool root-to-shoot ratio for SRC poplar (3 to 4 years old) in four different sites in Spain, finding an average value of 0.23. Berhongaray et al. [
73] found root-to-shoot values ranging from 0.27 to 0.70 in 2- to 4-year-old poplar, obtained through allometric equations and excluding the fine root biomass. The root-to-shoot value in our SRC is 0.79, if roughly calculated with the belowground biomass and divided by the aboveground biomass, which is much higher than the reported values. These data could suggest that the stand age and site characteristics are determinant, and it becomes difficult to compare root data on SRC stands of different ages [
67]. The study conducted by Oliveira et al. [
74] focused on younger pure stands in less productive sites. These issues suggest that our quantification of poplar roots in a 9-year-old converted ACS could have been hampered by sampling complications, which could eventually give unsatisfactory results.
4.3. Contribution of the C Output to the NBP
As for the carbon input, the SRC showed a higher but not significant carbon output, which was mainly due to the harvested poplar woody biomass. In the ACS, the carbon output was close to that of the SRC due to its higher heterotrophic respiration rate, which is high enough to close the gap between the ACS and SRC due to the two times higher biomass harvested in the SRC. For instance, in the SRC, there was a higher aboveground biomass production, but the carbon was lost through harvesting, while the respiration rate was significantly lower than in the ACS, thus leading to a positive NBP in the SRC. Conversely, the ACS, which had a lower aboveground production and a similar belowground accumulation compared to SRC, was found to have a carbon output closer to that of the SRC, thus leading to a negative NBP.
The poplar stools’ yields were discussed in the previous section with respect to the contribution of the C input since poplar stools were measured as C inputs and C outputs through harvesting. According to the levels of sorghum aboveground biomass discussed in the previous section, the sorghum grain yield of the ACS (164.3 ± 82.0 g DM m
−2) was also about 50% lower than in an open field condition in the area (data not published). It should be noted, however, that the sorghum yield recorded in the ACS was lower (−64%) than the sorghum yields of the area from the national statistic for Pisa Province (459 g DM m
−2) [
75]. Nassi o Di Nasso et al. [
62] reported an even higher long-term yield average of 670 g DM m
−2. Our low yield is due to the lower crop growth, especially next to the poplar rows, and also to the reduced cropped area.
After considering these data, our hypothesis was that the factor underlying the main differences in NBP among the two systems was the carbon output through heterotrophic respiration, which is linked with soil and crop management and site history [
76]. Our result regarding the total soil respiration for the ACS was 1560.2 ± 300.7 g C m
−2 year
−1 (
Table A4), a high value if compared with other open field cultivations. Sharma et al. [
77] measured an open field biomass sorghum (
Sorghum bicolor L. Moench) in southern USA, finding values of 872.1, 1138.4 and 882.2 g C m
−2 year
−1 in 2013, 2014 and 2015, respectively. Alberti et al. [
78] reported another warm season cereal, continuous maize, in Northern Italy, finding much lower Rh rates of 607 g C m
−2 year
−1 and 540 g C m
−2 year
−1 for 2007 and 2008, respectively. In a review paper, Subke et al. [
79] reported about 30% lower values for total respiration of 1077 g C m
−2 year
−1 for Mediterranean croplands, on average. However, there is a lack of data on the respiration measurement and partitioning of ACSs in Mediterranean environments and on the peculiar transition characteristics considered in our experiment, so we found no data that could be clearly compared with ours [
80]. Moreover, it should be noted that the C
Rh calculated by means of heterotrophic respiration could result in an underestimation of the C output, since it does not include the C lost through biomass degradation.
The SRC showed a cumulate Rh value close to the values reported for temperate forest ecosystems, ranging between 280–970 g C m
−2 year
−1 [
81]. Under Mediterranean conditions in Northern Italy, Ventura et al. [
64] measured, in a poplar SRC plantation, an Rh ranging from 700 ± 100 g C m
−2 year
−1 for 2008 and 900 ± 100 g C m
−2 year
−1 for 2009 in control non-fertilized plots. In the SRC, the lack of soil aeration, crop residue incorporation and, eventually, tree-shading may have led to a lower carbon loss through respiration. Indeed, in the SRC, the C
Rh was −37% compared to that in the ACS.
The SOM content in the ACS was closer to that of the SRC because of the former poplar stand, but the soil aeration and crop residue incorporation, due to tillage operations, may have led to an increase in heterotrophic respiration, which may have been due to the higher pool of carbon in the SOM. Indeed, in the ACS, the stumps were destroyed and ground one year before the beginning of the experiment. Thus, a very high quantity of woody biomass was incorporated into the soil (estimated to be equal to 1520 g C m
−2 or 15.2 t C ha
−1, data not shown). This could have boosted the heterotroph respiration activity in the soil, resulting in a higher rate of C
Rh. Beuschel et al. [
9] studied the conversion of a Short Rotation Coppice stand into a silvoarable system in Germany and found that the SOC and related soil quality parameters in the first 0–5 cm decrease in the alley in the first year after conversion. Thus, in the ACS, the incorporation of woody biomass and the consequent repeated tillage operations could have favored a higher mineralization rate than that in the SRC. No studies are available on soil CO
2 fluxes for a conversion from an SRC stand to an Alley Cropping System, since the few data available are on studies conducted on formerly arable lands that have been converted into SRCs and not vice versa [
82,
83].
In arable systems, soil carbon is usually depleted, because the systems have a lower plant biomass input due to harvesting, a fast SOM breakdown (SOM is more easily reached and disrupted by tillage soil aggregates) and an increased displacement of carbon-rich topsoil [
84]. However, crop management practices, such as ploughing and fertilization, may cause either soil C increases or losses [
19].
In our case, if the incorporation of poplar stumps in the ACS led to a peak in soil respiration in the short term, in the second year, we could expect a decrease in the Rh and a lower carbon loss [
85]. Nonetheless, if the repeated soil aeration and crop residue incorporation steadily boosted the heterotrophic activity in the soil, we could expect the opposite result, since the ACS loses more carbon even in the following years. Some studies suggest that the loss of a permanent cover and re-introduction of tillage may lead to a destruction of microorganisms’ habitats and a decrease in soil organic matter recycling, thus overcoming the beneficial effect of stump incorporation for SOM accumulation [
86]. These two opposite results could also be dependent on the woody nature of the poplar stumps incorporated. Alberti et al. [
78] and Aubinet et al. [
87] showed that crop residue respiration (maize and sugar beet) was mostly accomplished in the first months after the incorporation and was nearly finished within one year. Poplar stumps could take longer and show their effect on C
Rh in more than one year. We need to point out that the SOM content in topsoil could have influenced the soil respiration rates, as reported for temperate croplands [
55]. However, in our experiment, the SOM content sampled from the ACS and SRC in 2017 was very close, which is mainly related to them having the same management history for the years prior to the conversion (
Table 1). Since organic substrates present in litter or soil are decomposed through Rh [
88], carbon losses from the ecosystem can be correlated with the C concentration in topsoil [
55,
76,
89].