Abstract
Grassland management affects ecosystem services such as the conservation of C stocks. The aim of this study was to analyze the relation between vegetation production and soil C stocks for a set of seven temperate grasslands of various productivity levels. We estimated vegetation production directly through measurements of aboveground biomass (>5 cm), stubble and root biomass, and indirectly via plant community functioning. Soil C stocks were measured for bulk soil (organic C, SOC) and hot-water-extractable C (HWC) of topsoil. Plant community functioning was characterized by community-weighted mean (CWM) traits and functional diversity index. Results show a negative relation between biomass production and SOCstock. The tradeoff between productivity and SOCstock could be linked to plant community functioning and particularly Leaf Dry Matter content (LDMCCWM) which appeared to be the most relevant descriptor of plant community functioning. High SOCstock could be associated to low productivity, conservative strategy (high LDMCCWM), low soil labile C content and grassland age. Our results show a strong direct effect of management and grassland age on plant community, which in turn affects plant tissue quality and subsequent organic matter mineralization. Old permanent grasslands appeared less productive but represent an occasion for C storage and thus global change mitigation.
1. Introduction
Grasslands cover about 20% of the total area in Europe [1] where most of the area is dedicated to feed livestock. In view of that, grasslands play a key role in forage provision, but they also deliver a number of ecosystem services (ES) [2], including the regulation of air and soil quality, especially carbon (C) sequestration [3,4]. Indeed, grasslands have a high potential to mitigate climate change through C sequestration [5]. Around 12% of the global terrestrial C pool is located in temperate grasslands (calculated from [6]). In all ecosystems, C storage depends on the balance between C input and output. In terrestrial ecosystems, C input mainly results from CO2 fixation by plants and C output is the result of the release of CO2 into the atmosphere by the plant and micro-organism respiration, the volatilization of organic compounds and the leaching of dissolved organic C [7]. Additionally, in managed grasslands, a portion of the C output is due to the export of biomass by grazing or mowing. Soil carbon sequestration in grasslands is regulated by complex biogeochemical processes, which are in turn affected by both management and by multiple environmental factors themselves dependent on global change. There are still large uncertainties relative to the accurate estimation of C sequestration rates in grassland soils due to, for example, high spatial and temporal variability of soil C stocks. Nevertheless, a better understanding of grasslands functioning and linkages to C storage appears to be a major issue in the context of global change.
Grasslands are potential C sinks or sources depending on their management [8]. Short-term grasslands resulting from periodic tillage and sowing have a lower potential to store soil C than permanent grasslands due to an increase of soil respiration by tillage [8,9]. Management practices such as grazing or mowing and fertilization have also been reported to modify soil organic C (SOC) stocks [10,11]. Grazing, which stimulates primary production, often increases SOC accumulation compared with mowing [8]. The intensity of grassland management affects strongly C fluxes. On one hand, intensive management, with high nitrogen fertilization and frequent cutting, has been demonstrated to stimulate C sequestration [12,13,14,15]. On the other hand, an extensification through the cessation of fertilization and biodiversity restoration can reduce gross soil respiration and thus increase the rate of SOC accumulation [16]. By modeling C cycling in temperate grasslands, Soussana et al. [8] demonstrated that nitrogenous fertilizer might increase soil SOC stocks for a moderate application or reduce them for an intensive use in combination with high biomass exports. These effects of grassland management, in particular fertilization on C sequestration, can result from the modification of plant community functional composition [17]. The functioning of plant communities and plant ecological strategies (e.g., growth, nutrient uptake) can be associated to a set of plant functional traits such as leaf traits [18,19,20]. According to the “mass ratio hypothesis” [21], which postulates that species traits affect ecosystems in relation to the relative abundance of the species in the assemblage, abundance-weighted mean traits can be calculated at the community level [22]. Relationships between community-level leaf traits (traitsCWM) and biochemical processes are often unclear when reviewing the literature [23]. However, relationships have been established between specific leaf area (SLACWM), leaf dry matter content (LDMCCWM) or leaf nitrogen content (LNCCWM) and both net primary productivity [22,24] and litter decomposition rate [22,25,26]. The processes promoting high C inputs and slow decomposition of soil organic matter (SOM) are often conflicting [7,27] and may making it difficult to identify relevant plant traits as indicators of C sequestration. Exploitative (fast-growing) species typical of intensive management (i.e., fertilization and exports) are characterized by high SLA, high LNC and low LDMC [20,28] promoting high C inputs and thus C storage potential [27]. In contrast, conservative (slow-growing) species typical for extensive management and low fertilizer inputs, are characterized by low SLA, low LNC and high LDMC [20,28], producing long-lived tissues with a low decomposition rate [29] and may thus promote C storage by means of low C outputs (i.e., losses via heterotrophic respiration) [27]. Soil C storage in grasslands can also be affected by plant diversity, which is a community structure component [16,30]. Whereas many farming practices, designed to increase productivity such as fertilization, lead to decreases in the biodiversity in grasslands [31], convincing studies have demonstrated positive relationships between biodiversity and primary productivity [32,33]. This positive relationship has been explained by the selection of extreme trait values and also by functional niche complementarity [32]. High functional diversity may allow a more complete utilization of resources, which can lead to higher plant productivity [34] and promote soil C accumulation [35,36].
The present study focused on the relationship between two ES, namely conservation of C stocks and grassland productivity, through the analysis of plant community functioning. We used a set of seven temperate grasslands from an experimental farm in Normandy, differing in age (i.e., from a 1-year temporary grassland to old permanent grasslands >30 years), management practices, and thus their productivity. In this study, we examined annual net primary productivity, C distribution in the different plant compartments and in the different soil C pools (i.e., SOC and hot-water-extractable C, HWC, which is a labile C fraction) for each grassland field. The literature describes conflicting relations between provisioning services such as the provision of nutritional biomass and regulating services such as C sequestration. We hypothesized (Hyp1) a tradeoff between the service of soil C conservation and grassland productivity. As functional traits have been demonstrated as relevant tools to understand ecosystem functioning, we postulated (Hyp2) that among the easy measurable leaf traits, one trait could be identified as the best descriptor for both primary productivity and conservation of C stocks and thus constitute a relevant indicator for the study of the relationships between these two services.
2. Materials and Methods
2.1. Study Site and Experimental Design
The study was conducted on seven grassland fields located within an area of 3 km2 at the INRA Experimental Domain of Le Pin-au-Haras in the north-western part of France (Normandy, France, 48°73’ N, 0°17’ E, 191 m a.s.l.). The climate is temperate. The 30-year period (1982–2012) mean of annual precipitation was 727 mm and the average daily temperature was 10.0 °C. During the experiment, in 2012, annual precipitation was 864 mm and daily temperature was registered with 10.3 °C. Soil properties of the 0–20-cm horizon are described in Table 1. The soil of the seven grassland fields is classified as a Eutric Cambisol [37] developed on green clay with the topsoil (0–20 cm) being a sandy clay loam with a pHH2O ranging from 6.1 to 7.9 and a calcium carbonate equivalent ranging from 0.7 to 1.5% (Table 1). Each grassland field had a slope close to 0, except field 1 which had a slope of 5%. Management history of fields has been well documented for the 30 years. Briefly, fields 1 to 3 have been grasslands for more than 30 years, while fields 4 to 7 were croplands cultivated with corn before their conversion to grasslands respectively in 1991, 1995, 2008 and 1995 (see Table 2). The seven grassland fields varying in size (2.5 to 12 ha) were either grazed by cows (~1.5 livestock unit ha−1 year−1), mown (1 to 3 cuts per year) or both (see Table 2). Fertilization ranged from 0 to 185 kg N ha−1 year−1 and was provided through mineral and/or organic supplies. For grazed fields, N inputs by grazing animals were estimated according IPCC guidelines Tier 2 (IPCC 2006, Chapter 10.5.2) [38] where the annual amount of N excreted by livestock was estimated via annual N intake and N excretion, based on total feed intake and livestock density (LSU ha−1 year−1). In 2012, the annual stocking rate ranged from 0 to 1.45 livestock unit ha−1 year−1 with a maximum instantaneous stocking rate of 20.69 livestock unit ha−1, livestock units being based on the grazing equivalent of an adult dairy cow (i.e., 600 kg). In 2011, fields 1 and 5 were over-seeded with Lolium perenne L. and Trifolium repens L. For each field, a 1000-m2 square was defined inside a homogeneous zone of vegetation with a buffer zone of at least 20 m width. In each field, 10 sampling points were placed regularly along the diagonals of the 1000-m2 square. Sampling points were shifted by one meter between each sampling date.

Table 1.
Topsoil properties (0–20 cm) of the seven grassland fields.

Table 2.
Management and age of the seven grassland fields since last seeding, with establishment date, management regime (grazed/mowed), grazing intensity (annual stocking rate), mowing intensity (number of cuts per year), fertilization, N excreted by livestock (estimated using the equations from the 2006 IPCC guidelines) and over-seeding.
2.2. Plant Communities, Leaf Traits and Functional Diversity
Relative cover of plant species was estimated during July 2012 in each field for 10 sampling quadrats (0.25 m2 each) using species relative cover. Only dominant species accumulating at least 90% of the mean cover in plant communities were recorded for plant functional trait measurements. Leaf sampling for trait measurements was carried out according to Cornelissen et al. [39]. Five leaf traits were assessed: specific leaf area (SLA, leaf area: leaf dry mass), leaf dry matter content (LDMC, leaf dry mass: leaf fresh mass), leaf carbon content on a mass basis (LCC, C content in leaf: leaf dry mass), leaf nitrogen content on a mass basis (LNC, N content in leaf: leaf dry mass) and the leaf carbon to nitrogen ratio (LC:N). After sampling, leaf area was measured using an area meter (Li-3100 leaf area meter, Li-COR, Lincoln, NE, USA), and leaves were oven dried at 60 °C until constant mass and weighed. Total C and total N for grinded leaves were determined with an IsoPrime mass spectrometer (Elementar, Lyon, France) connected to an elemental analyzer (EA3000, Euro Vector, Milan, Italy).
Community functional parameters were calculated using the weighted mean of leaf traits (weighted by their relative cover in each quadrat) according to Garnier et al. [22]. Rao’s quadratic diversity index (FDQ) was calculated from the five leaf traits using the Excel macro developed by Lepš et al. [40] according to the equation:
where n is the number of dominant species in the sample, pi is the relative abundance of species i, pj is the relative abundance of species j, and dij describes the functional dissimilarity between species i and j. This functional diversity index can be interpreted as the average dissimilarity of two randomly chosen individuals in the studied sample [40,41].
2.3. Soil and Vegetation Sampling
In 2012, prior to each mowing event or grazing period, 10 soil and vegetation samples (10 replicates) were collected in each grassland field. The number of field sampling dates varied from 2 to 4 according to the management of each grassland field (mowing frequency or grazing periods, Table 2). Mowing dates and grazing periods were determined by the farm activity, accounting for both maximization of forage production and maintenance of forage quality (i.e., avoid decline due to grass phenology). At field sampling, aboveground biomass was collected from a 100-cm2 circle by separating two compartments: above 5 cm (clipped 5 cm above the soil surface, AGB) and below 5 cm (clipped at the ground, stubble). Soil and roots were collected immediately after clipping by using a stainless steel corer (24 cm2 × 10 cm depth). For fresh soil samples, visible roots, plant and animal residuals, and stones were picked out by hand. The root section was then washed, whereas “clean” fresh soil was sieved to <2 mm and a subsample was frozen at −20 °C for further HWC analyses. Aboveground biomass, stubble, root and soil samples were oven-dried at 60 °C for at least 48 h, weighed and ground to a fine powder. The precise dry soil mass of each soil core was recorded to calculate soil bulk density (BD; g dry soil volume−1). The aboveground net primary productivity (ANPP; t DM ha–1 year–1) was the sum of the dry mass aboveground vegetation harvested above 5 cm.
2.4. Soil and Vegetation Analysis
Soil inorganic matter was determined using ignition of the fine soil sample at 375 °C for 16 h in a muffle furnace [42]. C concentrations (in C mass per dry mass) in fine soil, soil inorganic matter, roots and aboveground vegetation were measured with an IsoPrime mass spectrometer (Elementar, Lyon, France) connected to an elemental analyzer (EA3000, Euro Vector, Milan, Italy). The SOC concentration (SOCconc in mass per soil dry mass) of the soil samples was determined from the soil inorganic C concentration (SICconc) and soil total C concentration (STCconc) as follows:
The BD of the fine soil <2 mm, necessary to estimate SOCstock (t ha–1 in 0–10 cm depth) was calculated as follows:
where ρ stones is the stone density (2.4 g cm−3).
In the seven grassland fields, soil BD ranged from 0.7 to 1.0 g cm−3 and stone contents from 0 to 50 kg m−3. The apparent SOCstock (t ha–1 in 0–10 cm depth) was calculated using the SOCconc and BD as follows:
Estimation of belowground C stocks (soil and roots) was calculated from samples obtained from a fixed depth in the organic soil layer. Seasonal variations in soil water content and animal trampling often affect BD and C stock estimations [43]. To compare fields, we used the equivalent soil mass correction [44] to the fixed depth method, taking the higher BD (BDmax) as a reference by using a correction factor (Cf = BDmax/BDsample).
Soil HWC was determined by adapting the procedures of Ghani et al. [45]. Briefly, for each fine soil sample, 3 g dry mass were placed in polypropylene centrifuge tubes with 30 mL distilled water. The tubes were shaken, capped and left for 16 h in an 80 °C hot water bath. The tubes were then shaken and subsequently centrifuged at 1160 g for 20 min before vacuum filtration of supernatants through Whatman No. 42 filter paper. An aliquot (50 µL) of the filtrate was dried on Chromosorb (W30-60 Mesh; Sercon, Crewe, UK), packed in tin cups and the C concentration was measured using an IsoPrime mass spectrometer (Elementar, Lyon, France) connected to an elemental analyzer (EA3000, Euro Vector, Milan, Italy). Root density was calculated as the amount of root dry mass per cm3 of soil corrected with Cf as for the SOCstock.
2.5. Statistics
Statistical analyses were computed using ‘R’ (version 3.0.2). Data were analyzed for variance analysis (one-way ANOVA, linear model). Prior to ANOVA, a Shapiro–Wilk test (99%) and a Bartlett test (99%) were performed on each set of data to assess data normality and homogeneity of variances, respectively. The SOCstock data did not fit the parametric test conditions and were inverse (1/X) transformed prior to ANOVA. Tukey’s tests were used to compare all treatments when the field effect was significant (p ≤ 0.05). Correlations between variables were evaluated by Pearson’s correlation coefficients. The relations between ANPP, SOC and HWC were assessed through linear regression analysis. The relations between ANPP Root C:ABG C, SOC, LDMCcwm and N fertilization were assessed with multiple nonlinear regression.
3. Results
3.1. Vegetation Productivity
Annual ANPP (Figure 1) was calculated as the sum of harvests (dry matter above 5 cm) throughout the year. It varied largely among fields (F = 44.5, p < 0.001) and increased gradually from field 1 (3.8 t DM ha−1 year−1) to field 7 (21.9 t DM ha−1 year−1). As expected, the fields recently sown and having received more fertilization and exploited more intensively were more productive than the older ones (not sown for at least 30 years) which were managed more extensively (see Table 2).

Figure 1.
Aboveground net primary productivity (ANPP, t DM ha−1), as the cumulative values, are means ± standard errors (n = 10) at sampling dates, for each grassland field. Sampling dates correspond to management events; grazing, mowing or both. Cumulated ANPPs with a common letter are not statistically different from each other (Tukey’s test, p < 0.05).
3.2. Vegetation and Soil Carbon Pools
C pools were measured in AGB (aboveground biomass above 5 cm), stubbles (aboveground biomass below 5 cm), roots and in soil at each sampling in the seven grassland fields (Figure 2). The AGB C pool, corresponded to the fraction that is exported several times a year through cutting or grazing (Figure 2a). Three other C pools, which are not exported, are shown as averages (Figure 2b,c). The amount of C in AGB varied from 165.2 to 937.6 g C m−2 (F = 35.3, p < 0.001), and it followed a same pattern as the annual ANPP (Figure 1), increasing from fields 1 to 7. For all fields, the majority (78.6 to 97.0%) of C was located belowground in the 0–10 cm depth soil pool (Figure 2c). The SOCstock values ranged from 3539 (field 7) to 8266 g C m−2 (field 1), with the highest amount corresponding to the least productive field (F = 54.9, p < 0.001). Compared to soil, roots represented a very small C pool (from 11.4 to 47.0 g C m−2), which was the highest in the three least productive fields (1, 2 and 3) (Figure 2b) (F = 17.1, p < 0.001), and all of which were characterized by high root density (Table 3). The amount of C stored in stubble was higher than the amount of C in roots, and differences of C content between these two compartments were higher in the most productive fields. SOCstock occurred to be negatively correlated with the ABG C and positively with root C, whereas the relationship was more pronounced for ABG C than for root C (Table 3). Root C and stubble C follow an opposite pattern, showing a negative correlation.

Figure 2.
Plant and soil C pools (g C m−2) in the seven grassland fields. (a) Annual ABG (biomass above 5 cm) cumulated C along a year, (b) Stubble and root C, (c) SOCstock. Values for AGB (biomass above 5 cm) corresponds to the sum of all samples collected along a year whereas stubble C, root C and SOCstock are means of sampling dates, where sampling dates correspond to management events; grazing, mowing or both. Values are means ± standard errors (n = 10). Means with a common letter within each panel are not statistically different from each other (Tukey’s test, p < 0.05).

Table 3.
Correlation matrix for Pearson’s coefficients involving soil and plant C pools (g C m−2) in the seven grassland fields (n = 70). Annual ABG C corresponds to biomass above 5 cm cumulated C along a year.
The SOCconc in soil and the HWC percentages in the SOC were assessed to compare soil C quality (Figure 3). The SOCconc (Figure 3a) varied among fields (F = 58.7, p < 0.001) and decreased with the productivity of grasslands with the same trend as SOCstock (Figure 2c). The SOCconc ranged from 2.9% of soil dry mass in field 7 to 6.8% in field 1. The quality of SOC, apprehended from the percentage of HWC in SOC, varied with fields (F = 14.3, p < 0.001). The grassland of field 7 was characterized by a high percentage of HWC in the SOC (3.99%) while this percentage was the lowest, 2.33%, for field 1, the less productive grassland (Figure 3b). In the other five fields, the percentages of HWC were intermediate (around 3.4%).

Figure 3.
Soil organic C (SOCconc) and hot-water-extractable soil C (HWC) in the seven grassland fields. (a) SOC expressed as a percentage of soil dry mass. (b) HWC expressed as a percentage of SOC. Values are means ± standard errors (n = 10). Means with a common letter within each panel are not statistically different from each other (Tukey’s test, p < 0.05).
The three parameters, SOC stocks, percentage of HWC in the SOC stocks and ANPP, presented significant relationships (Figure 4). The higher the annual productivity (ANPP), the lower the SOC stock and the higher the HWC content. Multivariable analyses show a negative relation between SOC and ANPP, where the latter is positively related to total N supply and vice versa for SOC (r2 = 0.58, p < 0.001) (Figure 5a). These analyses also show a positive relation between SOC and root C:ABG C ratio where both are negatively related to total N supply (r2 = 0.63, p < 0.001) (Figure 5b). The data suggest thus a compromise between N inputs, C exports and C storage potential.

Figure 4.
Relations among SOC stocks, percentage of HWC in the SOC and annual ANPP, for the seven fields. R2 gives the adjusted coefficients of regression models (*** p < 0.001).
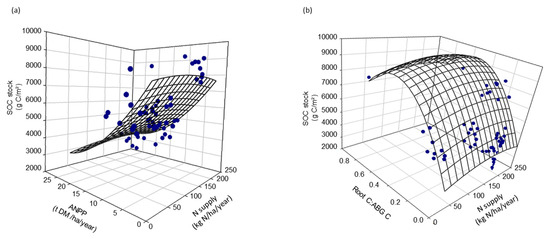
Figure 5.
Relations among total N supply (mineral and/or organic fertilization, and N inputs from grazing animals estimated using the equations from the 2006 IPCC guideline) fertilization and SOC stock and (a) ANPP (r2 = 0.58, p < 0.001) or (b) Root C:ABG C (r2 = 0.63, p < 0.001), for the seven fields assessed with multiple nonlinear regression.
3.3. Plant Community Floristic and Functional Description
Plant communities were dominated by two to five species, with field 3 being the most diversified (Table 4). Lolium perenne L. was the dominant species in five of the fields representing up to 95% of the dominant species relative cover in fields 5 and 7. A high relative cover of Holcus lanatus L. was observed in fields 2 and 3 (41 and 40%, respectively). Legumes took part in the dominant species only in fields 3 and 6 as Trifolium repens L. with a proportion of 11 and 5%, respectively.

Table 4.
Community structure, functional parameters and functional diversity in the seven fields.
The five community weighted mean (CWM) leaf traits varied significantly among fields (Table 4). SLACWM varied between 17.8 and 27.8 mm2 mg−1, with the lowest values corresponding to the fields submitted to grazing and over seeding (fields 1 and 5, Table 2) while highest were found in the exclusively mowed field (field 2). LDMCCWM decreased gradually from 261.5 mg g−1 in the less productive field 1 to 179.0 mg g−1 in the most productive field 7. The highest LNCCWM value (27.2 mg g−1) and lowest LC:NCWM value (16.7 mg g−1) were associated with field 3, which had the greatest proportion of Trifolium repens (11%). Relationships between leaf traits are presented in Table 5. SLACWM and LNCCWM were positively correlated with each other, and both were negatively correlated with LDMCCWM. LC:NCWM, an indicator of leaf tissue quality, was positively correlated with LDMCCWM and negatively with SLACWM. This ratio seemed mainly driven by LNCCWM (r = −0.93, p < 0.001) and did not correlate to LCCCWM.

Table 5.
Correlation matrix for Pearson’s coefficients involving community weighted mean (CWM) traits in the seven fields (n = 70).
The community functional diversity was evaluated by the FDQ index taking into account these five leaf traits (Table 4). FDQ showed the same trend as the number of dominant species and was the highest in field 3 (0.72), and the lowest in the productive fields 5 and 7 (0.10), dominated by Lolium perenne. The root density varied between fields (Table 4), from 2883 to 9007 g of root per cm3 of soil, with the lowest values corresponding to the youngest field (field 7).
3.4. Relationship Between Plant Community Functioning, Productivity and Soil C Stocks
Pearson’s correlations revealed strong relationships between vegetation community parameters and both productivity and soil C pools (Table 6). ANPP was negatively correlated with FDQ, and LDMCCWM but no relation between plant productivity and SLACWM or LNCCWM was observed. The SOCstock was correlated positively with LDMCCWM and LC:NCWM and negatively correlated with SLACWM and LNCCWM. Among functional parameters, LDMCCWM was the best functional indicator when focusing on the SOC with the highest correlation coefficient (r = 0.56, p < 0.001). A high HWC percentage in SOC appeared to be linked with fast growing communities, as suggested by its positive correlation with SLACWM and LNCCWM and negative correlation with LDMCCWM and LC:NCWM. Notably, no correlation was observed between functional diversity (FDQ) and soil C pools. Multivariable analyses indicated that LDMC related also to ANPP (r2 = 0.60, p < 0.001) (Figure 6a) and root C:ABG C ratio (r2 = 0.58, p < 0.001) (Figure 6b), which both related to SOC, i.e., the LDMC-biomass production linkage (i.e., ANPP and root C:ABG C ratio) increased with SOC, showing an optimum LDMC beyond SOC declines.

Table 6.
Correlation matrix for Pearson’s coefficients, for the plant community parameters and soil C pools of the seven fields (n = 70).
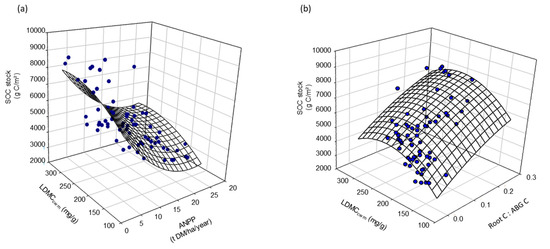
Figure 6.
Relations among SOC stock and LDMCcwm and (a) ANPP (r2 = 0.60, p < 0.001) or (b) Root C:ABG C (r2 = 0.58, p < 0.001), for the seven fields assessed with multiple nonlinear regression.
4. Discussion
The literature has often reported tradeoffs between livestock production and regulating services such as air or water quality or C sequestration [46,47]. Here, we focused on the linkage between the two ecosystem services (ES), productivity and soil C stocks. As ES provision does vary with management but also with local environmental drivers [48], we examined seven grassland fields within the same pedoclimatic (i.e., experimental farm in Normandy), differing in ages since last seeding and management practices with respect to C exports and fertilization. The overall analyses of annual net primary productivity (ANPP, variation from 8 to 21.9 t DM ha−1 year−1) highlighted that, indeed, productivity was negatively correlated with SOCstock.
4.1. Distribution of C Pools in the Plant–Soil System
Our results show that a very high proportion of total C stock was stored in the soil (89.3%) when considering the mean of biomasses before harvest and the 0–10-cm soil horizon. Overall, SOC content ranged between 2.9 and 6.8% (of the soil dry mass), largely above the threshold of 2% which is often considered as the threshold under which a serious decline in soil quality may occur [49]. Results are within the range of Ammann et al. [50] where 2.3 and 2.6% of C are allocated to aboveground vegetation and roots, while soil contains 95.1% of the plant–soil C stocks, when the mean aboveground biomass is considered before harvest and the 0–20 cm soil horizon in temperate grasslands. Our higher SOC contents were partly explained by the high proportion of clay which contribute to soil organic matter stabilization by the formation of aggregates that decrease C-turnover rates [51,52].
The aboveground biomass (above 5 cm), containing on average 8.9% of the total amount of C, did not constitute a storage pool because this compartment is removed one to several times a year by cutting or grazing. Stubbles remain after cutting. They accumulate C reserves that are mobilized to ensure regrowth by means of C re-allocation above the cutting level [53,54]. Nonetheless, stubbles are also implied in belowground C allocation through litter deposition and through belowground C allocation to roots. Roots contribute to the transfer of C to soil through root mortality and rhizodeposition such as exudation, mucilage production and sloughing from living roots [30,55]. Consequently, stubbles and roots represent key compartments of the grassland C cycle. However, in the present study, stubbles and roots (1.4% and 0.4% of the total C) constitute only a small proportion of the plant–soil C pools.
Nevertheless, we observed a positive correlation between root C and SOC stock that underlines the key role roots played in the C transfer to the soil. The relationship between the aboveground C pool and SOC stock suggests a link between productivity and conservation of C stocks through the compromise between investment of C to either root biomass or shoot biomass with increasing N supply and C exports (see Figure 5b) [56]. Indeed, C distribution in both pools varied among fields (Figure 2); the low productive and permanent grasslands had lower stubble C and higher root C allocation than the more productive and younger grasslands.
4.2. Direct and Indirect Effects Grassland Management, Productivity and C Stocks
Management practices have direct and indirect effects on ecosystem functioning. For instance, grassland renovation and frequency of exports act on ecosystem functioning via effects on floristic composition [23,31]. In the present study, most productive grasslands had low soil C stocks and were characterized by communities highly dominated by perennial ryegrass (Lolium perenne) while the three less productive fields with high soil C stocks differed in their flora (Table 3). For example, field 3, managed with a low grazing intensity and no fertilization supply, differed markedly from the two other low productive fields by a high relative cover (30%) of meadow buttercup (Ranunculus acris), which is known as a weed under aged extensively managed grasslands [57].
For these low productive old (>30 years) fields we observed the highest soil C stocks (i.e., 1 to 3) which were also the oldest (>30 years). Tillage is largely recognized for its negative impact on SOC stocks by means of stimulated soil respiration and soil structure damages [51,58,59,60]. Additionally, microbial abundance tends to decrease with grassland age [61], which may contribute to a decrease in the organic matter decomposition rate. Consequently, short duration grasslands, submitted to periodic tillage and re-sowing, tend to have higher productivity but lower soil C storage than permanent grasslands [8] due to lower C inputs to soil (e.g., root C, Figure 5b and Figure 6b) and higher litter decomposition rates [27]. Indeed, LDMC was identified as a pivotal trait influencing the quality of the litter produced, and hence litter decomposition and related ecosystem properties [25,62].
Even though C inputs to soil were not measured directly in the present work, the measurement of HWC in the topsoil provided information about C dynamics. This HWC pool, which is a component of the labile SOC, originates from soil microbial biomass, root exudates and lysates [63]. HWC thus constitutes the readily-decomposable soil organic matter that is more sensitive to changes than SOC [45] and can be monitored to estimate the supply of decomposable SOC in soils [64]. In the seven fields studied here, HWC in the 0–10-cm layer varied between 2.3 and 4.0% of SOC, which was in line with temperate grassland observations by Breulmann [65] where HWC represented 4% of SOC in the 0–30-cm topsoil, with the largest percentage detected in the 0–10-cm layer. The low HWC proportion observed in the less productive old fields characterized by high LDMCcwm may indicate that high C stocks result from slow degradation of plant residues. Inversely, a faster litter decomposition, combined with high root exudation and subsequent enhanced microbial biomass and activity [66], and thus enhanced mineralization [67], may explain the high HWC proportion in the highly productive fields. In view of that, the positive relationship between ANPP and HWC proportion as well as the negative relationship between them and SOC stocks suggest that plant community functioning drives SOC through the quality of organic matter, the mineralization rate and C exports.
Accordingly, C exports and fertilization have direct effects on C inputs to soil and C storage potential. Indeed, analyses show a negative relation between SOC and ANPP, where the latter is positively related to N supply and vice versa for SOC (Figure 5a,b). Data suggest thus a compromise between N inputs, C exports and C storage potential. Hence, management practices aiming to increase productivity by N fertilization may increase C inputs and C storage potential through enhanced primary production [13,14,36,68,69]. However, Soussana and Lemaire [46] have suggested that N fertilization has an optimum until which both biomass production and C sequestration increase, whereas high N fertilization decreases C sequestration by increasing the organic matter mineralization rate and C exports. Whereas in grasslands, which have been under constant management for a long-time (i.e., without improvement), we observe a decline in plant biomass production in parallel to an increase in C sequestration (see also De Deyn et al. [16]). This increase is often assigned to a reduction in organic matter mineralization due to a slow degradation of plant residues [70]. In the present study, old low productive fields seemed to have gone into this phase as suggested by the comparable lower ANPP, the higher LDMC and low HWC (i.e., indicators of higher litter decomposition rate of and C inputs).
4.3. Links Among Soil C Stocks, Productivity and Plant Community Functioning
Among the community weighted mean traits, LDMCCWM appeared to be the most relevant trait when analyzing the relationship between grassland functioning, ANPP and SOC. The negative correlation between SLACWM and LNCCWM with LDMCCWM and LC:NCWM is well known and has been reported also in the literature [18,19,20]. Similarly, a positive relationship have been observed between SOC, LDMCCWM and age as also observed for a Mediterranean old field succession [22] and in nutrient-poor natural grasslands [71]. Conservative species as defined by low SLA and LNC and high LDMC and LC:N promote C storage particularly below ground [72] and their presence may limit C output. Our productive grasslands are characterized by high LNCCWM and low LDMCCWM and this community trait pattern has already been related to low litter lignin content and rapid litter decomposability [20,23,24]. Traits related to the tradeoff between acquisition and conservation of resources [18,19,20] are involved in both the response to and affecting global change [73]. However, predicting future response at the species and the plant community levels to global change from past relation, are difficult to decipher as elevation of CO2 in atmosphere, warming and the drought can affect traits in different ways [74].
Last, even if low productive fields tend to have high functional diversity, we did not find evidence of a relationship between functional diversity (FDQ) and soil C stocks. This finding is not in line with previous studies demonstrating that plant species richness and functional richness expressed as the number of functional groups may promote C sequestration [30,72,75]. However, the low taxonomic richness found in the seven fields suggested overall low functional diversity indexes in the present study. A panel of grasslands along a gradient of diversity would be needed to test the impact of functional diversity on C stocks.
5. Conclusions
In our study, we could validate our first hypothesis (Hyp1), a tradeoff between two ecosystem services (ES) through the negative linear relationship between forage production and SOC. Our data also support the second hypothesis (Hyp2) as, among leaf traits, LDMCcwm, closely related to both primary productivity and the conservation of C stocks, appears particularly relevant for analyzing the relationship between these two services. The trait-based approach used here has allowed to demonstrate that plant community functioning, resulting from a combination of grassland age and management, affects forage production and soil C stocks in an opposite way, whereas LDMCCWM appeared as the best predictor of both ES and their relationships. The more the community is characterized by traits indicating resource conservation (e.g., high LDMC, low SLA), the lower are aboveground production and soluble soil C (HWC), and the higher are root biomass and soil C stock. Management practices act on the productivity of vegetation and therefore on C inputs as well as on the characteristics of plant communities including the quality of organic matter and therefore the C exports. Accordingly, grassland management plays a key role in mitigating atmospheric carbon dioxide though “best” management practices, being adopted to a compromise between forage production and conservation of soil C stocks.
Author Contributions
S.L.-L., A.M.-B. and J.-B.C. designed the study, C.K. carried out the experiment, C.K., S.L.-L. and A.M.-B. led the writing, J.-B.C. and K.K. contributed to the text. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Conseil Régional de Basse-Normandie.
Acknowledgments
We thank Y. Gallard and B. Blanchet from the INRAE Experimental Domain of Le Pin-au-Haras for their collaboration and UMR EVA technical staff for their skillful assistance. We are grateful to Laurence Cantrill for English language correction. We are most grateful to PLATIN’ (Plateau d’Isotopie de Normandie) core facility for all element and isotope analysis used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eurostat 2015 [Data file]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Land_cover_statistics (accessed on 7 April 2020).
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; p. 137. [Google Scholar]
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.B.; Silveira, M.L. Grassland Management Affects Delivery of Regulating and Supporting Ecosystem Services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Huguenin-Elie, O.; Delaby, L.; Klumpp, K.; Lemauviel-Lavenant, S.; Ryschawy, J.; Sabatier, R. The role of grasslands in biogeochemical cycles and biodiversity conservation. In Improving Grassland and Pasture Management in Temperate Agriculture; Marshall, A., Collins, R., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 1–27. [Google Scholar]
- Petri, M.; Batello, C.; Villani, R.; Nachtergaele, F. Carbon status and carbon sequestration potential in the world’s grasslands. In Grassland Carbon Sequestration: Management, Policy and Economics; Abberton, M., Conant, R., Batello, C., Eds.; FAO: Rome, Italy, 2010; pp. 19–31. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Soussana, J.F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Linsler, D.; Geisseler, D.; Loges, R.; Taube, F.; Ludwig, B. Temporal dynamics of soil organic matter composition and aggregate distribution in permanent grassland after a single tillage event in a temperate climate. Soil Till. Res. 2013, 126, 90–99. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Eze, S.; Palmer, S.M.; Chapman, P.J. Soil organic carbon stock and fractional distribution in upland grasslands. Geoderma 2018, 314, 175–183. [Google Scholar] [CrossRef]
- Ammann, C.; Flechard, C.R.; Leifeld, J.; Neftel, A.; Fuhrer, J. The carbon budget of newly established temperate grassland depends on management intensity. Agric. Ecosyst. Environ. 2007, 121, 5–20. [Google Scholar] [CrossRef]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland management and conversion into grassland: Effects on soil carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- Leifeld, J.; Ammann, C.; Neftel, A.; Fuhrer, J. A comparison of repeated soil inventory and carbon flux budget to detect soil carbon stock changes after conversion from cropland to grasslands. Glob. Chang. Biol. 2011, 17, 3366–3375. [Google Scholar] [CrossRef]
- Soussana, J.F.; Fuhrer, J.; Jones, M.; Van Amstel, A. The greenhouse gas balance of grasslands in Europe. Agric. Ecosyst. Environ. 2007, 121, 1–4. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Shiel, R.S.; Ostle, N.J.; McNamara, N.P.; Oakley, S.; Young, I.; Freeman, C.; Fenner, N.; Quirk, H.; Bardgett, R.D. Additional carbon sequestration benefits of grassland diversity restoration: Soil C sequestration and diversity restoration. J. Appl. Ecol. 2011, 48, 600–608. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billes, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Garnier, E.; Navas, M.L. A trait-based approach to comparative functional plant ecology: Concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 2012, 32, 365–399. [Google Scholar] [CrossRef]
- Klumpp, K.; Soussana, J.F. Using functional traits to predict grassland ecosystem change: A mathematical test of the response-and-effect trait approach. Glob. Chang. Biol. 2009, 15, 2921–2934. [Google Scholar] [CrossRef]
- Fortunel, C.; Garnier, E.; Joffre, R.; Kazakou, E.; Quested, H.; Grigulis, K.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 2009, 90, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Quested, H.; Eriksson, O.; Fortunel, C.; Garnier, E. Plant traits relate to whole-community litter quality and decomposition following land use change. Funct. Ecol. 2007, 21, 1016–1026. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Marti, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R.; et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Steinbeiss, S.; Beßler, H.; Engels, C.; Temperton, V.M.; Buchmann, N.; Roscher, C.; Kreutziger, Y.; Baade, J.; Habekost, M.; Gleixner, G. Plant diversity positively affects short-term soil carbon storage in experimental grasslands. Glob. Chang. Biol. 2008, 14, 2937–2949. [Google Scholar] [CrossRef]
- Gaujour, E.; Amiaud, B.; Mignolet, C.; Plantureux, S. Factors and processes affecting plant biodiversity in permanent grasslands. A review. Agron. Sustain. Dev. 2012, 32, 133–160. [Google Scholar] [CrossRef]
- Loreau, M. Biodiversity and ecosystem functioning: Recent theoretical advances. Oikos 2000, 91, 3–17. [Google Scholar] [CrossRef]
- Schaub, S.; Finger, R.; Leiber, F.; Probst, S.; Kreuzer, M.; Weigelt, A.; Buchmann, N.; Scherer-Lorenzen, M. Plant diversity effects on forage quality, yield and revenues of semi-natural grasslands. Nat. Commun. 2020, 11, e768. [Google Scholar] [CrossRef]
- Roscher, C.; Schumacher, J.; Gubsch, M.; Lipowsky, A.; Weigelt, A.; Buchmann, N.; Schmid, B.; Schulze, E.D. Using plant functional traits to explain diversity–productivity relationships (ed Chen HYH). PLoS ONE 2012, 7, e36760. [Google Scholar] [CrossRef]
- Cong, W.F.; Van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, E.F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; FAO: Rome, Italy, 2006; p. 132. [Google Scholar]
- IPCC 2006. Emissions from livestock and manure management. In IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; p. 87. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurlvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Lepš, J.; De Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical considerations matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. Eur. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Post, W.M.; Izaurralde, R.C.; Mann, L.K.; Bliss, N. Monitoring and verifying changes of organic carbon in soil. Clim. Chang. 2001, 51, 73–99. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Soussana, J.F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Dumont, B.; Ryschawy, J.; Duru, M.; Benoit, M.; Chatellier, V.; Delaby, L.; Donnars, C.; Dupraz, P.; Lemauviel-Lavenant, S.; Méda, B.; et al. Review: Associations among goods, impacts and ecosystem services provided by livestock farming. Animal 2019, 13, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Le Clec’h, S.; Finger, R.; Buchmann, N.; Gosal, A.S.; Hörtnagl, L.; Huguenin-Elie, O.; Jeanneret, P.; Lüscher, A.; Schneider, M.K.; Huber, R. Assessment of spatial variability of multiple ecosystem services in grasslands of different intensities. J. Environ. Manag. 2019, 251, e109372. [Google Scholar] [CrossRef] [PubMed]
- Loveland, P.; Webb, J. Is there a critical level of organic matter in the agricultural soils of temperate regions: A review. Soil Till. Res. 2003, 70, 1–18. [Google Scholar] [CrossRef]
- Ammann, C.; Spirig, C.; Leifeld, J.; Neftel, A. Assessment of the nitrogen and carbon budget of two managed temperate grassland fields. Agric. Ecosyst. Environ. 2009, 133, 150–162. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Till. Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Don, A.; Scholten, T.; Schulze, E. Conversion of cropland into grassland: Implications for soil organic-carbon stocks in two soils with different texture. J. Soil Sci. Plant Nutr. 2009, 172, 53–62. [Google Scholar] [CrossRef]
- Morvan-Bertrand, A.; Boucaud, J.; Prud’homme, M.P. Influence of initial levels of carbohydrates, fructans, nitrogen, and soluble proteins on regrowth of Lolium perenne L. cv. Bravo following defoliation. J. Exp. Bot. 1999, 50, 1817–1826. [Google Scholar] [CrossRef]
- Morvan-Bertrand, A.; Pavis, N.; Boucaud, J.; Prud’homme, M.P. Partitioning of reserve and newly assimilated carbon in roots and leaf tissues of Lolium perenne during regrowth after defoliation: Assessment by 13C steady-state labelling and carbohydrate analysis. Plant Cell Environ. 1999, 22, 1097–1108. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Poeplau, C. Estimating root: Shoot ratio and soil carbon inputs in temperate grasslands with the RothC model. Plant Soil 2016, 407, 293–305. [Google Scholar] [CrossRef]
- Bourdot, G.; Lamoureaux, S. Giant Buttercup (Ranunculus acris L.) management in dairy pastures-current problems and future solutions. In Proceedings of the New Zealand Grassland Association, West Coast, New Zealand, 1 January 2002; pp. 61–65. [Google Scholar]
- Liu, X.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2006, 52, 531–543. [Google Scholar] [CrossRef]
- Post, W.M.; Izaurralde, R.C.; West, T.O.; Liebig, M.A.; King, A.W. Management opportunities for enhancing terrestrial carbon dioxide sinks. Front. Ecol. Environ. 2012, 10, 554–561. [Google Scholar] [CrossRef]
- Powlson, D.S.; Whitmore, A.P.; Goulding, K.W.T. Soil carbon sequestration to mitigate climate change: A critical re-examination to identify the true and the false. Eur. J. Soil Sci. 2011, 62, 42–55. [Google Scholar] [CrossRef]
- Higashida, S.; Takao, K. Relations between soil microbial activity and soil properties in grassland. Soil Sci. Plant Nutr. 1986, 32, 587–597. [Google Scholar] [CrossRef]
- Kazakou, E.; Vile, D.; Shipley, B.; Gallet, C.; Garnier, E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Funct. Ecol. 2006, 20, 21–30. [Google Scholar] [CrossRef]
- Leinweber, P.; Schulten, H.R.; Körschens, M. Hot water extracted organic matter: Chemical composition and temporal variations in a long-term field experiment. Biol. Fert. Soils 1995, 20, 17–23. [Google Scholar] [CrossRef]
- Schulz, E. Influence of extreme management on decomposable soil organic matter pool. Arch. Agron. Soil Sci. 2002, 48, 101–105. [Google Scholar] [CrossRef]
- Breulmann, M.; Schulz, E.; Weibhuhn, K.; Buscot, F. Impact of the plant community composition on labile soil organic carbon, soil microbial activity and community structure in semi-natural grassland ecosystems of different productivity. Plant Soil 2012, 352, 253–265. [Google Scholar] [CrossRef]
- Guitian, R.; Bardgett, R.D. Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant Soil 2000, 220, 271–277. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Hill, P.W.; Jones, D.L. Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 2007, 290, 293–305. [Google Scholar] [CrossRef]
- Conant, R.T. Grassland soil organic carbon stocks: Status, opportunities, vulnerability. In Recarbonization of the Biosphere; Lal, R., Lorenz, K., Hüttl, R.F., Schneider, B.U., von Braun, J., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 275–302. [Google Scholar]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Fornara, D.A.; Steinbeiss, S.; McNamara, N.P.; Gleixner, G.; Oakley, S.; Poulton, P.R.; Macdonald, A.J.; Bardgett, R.D. Increases in soil organic carbon sequestration can reduce the global warming potential of long-term liming to permanent grassland. Glob. Chang. Biol. 2011, 17, 1925–1934. [Google Scholar] [CrossRef]
- Suter, M.; Edwards, P.J. Convergent succession of plant communities is linked to species’ functional traits. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 217–225. [Google Scholar] [CrossRef]
- Wardle, D.A.; Jonsson, M.; Bansal, S.; Bardgett, R.D.; Gundale, M.J.; Metcalfe, D.B. Linking vegetation change, carbon sequestration and biodiversity: Insights from island ecosystems in a long-term natural experiment: Islands and ecosystem processes. J. Ecol. 2012, 100, 16–30. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S., III; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Cantarel, A.A.M.; Bloor, J.M.G.; Soussana, J.-F. Four years of simulated climate change reduces above-ground productivity and alters functional diversity in a grassland ecosystem. J. Veg. Sci. 2013, 24, 113–126. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Quirk, H.; Yi, Z.; Oakley, S.; Ostle, N.J.; Bardgett, R.D. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J. Ecol. 2009, 97, 864–875. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).