Abstract
Conservation farming practices, such as no-tillage and crop residue retention, have been proposed as sustainable management practices. However, it remains unclear how different tillage practices and rice straw retention affect the soil bacterial community (SBC) and the soil C/N ratio in the long term. The objective of this study was to evaluate changes in SBC composition and abundance and soil properties (e.g., carbon (C), nitrogen (N)) and determine their relationship to the soil C/N ratio under long-term no-tillage and straw retention techniques. This study investigates the effect of a long-term field experiment begun in 2008 and continued until 2019 to measure the response of the SBC and soil properties and their relation to different tillage practices, including no-tillage (NT), no-tillage and straw mulching (NT-SM), conventional tillage (CT), conventional tillage and straw mulching (CT-SM), and conventional tillage and straw retention (CT-SR). Soil samples were collected at depths of 0–5 cm (A), 5–10 cm (B), and 10–20 cm (C) after rice harvesting in the early and late growing seasons in 2018–2019. The Illumina MiSeq sequencing and quantitative polymerase chain reaction (PCR) technology was used to analyze changes in SBC diversity in soil and determined the changes in the soil C/N ratio and their relationship with the SBC diversity. The results showed that the Proteobacteria, Acidobacteria, and Chloroflexi were the dominant phyla in the soil and accounted for 61.26%, 59.39%, and 55.62% of the total bacteria in the A, B, and C soil layers, respectively. The NT treatment increased SBC diversity, the number of operational taxonomic units (OTUs), and the proportion of Proteobacteria across the soil depths. Similarly, straw retention also significantly improved SBC diversity, soil organic C (SOC), total N (TN), soil C/N ratio, and the abundance of Proteobacteria and Acidobacteria in the soil layers A and B. The NT-SM treatment increased the SOC, TN, and soil C/N ratio by 30%, 21%, and 6% in 2018 and by 33, 25% and 7% in 2019, respectively, across the seasons and layers compared to the CT treatment. The NT-SM treatment had the highest soil bacterial diversity index, and the CT-SR treatment had the highest soil bacterial abundance and number of OTUs. The redundancy analysis showed that Acidobacteria were highly positively correlated with the soil C/N ratio. The results demonstrate that conservation tillage practices, i.e., no-tillage and straw retention, increase the SBC diversity and soil C/N ratio, thereby enhancing soil organic C and total N and changing soil microbial ecology. As a result, sustainable crop production and profitable agro-ecosystems are ensured.
1. Introduction
Soil microorganisms provide important ecosystem services, which are necessary to maintain agricultural productivity and ecosystem health [1,2]. Soil bacteria account for about 70–90% of the total amount of soil microorganisms [3], which participate directly or indirectly in soil biochemical processes and contribute to nutrient cycling and energy transformation in soil. Examples include the decomposition and synthesis of organic substances, which improve the soil structure due to the formation of organic matter [4]. The abundance and community structure of soil microorganisms, including bacteria, fungi, microbivores (protozoa and nematodes), and predators (nematodes) highly depend on soil management practices [5,6]. Changes in the soil bacterial community (SBC) can affect mineralization and decomposition of organic matter [7]. Therefore, an understanding of the changes in SBC and the relationship with the soil C/N ratio for different soil management practices may lead to better management of the SBC to achieve sustainable crop production [8,9]. In-addition, the SBC plays a pivotal role in soil ecological processes, including the decomposition of organic matter and the formation of soil aggregates, thereby affecting soil fertility and improving soil ecosystems [10,11]. The SBC has a primary role in the degradation of plant residues and the transformation of organic matter by secreting specific extracellular enzymes for the decomposition of macromolecular organic substances into monomer substances for plant absorption and utilization. This mechanism improves the turnover and circulation of soil nutrients, such as carbon (C) and nitrogen (N) [12]. It was reported that the SBC composition was affected by soil pH, organic matter content, and soil tillage practices [13,14]. Many studies have shown that the soil fertility status, especially the soil C and N content, is an important factor affecting the abundance of the bacterial community.
Changes in the soil physicochemical properties due to conservation tillage resulted in changes in the SBC composition [15,16]. Conservation tillage practices i.e., no-tillage, mulch tillage, strip or zonal tillage, ridge till (including no-till on ridges), reduced or minimum tillage, straw retention on the soil surface, and crop rotation minimize soil disturbance and maintain or improve the soil quality, thereby increase crop productivity [17,18,19].The most common conservation tillage practices are no-tillage and straw retention on the soil surface [18]. No-tillage is a tillage method that requires no actual tillage of land. Further, in no-tillage systems plant residue is left unharmed in fields so it can naturally decay over time, thus stores more C in soil for long time, rather than breaking it down side faster and letting C escape into the atmosphere [19,20]. Straw mulching or retention consists of placing a uniform layer of straw and incorporating it into the soil [20]. Straw retention practices have a major effect on soil water holding capacity and C conservation [21,22]. Moreover, other studies have found that no-tillage and straw retention increases soil microbial biomass, bacterial diversity, and enzymatic activities in soil [19,20,21,22]. The soil chemical properties of the surface layer are generally more favorable under no-tillage than tillage conditions, and the C storage is also improved due to minimal soil disturbance and the slow decomposition of organic C [23,24,25]. In-addition, significant changes were observed in the soil bulk density, soil aeration, soil water holding capacity, and water aggregate stability under no-tillage, straw retention, biochar and organic manure fertilization and these practices ultimately improved soil fertility and health [26,27,28]. Several studies have found that conservation tillage had significant effects on the physicochemical properties of paddy fields, such as improvements in the soil aggregation and permeability, soil organic C, N, bacterial community composition, and a reduction in the soil bulk density of the topsoil layer [29,30,31]. The SBC diversity and abundance exhibited significant changes in the topsoil layer under no-tillage with rice straw retention [32,33]. Lin et al. [34] stated that the abundance and diversity of SBC under no-tillage with straw mulching were significantly higher than traditional tillage practices. In contrast, in other studies, no-tillage with straw retention did not change soil microbial biomass, diversity, and soil enzymatic activities [31,32]. These results may be attributed to the study area, climatic conditions, soil nutrients status, and temporal and spatial factors. There is a lack of knowledge on the effect of different conservation tillage practices, i.e., no-tillage and rice straw retention, on soil bacterial diversity and community composition at different soil depths (layer).
Additionally, soil organic carbon (SOC) and total nitrogen (TN) are key indicators of soil fertility [33].The soil C/N ratio is a sensitive indicator of soil quality, and the soil microbial activities and plant growth are affected by the soil C/N ratio and play a vital role in soil nutrient status and cycling [34]. The soil C/N ratio is significantly affected by external C and N inputs, which regulate the soil C and N cycle. Generally, the C/N ratio of rice straw is approximately 60/100, and that of soil is 3/50. Hence, rice straw addition improves the soil C/N ratio. In the process of depolymerization and mineralization of organic matter, microorganisms absorb C and N for self-regulation. Changes in the soil C/N ratio are closely related to SBC [35]. Similarly, Liu et al. [36] reported that an improvement in the soil C/N ratio might have adverse effects on the reproduction of soil microorganisms. Further, changes in soil C/N ratio affect soil microbial biomass and the fixation and release of nutrients from organic fertilizers, thus affecting soil fertility [37,38]. The soil C/N ratio also significantly influences soil microbial activity, organic matter decomposition and accumulation, the soil C and N cycle, and nutrient availability. Therefore, changes in the soil C/N ratio and SBC abundance can be used to predict soil fertility. It has been demonstrated that conservation tillage practices, such as no-tillage and straw retention, have significant impacts on soil microbial diversity and soil quality. However, the long-term impact of no-tillage and straw retention on soil bacterial community diversity and abundance and its relationship with changes in the soil C/N ratio is not fully understood, particularly in the humid, warm climate region of Guangxi in southern China.
The objectives of this study are (1) to determine changes in the SBC composition and abundance in response to long-term no-tillage and straw retention, (2) to determine changes in soil organic C and N and their relationship with changes in the SBC composition under long-term conservation tillage practices, and (3) to determine the bacterial diversity and community composition in different soil layers.
2. Materials and Methods
2.1. Site Description
The long-term experiment was performed at the experimental research station of Guangxi University (22°49′12″ N, 108°19′11″ E; 75 m). The climate is classified as a subtropical monsoon climate region, with a mean annual rainfall of 990 mm and a mean annual temperature of 21.6 °C (local weather station). The soil (0–20 cm) is an Ultisol, which is slightly acidic, with a pH of 5.94 (H2O). A soil test indicated the following contents: the SOC was 30.96 g kg−1, the TN was 1.04 g kg−1, and the available N, phosphorus (P), and K were 155.29 g kg−1, 212.08 g kg−1, and 121.44 g kg−1, respectively.
2.2. Experimental Design
The long-term field experiment in two rice growing seasons began in the fall of 2008 and continued until 2019; the system consisted of the early season (March to July) and the late season (July to November). The experiment was a randomized complete block design (RCBD) with five treatments and three replications. The plot size was 6 m × 6 m. The treatments in this study included (1) no-tillage (NT), (2) no-tillage and straw mulching (NT-SM), (3) conventional tillage (CT), (4) conventional tillage and straw mulching (CT-SM), and (5) conventional tillage and straw retention (CT-SR). In the NT treatment, there was no soil disturbance except for rice planting. The NT-SM treatment involved no-tillage, and after the rice harvest in each season, the plot area was evenly covered by the rice straw. In the CT-SM treatment, after rice harvesting, all the straw was removed from the plot area, and the plot was plowed with a micro-tiller (tillage); after plowing, the rice straw was evenly distributed on the surface of the plot area. In the CT treatment, after the rice harvest in each season, the rice straw was completely removed from the plot area, and the area was plowed (0–20cm) with a micro-tiller. In the CT-SR treatment, the rice straw was evenly distributed on the soil surface, and the area was plowed with a micro-tiller to incorporate the rice straw into the soil. The plots were surrounded by ridges (30 cm high and 20 cm wide) covered by thin polythene plastic to prevent water and fertilizer from other plots to enter the plot area. The rice seeds were started in plastic seedling trays, and the 25-day-old seedlings with uniform size were transplanted into the field.
The same dose of NPK fertilizer at a ratio of 232:98:180 (kg ha−1) was used for each treatment, and each plot received 1800 g urea, 2220 g superphosphate, and 1080 g potassium. N and K were applied three times, i.e., 50% of the amount was applied initially, 30% was applied seven days after transplanting, and 20% was applied at the jointing stage. All superphosphate was applied as a basal dose one day before transplanting. Uniform flooding (about four cm deep) was continued from transplanting until physiological maturity. Throughout the growing season, standard agricultural practices, such as irrigation and applications of insecticides and herbicides, were performed in the same manner for all pots during both seasons.
2.3. Soil Sampling
Soil samples were collected from three soil depths, i.e., 0–5 cm (A), 5–10 cm (B), and 10–20 cm (C) at the end of the 11-year study after post-harvest in the early and late seasons in 2018–2019. Five subsamples were collected randomly from each plot and were mixed evenly to create a bulk sample. There were 45 samples (five treatments with three replicates at three soil depths). Each soil sample was homogenized, and the plant roots and large rocks were removed. All the samples were divided into two parts: the first part was stored at −80 °C and was used to determine the SBC structure and for DNA extraction and the second part was air-dried and used for the determination of the soil chemical properties.
2.4. Soil Analysis
Soil organic C was determined by the oxidation method with K2Cr2O7-H2SO4. For the chemical analysis, 0.5 g of the soil was digested with 5 mL of 1M K2Cr2O7 and concentrated H2SO4 and heated at 175 °C for 5 min, followed by titration of the digests with FeSO4 [39]. For the TN analysis, 200 mg of the samples were digested using the salicylic acid–sulfuric acid–hydrogen peroxide method described by Ohyama et al. [40], and the TN was determined using the micro-Kjeldahl procedure [41]. The soil C/N ratio was calculated by dividing the SOC concentration by the soil TN concentration.
2.5. DNA Extraction, Bacterial 16S rRNA Gene Amplification and MiSeq Sequencing
Soil DNA was extracted from 0.25 g of wet soil using an E.Z.N.ATM Mag-Bind Soil DNA Kit (Shanghai Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s protocol. The DNA extracts were quantified with a nano spectrophotometer. An aliquot (50 ng) of DNA from each sample was used as a template for bacterial 16SrRNA gene amplification. Briefly, the bacterial hypervariable domain V3–V4 was amplified with region-specific primers (341F:5′-CCTACGGGNGGCWGCAG-3′ and 805R:5′-GACTACHVGGGTATCTAATCC-3′) that included the Illumina (San Diego, CA, USA) flow-cell adapter sequences [42]. The index sequences were added, and enrichment was performed after extraction. The polymerase chain reaction (PCR) amplification procedure was as follows: 94 °C for 3 min, 94 °C for 30 s, 45 °C for 20 s, 65 °C for 30 s 5 cycles, 94 °C for 20 s, 55 °C for 20 s, 72 °C for 30 s, 20 cycles, and extension at 72 °C for 5 min. A Qubit 3.0 (Thermo Fisher Scientific, Waltham, MA, USA) and Bio-RAD T100TM(Bio-Rad Laboratories, Hercules, CA, USA) Thermal Cycler were used to quantify the concentration and determine the purity of the library to ensure quality. Subsequently, the library was sequenced with an Illumina MiSeq instrument.
2.6. Processing of Illumina Sequencing Data
According to the overlap of the clean data, we spliced the paired reads using PEAR [43] software to merge the sequences. We used the software QIIME and MOTHER (Northern Arizona University: United State) to filter and remove the chimera of the sequences at the connection, and clustering into operational taxonomic units (OTUs) based on 97% pair-wise identity was performed using UCLUST [44]. Taxonomic classification of the representative sequence for each OTU was performed using the RDP classifier or QIIME’s Closed Reference strategy against the 16S rRNA database.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was performed to determine the differences in the soil properties and SBC composition among the five treatments using Statistics 8.1 analytical software. The data were first checked for normal distribution and then the assumptions were followed. A Venn graph was used to count the number of common and unique OTUs of the samples and determine the similarity and overlap of the number of OTUs of the environmental samples. The abundance index and diversity index of the bacterial community were calculated using Mothur software. The species were classified using R software R Foundation for Statistical Computing (Math Soft Company: New Zealand).We used redundancy analysis (RDA) to analyze the strength of the association between the soil properties and SBC diversity [45]; the analyses were conducted in R 3.2 software. The Simpson index [46] was calculated as follows:
where λ is the Simpson diversity index and is the proportion of an individual peak height relative to the sum of all peak heights. The Shannon’s diversity index [47] was calculated as follows:
and this index is commonly used to characterize species in a community. The Pielou evenness index [48] was derived from Shannon’s diversity index and was calculated as follows:
where Ὴ max = In (S) and S represents the total number of species. The other data were analyzed using Excel 2016(Microsoft Corporation, Redmond, WA, USA) and DPS software (DPS Software Ltd., Enfield, UK).
3. Results
3.1. Sequencing Reads and Bacterial Diversity
A total of 1,043,940 effective sequences were obtained from the Illumina MiSeq high-throughput sequencing, as shown in Table 1. There were 349,635 sequences in soil layer A, 341,446 sequences in soil layer B, and 352,859 sequences in soil layer C. In layer A, the number of sequences was highest for the CT-SR treatment (72,301) and the lowest for the NT-SM treatment (66,550). In soil layer B, the number of sequences was the maximum for the NT treatment (71,404) and lowest for the NT-SM treatment (66,134). In soil layer C, the number of sequences was highest for the CT-SM treatment (76,541) and the lowest for the NT-SM treatment (63,036). The length of the high-quality sequences was in the range of 400–430 bp, which is approximately equal to the length of the 16SrRNA v3–v4 region (about 400 bp). The library coverage rate of the soil samples was 95%, indicating that the data of the soil bacterial library reflect the microbial community in the samples.

Table 1.
The number of sequences at different soil depths for different conservation tillage practices.
3.2. Soil Properties
Tillage practices and straw retention significantly affected the SOC, TN, and soil C/N ratio after harvesting of the rice crop in the early and late growing seasons in 2018–2019 after long-term (11 years) experimentation in the same field (Table 2). The SOC, TN, and soil C/N ratio showed a decreasing trend with the soil depth from soil layer (0–5) A to (5–10) B to (10–20) C. The results were similar for both seasons. The average values of the SOC, TN, and soil C/N ratio for both seasons were 33.09%, 32.82%, and 3.51% lower in 2018 and 38.05%, 37.85%, and 5.23% lower in 2019, respectively, in soil layer C than soil layer A.

Table 2.
Changes in soil chemical traitsat different soil depths under different conservation tillage practices.
In soil layer A, the SOC, TN, and the C/N ratio were significantly higher in the NT-SM treatment than in all other treatments. Averaged across the seasons, the SOC, TN, and the C/N ratio were 20.95%, 16.15%, and 5.86%, respectively higher in 2018 and 22.52%, 17.85%, and 6.15% higher in 2019 in the NT-SM treatment than the NT treatment. The SOC, TN, and the C/N ratio were 33.54%, 22.85%, and 6.98%, respectively higher in 2018 and 35.85%, 25.58%, and 7.84% higher in 2019 in the NT-SM treatment than the CT treatment in soil layer A. Similarly, in soil layer B, the SOC, TN, and C/N ratio was significantly higher in the CT-SM treatment than in all other treatments, as shown in Table 2. The SOC and TN content in soil layer B was 18% and 12%, respectively higher in 2018 and 20%, and 26% higher in 2019 in the CT-SM treatment than the NT treatment across the seasons. However, the difference between the NT-CM and CT-SR treatments was statistically (p < 0.05) non-significant. In-addition, the conservation tillage practices also increased the SOC and TN in soil layer C. The CT-SR treatment significantly increased the SOC and TN by 10% and 12% in 2018 and 17% and 15% in 2019, respectively, compared with the NT treatment across the seasons.
3.3. SBC Composition and Structure
3.3.1. Soil Bacterial Diversity and Abundance Index
The Chao1 index and abundance-based coverage estimator (ACE) index represents the SBC abundance in the soil; the higher the value, the higher the species abundance is. Similarly, Shannon and Simpson indices indicate the degree of diversity of the SBC. The higher the Shannon index, the higher the SBC diversity is, and the higher the Simpson index, the lower the bacterial community diversity is.
In the present study, the tillage practices and straw retention significantly affected the soil microbial community composition and diversity, which showed decreasing, trends with increasing soil depth (Table 3). In soil layer C, the Shannon, ACE, and Chao1 indices were 7.19%, 21.31%, and 21.80% lower, respectively than in soil layer A, and the Simpson index was 44.90% higher in soil layer C than in soil layer A as shown in Table 4.

Table 3.
Changes in bacterial abundance and diversity indices at different soil depths for different conservation tillage practices.

Table 4.
Changes in bacterial abundance and diversity indices under different conservation tillage practices.
Table 3 shows that the conservation tillage practices significantly affected the SBC abundance and diversity in soil layers A, B, and C. In soil layer A, the NT-SM treatment resulted in the highest diversity index of the soil bacteria, whereas the CT-SM treatment exhibited the highest abundance of soil bacteria. In soil layer B, the CT-SM treatment had the highest diversity index of soil bacteria, whereas higher soil bacterial abundance was observed in the CT-SR treatment. The NT-SM treatment exhibited the highest bacterial diversity, whereas the CT-SR treatment had the highest bacterial abundance in soil layer C.
3.3.2. Bacterial Community Diversity
The total number of OTUs was obtained from the soil samples using Illumina MiSeq sequencing analysis. The Venn diagram (Figure 1) shows that the total number of OTUs in the soil layers A, B, and C was 23,221, 22,864, 18,939, respectively, indicating decreasing trend from soil layer A to C. The number of unique OTUs was 3559, 1952, and 2175 in soil layers A, B, and C, respectively. The results showed that the number of total OTUs and the number of unique OTUs were maximum in soil layer A.
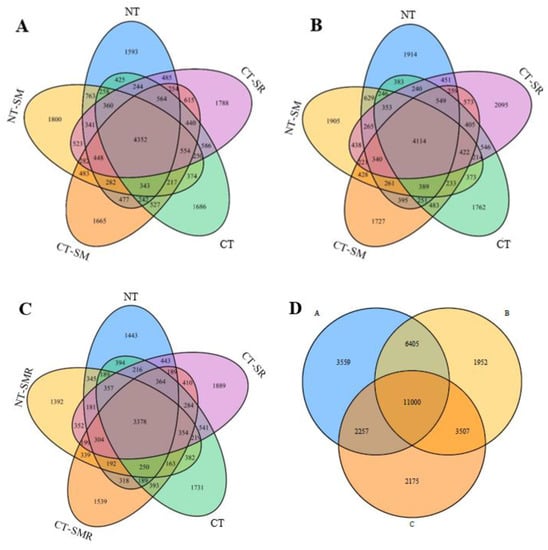
Figure 1.
Venn diagrams showing the bacterial operational taxonomic units (OTUs) at a 3% sequence dissimilarity level for the five tillage treatments and three soil depths. Note: NT: no-tillage, NT-SM: no-tillage and straw mulching, CT: conventional tillage, CT-SM: conventional tillage and straw mulching, CT-SR: conventional tillage and straw retention; (A): 0–5 cm soil depth, (B): 5–10 cm soil depth, (C): 10–20 cm soil depth, and (D): 0–20 cm soil depth.
In soil layer A, the number of OTUs in the NT, NT-SM, CT-SM, CT, and CT-SR treatments was 11,431, 11,630, 11,745, 11,422, and 12,086, respectively. The number of unique OTUs was 1593, 1800, 1665, 1686, and 1788 respectively, and the number of common OTUs was 4325. Similarly, in soil layer B, the number of OTUs in the NT, NT-SM, CT-SM, CT, and CT-SR treatments was 11,039, 10,831, 11,445, 10,963, and 11,485, respectively; the number of unique OTUs was 1914, 1905, 1727, 1762, and 2095, respectively, and the number of common OTUs was 4114. In soil layer C, the number of OTUs in the NT, NT-SM, CT-SM, CT, and CT-SR treatments was 8752, 8596, 8865, 9404, and 9680, respectively, the number of unique OTUs was 1443, 1392, 1539, 1731, and 1889, and the number of common OTUs was 3378. The results indicated that the number of OTUs was highest for the CT-SR treatment at all soil depths.
3.3.3. Bacterial Community Composition
The bacterial community analysis of the soil samples of the different tillage treatments and different soil depths indicated the presence of 19 phyla, as shown in Figure 2. In layer A, the dominant bacterial phyla included Proteobacteria (44.88%) (average relative abundance), Acidobacteria (11.58%), and Chloroflexi (4.8%) (Figure 2A). Similarly, in soil layer B, Proteobacteria (40.78%), Acidobacteria (12.78%), and Chloroflexi (6.33%) were dominant (Figure 2B). In soil layer C, Proteobacteria, Acidobacteria, Chloroflexi, and Firmicutes were dominant and accounted for 36.64%, 11.56%, 7.42%, and 6.94% of the total, respectively. The results showed that Proteobacteria, Acidobacteria, and Chloroflexi were the dominant phyla in soil layers A, B, and C. The analysis showed that the percentage of Proteobacteria decreased significantly with the increase in soil depth, whereas the percentage of Acidobacteria increased with the increase in soil depth. The proportions of the three dominant phyla were 61.26%, 59.39%, and 55.62% in soil layers A, B, and C, respectively; a decreasing trend with increasing soil depth was observed (Figure 3).
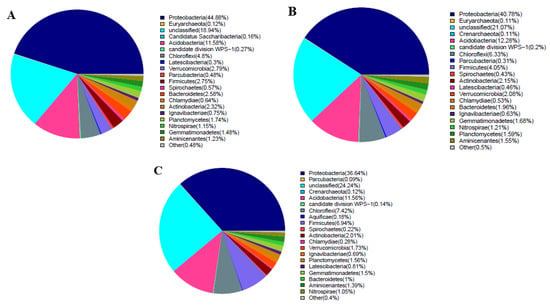
Figure 2.
Bacterial community composition at soil depths of 0–5 cm (A), 5–10 cm (B), and 10–20 cm (C).
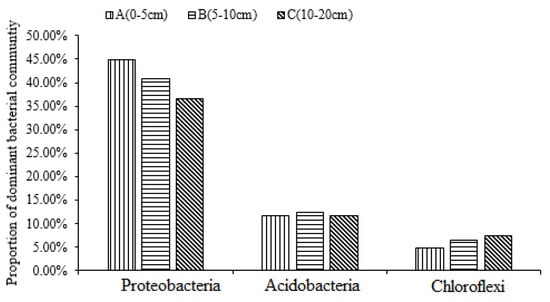
Figure 3.
The dominant soil bacterial community at different soil depths.
The bacterial community composition at the phylum level in the three soil layers for different tillage practices are shown in Figure 4. The proportions of the dominant bacterial phyla were different at different soil depths; however, the diversity was the same. At all soil depths, the proportion of Proteobacteria was significantly lower in the CT treatment than in all other treatments. In soil layer A, the proportion of Proteobacteria was significantly higher (by 7.9%) in the NT treatment than the CT treatment, as well as from all other treatments. The NT-SM treatment had the highest proportion of Acidobacteria, which was 29.56% higher than that in the NT treatment. The Chloroflexi proportion in the CT-SR treatment was 44.33% and 19.13% higher than in the NT and CT treatments, respectively. Similarly, in soil layer B, the Proteobacteria proportion in the CT-SR treatment was 5.65% and 7.26% higher than in the NT and CT treatments, respectively. The CT-SM treatment had the highest Acidobacteria proportion among the treatments. The Chloroflexi proportion in the NT-SM treatment was 34.13% and 11.94% higher than in the NT and CT treatments, respectively. Additionally, in soil layer C, the NT treatment had the highest Proteobacteria proportion (10.58% higher than in the CT treatment), and the proportions of Acidobacteria and Chloroflexi were highest in the CT treatment.
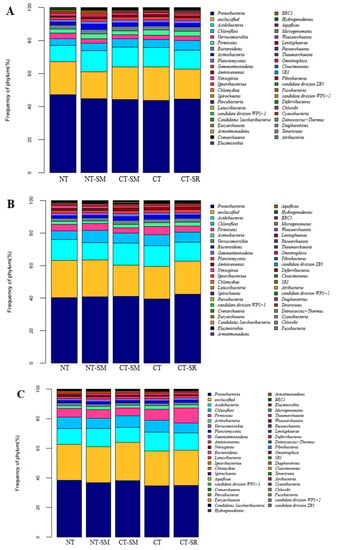
Figure 4.
Soil bacterial community composition at soil depths of 0–5 cm (A), 5–10 cm (B), and 10–20 cm (C) for different tillage practices. Note NT—no-tillage, NT-SM—no-tillage and straw mulching, CT—conventional tillage, CT-SM—conventional tillage and straw mulching, CT-SR—conventional tillage and straw retention.
3.4. Relationship between Bacterial Community Composition and Soil Properties
An RDA was performed to determine the strength of the association between the soil C and nitrogen N contents and the diversity of the SBC. Figure 5 shows the relationship between the bacteria communities (at the phylum level) and the soil properties for the different treatments. In soil layer A, the five treatments (NT, NT-SM, CT-SR, CT, and CT-SR) occurred in different quadrants, which indicated that the different tillage treatments had significant effects on the SBC (Figure 5A). Moreover, the RDA showed that the soil chemical properties (TN, SOC, and C/N ratio) in all treatments affected the SBC; however, a significantly higher correlation existed for TN, SOC, and C/N with NT-SM. In soil layer A, Proteobacteria were positively correlated with SOC (r = 0.05) and TN (r = 0.12) and negatively correlated with the C/N ratio (r = −0.11). Acidobacteria were highly correlated with SOC (r = 0.35), TN (r = 0.22), and C/N (r = 0.52), and Chloroflexi were negatively correlated with SOC (r = −0.10) and TN (r = −0.19) and positively correlated with the C/N ratio (r = 0.11) in soil layer A. Similarly, in soil layer B the NT, NT-SM, CT-SM, CT, and CT-SR treatments existed in different quadrants, indicating that the tillage practices had significant effects on the soil properties and bacterial community (Figure 5B). The RDA showed the highest correlation for SOC, TN, and C/N for in CT-SR treatment in soil layer B. In soil layer B, the dominant phylum Proteobacteria was positively correlated with SOC (r = 0.39), TN (r = 0.34), and C/N (r = 0.15). Similarly, Acidobacteria were also positively correlated with SOC (r = 0.11) and C/N (r = 0.42) and negatively correlated with TN (r = −0.25). Chloroflexi were positively correlated with SOC (r = 0.14) and TN (r = 0.02) but negatively correlated with the C/N ratio (r = −0.14). Moreover, in soil layer C, the NT, NT-SM, CT-SM, CT, and CT-SR treatments existed in different quadrants, demonstrating that the tillage practices had significant effects on the soil properties and bacterial community. A significant positive correlation was observed between the SOC, TN, and C/N ratio and the bacterial community for the CT-SR treatment (Figure 5C). The Proteobacteria and Chloroflexi were negatively correlated with SOC (r = −0.44 and r = −0.15), TN (r = −0.44 and r = −0.13), and C/N (r = −0.16 and r = −0.38), respectively, whereas Acidobacteria were positively correlated with SOC (r = 0.23), TN (r = 0.19), and the C/N ratio (r = 0.11) in soil layer C. The RDA results demonstrated that returning rice straw to the field had the most significant influences on soil C, TN, and the C/N ratio.
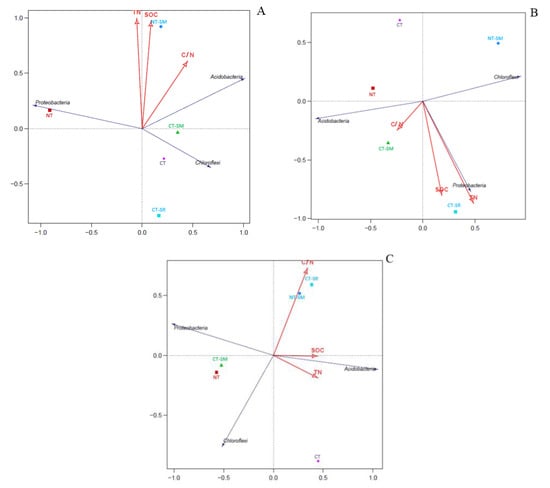
Figure 5.
Redundancy analysis ordination diagram showing the strength of association between the dominant bacterial phylum and the soil chemical properties (represented by red arrows) for different tillage practices and soil depths, i.e., 0–5 cm, (A), 5–10 cm (B), and 10–20 cm (C). SOC—soil organic carbon, TN—total nitrogen, C/N—carbon to nitrogen ratio.
4. Discussion
Conservation farming practices, such as no-tillage and crop residue retention, have been proposed as sustainable management practices because they increase the proportion of beneficial soil micro-organisms and improve soil quality. Soil micro-organisms provide important ecosystem services, which are necessary to maintain agricultural productivity and ecosystem health [1,2]. The SBC is the most important component of soil biology and is vital for improving soil properties, soil quality, and crop growth. SBC abundance is an indicator of soil health [46,47].
4.1. Bacterial Community Structure and Soil C/N Ratio
In this study, the bacterial diversity and abundance index showed a decreasing trend with increasing soil depth, which was consistent with the result reported by Zhang [48]. Moreover, the Venn diagram of OTU clustering showed that the number of OTUs also exhibited a decreasing trend with increasing soil depth: A > B > C. Proteobacteria, Acidobacteria, and Chloroflexi were the dominant bacterial phyla at a depth of 0–20 cm, and the proportion of these phyla decreased with increasing soil depth. The proportion of Proteobacteria was about 40% at all depths, which agrees with other studies [49,50].
Proteobacteria are the dominant phylum of soil bacteria and play a vital role in N fixation and soil quality [51]. Chloroflexi is bacteria that use CO2 as a C source to produce energy for photosynthesis, and they take part in the transformation of C, which is difficult to degrade under low nutrient conditions [52]. Acidobacteria is abundant in soil, and Acidobacteria in the sediment participate in the C cycle and humus decomposition. Similar to our findings, Gao et al. [53] reported that Proteobacteria, Actinobacteria, and Acidobacteria were the most abundant bacterial phyla under different fertilization treatments in a continuous soybean cropping system. The possible reason for the higher abundance of bacteria in the surface soil may be higher nutrient circulation and accumulation [54], resulting in higher soil fertility. In this study, the bacterial diversity and community abundance in the surface soil were higher than that in the deep soil.
4.2. Effects of No-Tillage on Soil Properties and Bacterial Community Composition
Our results are consistent with previous studies, which reported that the SOC, TN, soil C/N ratio, and bacterial community composition were improved significantly by long-term NT practices. A possible explanation is that NT practices reduce soil disturbance, slow the decomposition of soil organic matter, and increases the soil C content [55]. Further, NT and residue management practices increase the soil moisture content because residue left on the soil surface forms a barrier that prevents loss of soil moisture and nutrients [56]. In the present study, SOC was significantly higher in the NT treatment than the CT in soil layer A, whereas SOC was significantly lower in the NT treatment than the CT treatment in soil layers B and C (Table 2). Similarly, the NT treatment resulted in a larger soil C/N ratio in soil layer A but not in soil layers B and C, as shown in Table 2. Similar to our findings, many studies showed that NT practices increased the organic C content in the surface soil [56,57]. Liu et al. [58] showed that long-term NT practices increased SOC significantly at a soil depth of 0–10 cm but resulted in a slight decrease in SOC at a depth of 10–40 cm. Soil TN content in this study was significantly higher in NT treatment (Table 2). The possible reason is that NT practices reduce N losses, such as volatilization and leaching, due to less disturbance of the soil [59,60]. Similar to our findings, Lopez-Fando et al. [61] stated that NT significantly increased the total N content at a depth of 0–5 cm but resulted in a decrease at a depth of 5–30 cm. NT practices resulted in a higher soil C/N ratio than traditional tillage at a soil depth of 0–5 cm but a lower C/N ratio at a depth of 5–20 cm [62]. Our results and those reported in the literature indicate that NT practices resulted in decreases in the soil C and N with increasing soil depth.
Our study showed that NT practices not only resulted in a significant increase in soil C content but also significantly improved the bacterial diversity and abundance in the soil. The possible reason is that conventional deep plowing destroys the structure of the soil aggregates, resulting in C losses from the soil surface C and ultimately affecting the diversity and community composition of soil microorganisms [63]. Our results also agree with the findings of Lu et al. [32]. Further, our study revealed that soil bacterial diversity was higher in the NT treatment than the CT treatment, but the soil bacterial abundance was lower. It was also found that NT did not change the dominant groups of soil bacteria, but the proportion of the dominant groups changed significantly. The NT treatment increased the proportion of Proteobacteria in the A and C layers but decreased the proportion of Acidobacteria and Chloroflexi in the A, B, and C layers.
4.3. Effects of Straw Retention on Soil Properties and Bacterial Community Composition
Straw retention provides available soil nutrients such as C and N, and thus plays a key role in improving soil fertility [64,65]. Our results revealed that straw retention had a positive effect on the SOC and TN content of the soil (Table 2). This might be attributed to organic fertilizer, such as crop residues, which provided soil nutrients to the soil after decomposition [66]. Our results are also in line with those of Wei et al. [66], who reported that, in the North China Plain, NT and straw retention significantly increased the SOC content in the surface layer (0–10 cm), but the SOC content decreased with increasing soil depth from 10 to 50 cm. Many previous studies concluded that no-tillage with straw retention could significantly increase the SOC content and soil C/N ratio [67,68]. The likely reason is that a large amount of straw on the soil surface slows the decomposition rate. The rate of decrease in the soil C/N ratio with increasing soil depth depends on the amount of straw that is retained in the field [69]. The results showed that the SOC, TN, and C/N ratio of the NT-SM treatment were higher or significantly higher than those of the NT treatment. In the CT-SR treatment, the SOC and soil C/N ratio in the A and B layers were significantly increased. Our results showed that regardless of whether NT or CT was used, straw retention increased the SOC and TN content.
The SBC plays a key role in regulating soil processes, and the biomass and composition of soil bacteria affect soil sustainability [70]. Straw can provide energy and nutrients, i.e., carbohydrates, proteins, vitamins, and polyphenol for soil bacterial growth [65,66,67,68,69,70,71]. Our results also revealed that straw retention had a positive effect on soil bacterial abundance and community composition (Figure 2). Long-term straw retention practices significantly improve soil bacterial diversity and community abundance compared with control treatments [72]. In our study, straw retention significantly increased soil bacterial diversity and community composition. The NT-SM treatment had the highest diversity index, but the soil bacterial abundance was higher in the CT-SR treatment. The results also showed that straw retention affected the bacterial community structure. In the 0–10 cm soil layer, the Chloroflexi and Acidobacteria had significantly higher abundance for straw returning plots, whereas, non-significant changes were observed in the soil depth 10–20 cm.
4.4. Relationship between Soil Properties and Bacterial Community Structure
Increases in soil pH, organic matter, and soil available nutrients and decreases in electrical conductivity have been shown to improve bacterial community abundance and diversity. Similar to our findings, Zhao et al. [73] used RDA and reported complex correlations between soil properties and bacterial community abundance. An increase in the soil C/N ratio may promote the proliferation of soil microorganisms in arable land [36]. In this study, we found complex correlations between the dominant bacterial communities and soil environmental factors, such as organic C, TN, and the C/N ratio. In soil layer A, the only phylum that was negatively correlated with the C/N ratio in the group of dominant bacterial phyla was Proteobacteria; in soil layer B (C), only Chloroflexi (Proteobacteria and Chloroflexi) were negatively correlated with the C/N ratio. Therefore, the results showed that the SBC was not only affected by environmental factors but also by the soil depth and cultivation practices. Wang et al. [74] stated that the TN content of the soil significantly influenced the soil microbial community structure. Proteobacteria can affect different soil properties, i.e., SOC and TN, while changes in soil properties had little effect on Proteobacteria distribution and relative abundance. Further, Acidobacteria is mainly found in soil and sediment because they are acidophilic bacteria. Soil pH is the main soil indicator affecting the bacterial community, but in this study, we did not consider the soil pH and the correlation with the SBC.
5. Conclusions
In this eleven-year field experiment, we observed that conservation tillage practices significantly affected the SOC, TN, soil C/N ratio, and SBC. The soil C/N ratio, bacterial diversity, and richness index decreased with increasing soil depth. Proteobacteria, Acidobacteria, and Chloroflexi were the three dominant groups in the paddy soil. Soil tillage and straw retention significantly changed the soil C/N ratio, bacterial diversity, and structure. The SOC, TN, and soil C/N ratio were higher in the NT treatment than the CT treatment at a soil depth of 0–5 cm but lower at soil depths of 5–10 cm and 10–20 cm. The soil bacterial diversity index and OTUs were higher in the NT treatment than the CT treatment, but the bacterial abundance index was lower. Straw retention also enhanced soil bacterial diversity and abundance. The highest soil bacterial diversity was observed in the NT-SM treatment, whereas the highest soil bacterial abundance and OTUs were found in the CT-SR treatment. Moreover, the RDA showed that the SBC was highly correlated with the soil C/N ratio. The NT-SM had a significant effect on the SOC, TN, and C/N ratio at the soil depth of 0–5 cm, whereas the CT-SR treatment had a significant effect on the SOC, TN, and C/N ratio at soil depths of 5–10 cm and 10–20 cm. The results of this study demonstrate that conservation tillage practices, i.e., no-tillage and straw retention, increase the SBC diversity and C/N ratio, thereby enhancing soil nutrients and changing soil microbial ecology. As a result, sustainable crop production and profitable agro-ecosystems are ensured.
Author Contributions
Y.L. and L.J. conceived the main idea of research. Y.L. and A.I. wrote the manuscript. A.I., L.J., and L.H. revised the manuscript and provided suggestions. In addition, Y.L., I.A., and B.Y. analyzed the data. Q.Z., and S.U., and S.W. conducted assessments and data collection. All authors have read and agreed to the published version of the manuscript.
Funding
National Key Research and Development Project of China: 2018YFD020030503.
Acknowledgments
This research was financially supported by the National Key Research and Development Project of China (2018YFD020030503). We wish to thank our cooperators from the Guangxi University, Agriculture Station for the help with conducting and managing this experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Minoshima, H.; Jackson, L.E.; Cavagnaro, T.R.; Sánchez-Moreno, S.; Ferris, H.; Temple, S.R.; Goyal, S.; Mitchell, J.P. Soil food webs and carbon dynamics in response to conservation tillage in California. Soil Sci. Soc. Am. J. 2007, 71, 952–963. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Wagg, C. Soil microbial diversity and agro-ecosystem functioning. Plant Soil 2013, 363, 1–5. [Google Scholar] [CrossRef]
- Wang, Y.K.; Hong, K. DGGE analysis of PCR products of 16SrDNA V3 fragment of mangrove soil bacterial community. Acta Microbiol. Sin. 2005, 45, 201–204. [Google Scholar]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Coleman, D.C. From peds to paradoxes: Linkages between soil biota and their influences on ecological processes. Soil Biol. Biochem. 2008, 40, 271–289. [Google Scholar] [CrossRef]
- Li, Q.; Bao, X.L.; Lu, C.Y.; Zhang, X.K.; Zhu, J.G.; Jiang, Y.; Liang, W.J. Soil microbial food web responses to free-air ozone enrichment can depend on the ozone-tolerance of wheat cultivars. Soil Biol. Biochem. 2012, 47, 27–35. [Google Scholar] [CrossRef]
- Scharroba, A.; Dibbern, D.; Hünninghaus, M.; Kramer, S.; Moll, J.; Butenschoen, O.; Bonkowski, M.; Buscot, F.; Kandeler, E.; Koller, R.; et al. Effects of resource availability and quality on the structure of the micro-food web of an arable soil across depth. Soil Biol. Biochem. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Wardle, D.A. Communities and Ecosystems: Linking the Aboveground and Belowground Components; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Treonis, A.M.; Austin, E.E.; Buyer, J.S.; Maul, J.E.; Spicer, L.; Zasada, I.A. Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl. Soil Ecol. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Wall, D.H.; Bardgett, R.; Behan-Pelletier, V.; Herrick, J.E.; Jones, H.; Ritz, K.; Six, J.; Stone, D.; Van Der Putten, W.H. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Lemanceau, P.; Maron, P.A.; Mazurier, S.; Mougel, C.; Pivato, B.; Plassart, P.; Ranjard, L.; Revellin, C.; Tardy, V.; Wipf, D. Understanding and managing soil biodiversity: A major challenge in agroecology. Agron. Sustain. Dev. 2015, 35, 67–81. [Google Scholar] [CrossRef]
- Eo, J.; Park, K.C. Long-term effects of imbalanced fertilization on the composition and diversity of soil bacterial community. Agric. Ecosyst. Environ. 2016, 231, 176–182. [Google Scholar] [CrossRef]
- Brennan, E.B.; Acosta-Martinez, V. Cover cropping frequency is the main driver of soil microbial changes during six years of organic vegetable production. Soil Biol. Biochem. 2017, 109, 188–204. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Woodhouse, J.N.; Curlevski, N.J.; Hayward, M.W.; Brown, M.V.; Neilan, B.A. Soil-foraging animals alter the composition and co-occurrence of microbial communities in a desert shrubland. Int. Soc. Microb. Ecol. J. 2015, 9, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Creamer, R.E.; Hannula, S.E.; Van Leeuwen, J.P.; Stone, D.; Rutgers, M.; Schmelz, R.M.; de Ruiter, P.C.; Hendriksen, N.B.; Bolger, T.; Bouffaud, M.L. Ecological network analysis reveals the inter-connection between soil biodiversity and ecosystem function as affected by land use across Europe. Appl. Soil Ecol. 2016, 97, 112–124. [Google Scholar] [CrossRef]
- Yin, C.; Jones, K.L.; Peterson, D.E.; Garrett, K.A.; Hulbert, S.H.; Paulitz, T.C. Members of soil bacterial communities sensitive to tillage and crop rotation. Soil Biol. Biochem. 2010, 42, 2111–2118. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Gao, W.S. On the basic principles and development trend of conservation tillage technology. China Agric. Sci. 2007, 40, 2702–2708. [Google Scholar]
- Jaskulska, I.; Jaskulski, D. Strip-Till One-Pass Technology in Central and Eastern Europe: A MZURI Pro-Til Hybrid Machine Case Study. Agronomy 2020, 10, 925. [Google Scholar] [CrossRef]
- Badagliacca, G.; Petrovičovà, B.; Pathan, S.I.; Roccotelli, A.; Romeo, M.; Monti, M.; Gelsomino, A. Use of solid anaerobic digestate and no-tillage practice for restoring the fertility status of two Mediterranean orchard soils with contrasting properties. Agric. Ecosyst. Environ. 2020, 300, 107010. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Liu, D.; Li, Z.; Zhang, G.; Tao, Y.; Xie, J.; Pan, J.; Chen, F. Straw mulching reduces the harmful effects of extreme hydrological and temperature conditions in citrus orchards. PLoS ONE 2014, 9, e87094. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Kabiri, V.; Raiesi, F.; Ghazavi, M.A. Tillage effects on soil microbial biomass, SOM mineralization and enzyme activity in a semi-arid Calcixerepts. Agric. Ecosyst. Environ. 2016, 232, 73–84. [Google Scholar] [CrossRef]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Lacoux, J.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J.E.; et al. Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Staley, T.E. Soil microbial and organic component al-teration in a no-tillage chrono-sequence. Soil Sci. 1988, 52, 998–1005. [Google Scholar] [CrossRef]
- Ali, I.; He, L.; Ullah, S.; Quan, Z.; Wei, S.; Iqbal, A.; Munsif, F.; Shah, T.; Xuan, Y.; Luo, Y.; et al. Biochar addition coupled with nitrogen fertilization impacts on soil quality, crop productivity, and nitrogen uptake under double-cropping system. Food Energy Secur. 2020, 3, e208. [Google Scholar] [CrossRef]
- Obia, A.; Cornelissen, G.; Martinsen, V.; Smebye, A.B.; Mulder, J. Conservation tillage and biochar improve soil water content and moderate soil temperature in a tropical Acrisol. Soil Tillage Res. 2020, 197, 104–121. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Khan, A.; Wei, S.; Akhtar, K.; Ali, I.; Ullah, S.; Munsif, F.; Zhao, Q.; Jiang, L. Organic manure coupled with inorganic fertilizer: An approach for the sustainable production of rice by improving soil properties and nitrogen use efficiency. Agronomy 2019, 9, 651. [Google Scholar] [CrossRef]
- Bu, R.; Ren, T.; Lei, M.; Liu, B.; Li, X.; Cong, R.; Lu, J. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under rice-rice-rapeseed rotation system. Agric. Ecosyst. Environ. 2020, 287, 106–681. [Google Scholar] [CrossRef]
- Yang, M.F. Effects of Different Tillage Measures and Straw Returning on Soil Nutrients, Microorganisms and Carbon Pools in Rice Wheat Dual Cropping Farmland; College of Resources and Environmental Science, Nanjing Agricultural University: Nanjing, China, 2013. [Google Scholar]
- Xiang, X.H.; Wei, W.; Zhang, X.Y. Effects of conservation tillage on soybean growth and soil microbial diversity. Soybean Sci. 2013, 32, 321–327. [Google Scholar]
- Lu, D.; Lei, J.; Wei, Y.Y. Effects of short-term no tillage and ridge cultivation on microbial community and diversity index of paddy soil. J. Southwest Agric. 2015, 28, 1670–1674. [Google Scholar]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fertil. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Lin, Y.H.; Cha, Y.L.; Mao, K.M. Effect of wheat straw coverage on the number of microorganisms in different tobacco planting soils. Crop Res. 2012, 26, 664–667. [Google Scholar]
- Wang, L.L.; Dong, M.; Zhang, L. Effects of organic fertilizers with different carbon nitrogen ratios on soil microbial biomass in organic agriculture. Chin. J. Ecol. Agric. 2013, 21, 1073–1077. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.C.; Huang, Y.M. Characteristics of soil bacterial community in different arbor forests on the Loess Plateau Based on 454 high-throughput sequencing. Chin. Environ. Sci. 2016, 36, 3487–3494. [Google Scholar]
- Spedding, T.A.; Hamel, C.; Mehuys, G.R. Soil microbial dynamics in maizegrowing soil under different tillage and residue management systems. Soil Biol. Biochem. 2004, 36, 499–512. [Google Scholar] [CrossRef]
- Huang, B.; Sun, W.X.; Zhao, Y.C. Temporal and spatial variability of soil organic matter and total nitrogen in an agricultural ecosystem as affected by farming practices. Geoderma 2007, 139, 336–345. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Chinese Agriculture Press: Beijing, China, 2000; pp. 263–270. [Google Scholar]
- Ohyama, T.; Ito, M.; Kobayashi, K.; Araki, S.; Yasuyoshi, S.; Sasaki, O.; Yamazaki, T.; Soyama, K.; Tanemura, R.; Mizuno, Y. Analytical procedures of N, P, K contents in plant and manure materials using H2SO4-H2O2 Kjeldahl digestion method. Bull. Fac. Agric. Niigata Univ. 1991, 43, 110–120. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis—Advanced Course; University of Wisconsin: Madison, WI, USA, 1956; p. 991. [Google Scholar]
- Bates, S.T.; Cropsey, G.W.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial communities associated with the lichen symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End read merger. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef]
- Robert, C.E. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar]
- Wei, T. Package ‘Corrplot’. 2016. Available online: https://cran.r-project.org/web/packages/corrplot/corrplot.pdf (accessed on 15 May 2020).
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423, 623–656. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Insam, H.; Hutchinson, T.C.; Reber, H.H. Effects of heavy metal stress on the metabolic quotient of the soil microflora. Soil Biol. Biochem. 1996, 28, 691–694. [Google Scholar] [CrossRef]
- Li, T.; Sun, Z.; He, C.; Ge, X.; Ouyang, Z.; Wu, L. Changes in soil bacterial community structure and microbial function caused by straw retention in the North China Plain. Arch. Agron. Soil Sci. 2020, 66, 46–57. [Google Scholar] [CrossRef]
- Zhang, X. Effect of Different Tillage Measures on Soil Microbial Functional Diversity in the Loess Plateau of Central Gansu; Gansu Agricultural University: Lanzhou, China, 2017; pp. 1–58. [Google Scholar]
- Kou, W.B.; Huang, Z.Y.; Zhang, J. Composition and structure of bacterial community in Poyang Lake: A case study of Songmen mountain. J. Ecol. 2015, 35, 7608–7614. [Google Scholar]
- Nacke, H.; Thurmer, A.; Wollherr, A. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef]
- Luo, P.Y. Effects of Long-Term Fertilization on Microbial Communities in Brown Soil under Rotation Conditions; Shenyang Agricultural University: Shenyang, China, 2014. [Google Scholar]
- Zhang, J.; Ke, W.J.; Liu, J.; Wang, L.H.; Chen, H.; Peng, T.; Zhao, Q.Z. Influence of water controlling depth on soil micro-flora and bacterial community diversity in paddy soil. Chin. J. Eco-Agric. 2019, 27, 277–285. [Google Scholar]
- Gao, S.C.; Guan, D.W.; Ma, M.C. Effect of Fertilization on bacterial community of black soil in Northeast China under soybean continuous cropping. China Agric. Sci. 2017, 50, 1271–1281. [Google Scholar]
- Ren, W.J.; Liu, D.Y.; Wu, J.X. Effects of no till and high stubble and seedling throwing on soil fertility and microbial community in rice field. J. Appl. Ecol. 2009, 20, 817–822. [Google Scholar]
- Liu, J.J.; Sui, Y.Y.; Yu, Z.H. High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Xue, J.F.; Zhao, X.; Dikgwatlhe, S.B. Research progress on the effect of conservation tillage on carbon and nitrogen in farmland. Acta Ecol. Sin. 2013, 33, 6006–6013. [Google Scholar]
- Sainju, U.M.; Singh, B.P.; Whitehead, W.F. Long-term effects of tillage, cover crops, and nitrogenfertilization on organic carbon and nitrogen concentrations in sandy loam soils in Georgia, USA. Soil Tillage Res. 2002, 63, 167–179. [Google Scholar] [CrossRef]
- Lopez-Fando, C.; Pardo, M.T. Use of a partial-width tillage system maintains benefits of no-tillage in increasing total soil nitrogen. Soil Tillage Res. 2012, 118, 32–39. [Google Scholar] [CrossRef]
- Puget, P.; Lal, R. Soil organic carbon and nitrogen in a Mollisol in central Ohio as affected by tillage and land use. Soil Tillage Res. 2005, 80, 201–213. [Google Scholar] [CrossRef]
- Beare, M.H.; Hendix, P.F.; Coleman, D.C. Water stable aggregates and organic matter fraetions in coventional and no-till soils. Soils Sci. Soc. Am. 1994, 58, 777–786. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics, crop yield and nitrogen recovery. Adv. Agron. 2000, 68, 197–319. [Google Scholar]
- Singh, B.; Shan, Y.H.; Johnson-Beebout, S.E. Crop residue management for lowland rice-based cropping systems in Asia. Adv. Agron. 2008, 98, 117–119. [Google Scholar]
- Wei, Y.H.; Zhao, X.; Zhai, Y.L. Effects of Tillage Methods on soil carbon sequestration in North China. J. Agric. Eng. 2013, 29, 87–95. [Google Scholar]
- Baker, J.M.; Ochsner, T.E.; Venterea, R.T. Tillage and soil carbon sequestration-What dowe really know? Agric. Ecosyst. Environ. 2007, 118, 1–5. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. No-tillage and soil-profile carbon sequestration: An on-farm assessment. Soil Sci. Soc. Am. J. 2008, 72, 693–701. [Google Scholar] [CrossRef]
- Torbert, H.A.; Potter, K.N.; Morrison, J.E. Tillage intensity and fertility level effects on nitrogenand carbon cycling in a vertisol. Commun. Soil Sci. Plant Anal. 1997, 28, 699–710. [Google Scholar] [CrossRef]
- Segal, L.M.; Miller, D.N.; Mcghee, R.P.; Loecke, T.D.; Cook, K.L.; Shapiro, C.A. Bacterial and archaeal ammonia oxidizers respond differently to long-term tillage and fertilizer management at a continuous maize site. Soil Tillage Res. 2017, 168, 110–117. [Google Scholar] [CrossRef]
- Bai, Z.G.; Thomas, C.; Ruiperez, G.M.; Batjes, N.H.; Paul, M.; Bünemann Else, K. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Appl. Soil Ecol. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Yuan, H.C.; Qin, H.L.; Liu, S.L. Effects of long-term fertilization on bacterial community structure and quantity in red soil paddy soil. China Agric. Sci. 2011, 42, 4610–4617. [Google Scholar]
- Zhao, M.Q.; Zhou, N.N.; Du, Q.J. Effect of rice vegetable rotation on soil bacterial community under the condition of straw returning to the field. North. Hortic. 2018, 14, 109–117. [Google Scholar]
- Wang, W.X.; Luo, D.; Shi, Z.M. Effect of afforestation on soil microbial community structure in the arid valley of Minjiang River. J. Ecol. 2014, 34, 890–898. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).