1. Introduction
Lettuce (
Lactuca sativa L.) is a widely cultivated leafy vegetable belonging to the
Asteraceae family. Lettuce is one of the main greenhouse-grown vegetables, being recognized as highly productive and economically valuable [
1]. As reported by FAOSTAT [
2], world production of lettuce and chicory consists of around 27 Mt, 73.7% of which is produced in Asia and 9.7% in Europe. Cultivation of lettuce and chicory in Italy covers around 34,460 ha and yields an annual production of 768,055 t. Leafy Lettuce (
L. sativa, var.
acephala) is very important among fresh-cut, minimally processed vegetables, increasingly requested for fast-food, catering and home consumption [
3]. Due to its content in terms of vitamins, minerals and biologically active compounds such as photosynthetic pigments and phenols, lettuce has also been recognized as an important functional food [
4].
However, lettuce also was shown to have sometimes high nitrates concentration in leaves [
5,
6]. Nitrates (NO
3−) per se are relatively nontoxic compounds; yet, around 5–10% of ingested nitrates are converted to nitrites (NO
2−) in the gastrointestinal tract [
7]. Nitrites are highly toxic compounds that can lead to many disorders in humans [
7] including blue baby syndrome (methaemoglobinemia in infants) as well as other diseases [
8,
9]. For these reasons, the reduction of nitrate intake associated with vegetables consumption in the diet is strongly recommended [
10].
Such unfavorable effects derived from NO
3− accumulation are highly dependent on environmental and management factors occurring during the cultivation of lettuce [
11]. Among these, major drivers are the quality and intensity of light, temperature, and nitrogen availability [
12]. Growing leafy vegetables in soilless systems requires careful management of fertilization because of the limited growing substrate and the high density of plants [
13]. Optimization of the nutrient solution concentration is required to maximize the yield and quality of lettuce [
14,
15]. When consistent rates of mineral N are used during the production cycle, especially in a cool climate and with low light intensity, the conversion of NO
3− to organic N pools (i.e., proteins) is restricted and high levels of NO
3− could accumulate in lettuce leaves [
12,
16]. Concentration limits of NO
3− in lettuce leaves are currently regulated by EU Reg. No. 1258/2011 [
17], according to the period of harvesting and the system of cultivation (
Table A1 in the
Appendix A).
Beside traditional soil-based production systems, whether in open-field conditions or under protection structures (i.e., greenhouses or tunnels), lettuce is cultivated also in soilless systems (i.e., hydroponics or aquaponics systems). The soilless lettuce is cultivated increasingly in Italy; in 2019, its production may be estimated at around 3000 Mg, or about 2% of total greenhouse lettuce production (154,465 Mg) [
18]. Many hydroponic systems have been used in recent years, such as gravel beds, floating systems, and nutrient film techniques [
19]. The advantages of using these systems are that they (i) originate in a clean raw material with low microbiological contamination due to reduced contact between leaves and growing substrate [
20] and (ii) yield ready-to-use leaf vegetables requiring gentle washing procedures that cause only minimal stress to the leaf tissue [
21]. Hydroponic methods are very attractive to growers because they entail lower labor costs than those of conventional soil-based methods. In addition, no weeding is required, and the harvesting is easier and faster than in soil-based cultivation [
22].
In recent years, an innovative way to market lettuce and other vegetables has been proposed, consisting in selling entire live plants together with their peat blocks and packaged in a transparent corn-starch bag, thus forming an entirely biodegradable and compostable product. At home, this fresh product can be preserved for a week in a cool, light place, adding water as needed and picking the leaves for immediate consumption. The exhausted bag and peat blocks can be disposed of in household wet waste or in a composter, thus reducing the impact on the environment and lessening the ecological footprint.
To produce lettuce with peat blocks, two main systems are now used. The “Nutrient Film Technique” (NFT) is a hydroponic system in which a thin film of nutrient solution flows continuously over the roots and is then recirculated. Thus, it differs from the “ebb-and-flow” system in which plants cultivated in a growth medium are placed in a tray that is periodically filled with the nutrient solution [
23]. An innovative method, intermittent NFT, used in this study, lies midway between the two systems: plants growing on peat blocks are placed in a tray with an intermittent flow of nutrient solution. This intermittence allows greater oxygenation of the root systems and therefore a better health status of the plants.
Nevertheless, these systems require careful fertilization management because of the small amount of the growing substrate per plant [
13]. It follows that optimizing nutrient solution concentration and fertilization schedule is a major issue in order to (i) maximize lettuce yield [
14,
15] and (ii) avoid NO
3− accumulation [
10].
The leading objectives of the present study were (a) to identify the optimal growth cycle duration for maximum yield performance in the innovative system of lettuce production (modified intermittent NFT) and (b) to assess the effects of different fertilization strategies under intermittent NFT on nitrate accumulation in lettuce leaves. The initial hypothesis was that reducing the fertilization rate during the last days of the growing cycle would produce a high yield of lettuce and also safe nitrate concentrations in leaves.
2. Materials and Methods
2.1. Experiment and Treatments
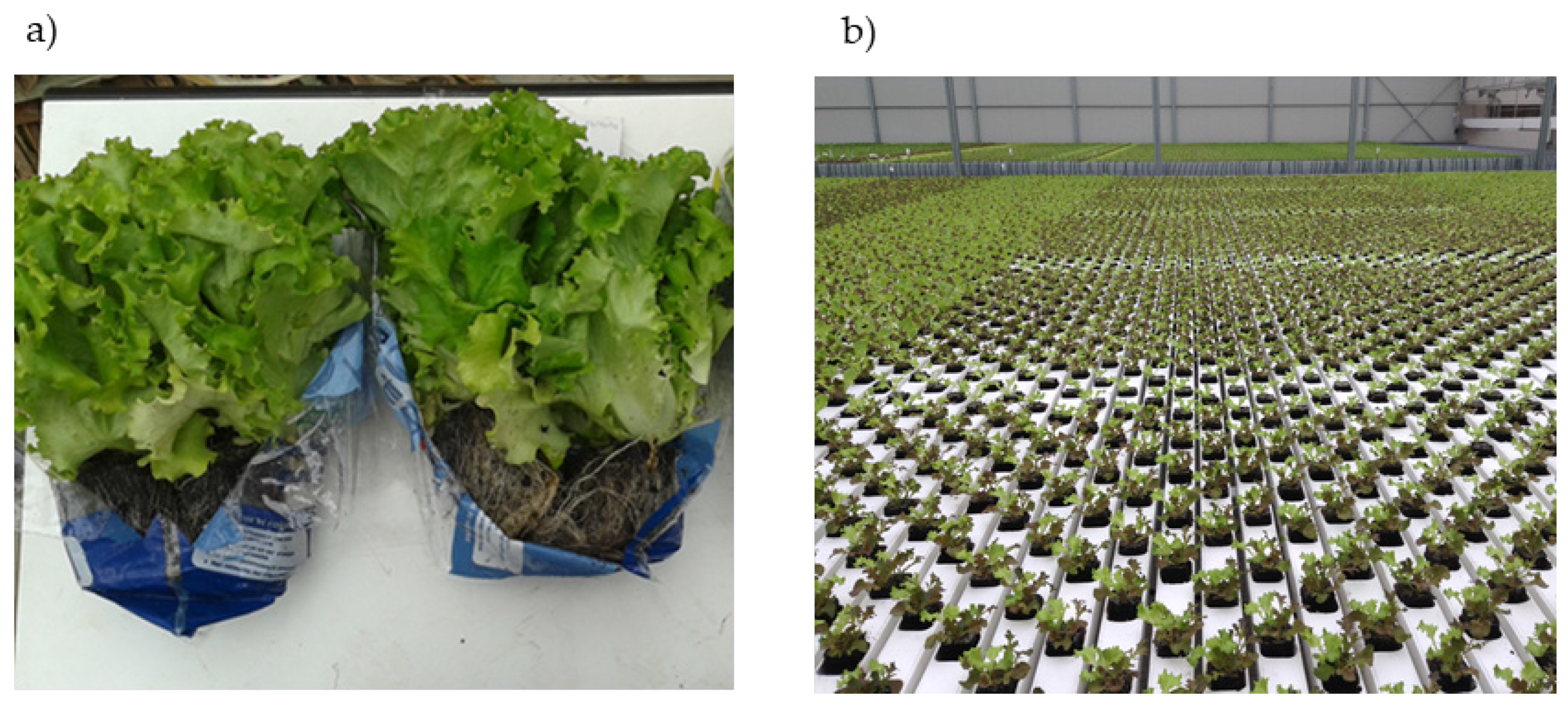
The experiment was carried out from October 2013 to April 2014 at the Sempre Fresco S.r.l. greenhouse plant located in Guidizzolo, Mantova, Italy (lat. 45.31718 N, long. 10.55832 E), in which an innovative intermittent NFT system has been adopted to produce lettuce laid out and marketed in a new way, potentially more pleasing to consumers. The marketed item consists of a “triple pack” of lettuce plants of three types (Batavia, Romana (cv.
longifolia), and Lollo (cv.
crispa)) grown in the same block and sold still alive with roots and in their peat block. The weight of the entire package is about 400 g, with 200 g of edible portion, corresponding to about two servings of mixed salad (
Figure 1a).
The intermittent NFT system was implemented as follows. The growing cycle was started using approximately 30-day-old seedlings from the nursery in blocks of compressed peat. The blocks with lettuce seedlings having 3–4 leaves each were placed in perforated plastic cable ducts. Each duct was 12.1 m long, had 57 holes for 57 sticks (
Figure 1b), and was placed on a raised surface inclined at a slope of about 3%, thus allowing the water to flow from side to side in the duct. At the beginning of the growing cycle, the ducts with the transplanted seedlings were placed at one end of the greenhouse bench; during the cropping cycle, the ducts were moved automatically to the opposite end of the bench, arriving at the end just in time for harvesting. The ducts were moved by a system of chains located below the bench, which fit into the bottom of the ducts and pushed them forward. The duration of the run was set according to the length of the production cycle, which depends on the season and ranges from 25 to 45 days. At the upper end of each duct there was a tube through which the nutrient solution was fed: the tube entered the slightly sloped duct and ran through it wetting the peat blocks and the lettuce roots. The excess nutrient solution was collected at the bottom of the duct and recycled.
Greenhouse dimensions were 130 m long, 80 m wide, and 5 m high. The framework of the greenhouse consisted of arches of galvanized-iron pipe covered by polyethylene sheeting 0.20 mm thick. The greenhouse environmental conditions (air temperature and humidity, light intensity) were constantly monitored during the trials from transplanting to harvesting; the pH of the nutrient solution was maintained within a range from 5.5 to 6.6 and electrical conductivity (EC) ranged from 1.92 to 2.01 mS cm−1.
Using intermittent NFT, we carried out two greenhouse experiments in two different seasons: (1) the autumn trial, performed from 3rd October to 1st November, and (2) the spring trial, from 8th March to 6th April.
2.1.1. Autumn Trial
The autumn elemental experiment was designed as a randomized complete block (RCB) with 4 replicates and 6 treatments, as follows: T1, continuous fertilization; T2, fertilization stopped during the last 2 days; T3, fertilization stopped during the last 4 days; T4, fertilization stopped during the last 6 days; T5, fertilization stopped during the last 8 days; T6, fertilization stopped during the last 10 days. The total number of plots was 24. Each stick contained one plant of lettuce (cv. Tourbillon, Batavia type). The experiment was repeated six times, investigating the same fertilization scheme for six durations of the growing cycle, from 25 to 30 days after transplanting (DAT).
The two concentrated nutrient solutions (A and B) were stored in two reservoirs with capacity of 1500 L each, and distributed after dilution. The composition of the concentrated nutrient solutions (A and B) is indicated in
Table 1. The diluted nutrient solution had the following physicochemical characteristics: nitrogen concentration (NO
3− + NH
4+) 13.8 mmol L
−1; pH 5.5; EC 2.01 mS cm
−1. The fertilization program consisted of 15 irrigations per day, each lasting 3 min, at a flow rate of 0.4 L min
−1. Each plant received 0.31 L of water per day. Excess nutrient solution collected from the greenhouse benches was reused after adjustment of pH and EC and sterilization by UV lamps.
The environmental conditions in the greenhouse were monitored during the growing cycle (
Figure A1 in the
Appendix A). Relative air humidity was maintained between 70 and 80% (77% on average during the entire growing cycle). Temperature and light intensity were more variable, as they were influenced by external conditions, although a minimum threshold of 12 °C at night and 16 °C during the day were set for starting automatic heating. The daily mean temperature over the period was 17.2 °C and the mean light intensity was 137 klx h
−1. The light intensity was measured with a digital photometer (LI-250A, LI-COR Inc., Lincoln, NE, USA) equipped with a photometric sensor.
2.1.2. Spring Trial
The spring elemental experiment was arranged as a randomized complete block (RCB) with 4 replicates and 6 treatments, as follows: T1, continuous fertilization; T2, fertilization stopped during the last 2 days; T3, fertilization stopped during the last 4 days; T4, fertilization stopped during the last 6 days; T5, fertilization stopped during the last 8 days; T6, fertilization stopped during the last 10 days. Similar to the autumn trial, the total number of plots was 24 and the experiment was repeated 6 times in order to investigate responses from 25- to 30-day growing cycles, using lettuce seedlings (cv. Tourbillon, Batavia type).
The concentrated nutrient solutions (A and B) were stored as in the autumn trial and their composition is indicated in
Table 1. The diluted nutrient solution had the following physicochemical characteristics: nitrogen concentration (NO
3− + NH
4+) 13.8 mmol L
−1; pH 6.6; EC 1.92 mS cm
−1. The fertilization program consisted of 23 irrigations per day, each of them lasting 2 min, at a flow rate of 0.4 L min
−1. Each plant received 0.32 L of water per day. Excess nutrient solution collected from the greenhouse benches was reused after adjustment and sterilization as in the previous trial.
Relative humidity was maintained almost constant, with a mean of 73% during the growing cycle. Light intensity, and to a greater degree air temperature, were not as steady, as they were influenced by external conditions, with no artificial heating being used. The daily mean temperature over the period was 14.4 °C and the mean light intensity was 188 klx h
−1, as per instrumental detection (
Figure A2 in the
Appendix A).
2.2. Plant Sampling and Yield Measurements
In the autumn trial, the harvest of lettuce plants took place at 25, 26, 27, 28, 29, and 30 DAT, from 27th October to 1st November 2014. Two plants from each plot were sampled randomly and the fresh biomass yield (FBY) was immediately determined by weighing it. Dry biomass yield (DBY) was then calculated by multiplying FBY by dry matter content (DMC), as obtained after drying the fresh biomass in a forced oven at 65 °C until constant weight.
Using the same procedure in the spring trial, two plants of lettuce from each plot were sampled randomly at 25, 26, 27, 28, 29, and 30 DAT, from 1st April to 6th April 2014 in order to determine FBY, DMC, and DBY.
2.3. Nitrate Concentration in the Plant
The concentration of NO
3− in the lettuce was determined on fresh and dry matter basis by ion chromatograph (Dionex DX−120) provided by the pre-guard column AG9HC (4 mm × 50 mm) and the column AS9HC (4 mm × 250 mm). The analytical parameters were sodium carbonate/sodium bicarbonate solution as eluent; flow 1.0 mL min
−1; pressure 1650−1890 psi; total conductivity 24–28 µS cm
−1; nitrate retention time 6.95–7.28 min [
24].
Dried lettuce leaf samples were ground at 2 mm. Extraction was performed according to the method of Santamaria et al. [
24], which is based on cold extraction of nitrates using a solution of sodium carbonate 0.5 M and sodium bicarbonate 0.5 M, the same solution used as eluent. Then, 1 g of dried lettuce leaf was added to 50 mL of extraction solution and shaken for 20 min at 150 rpm. The extract was filtered with Whatman paper No. 1 and then with a 0.45-µm syringe filter combined with an On-Guard IIP (Dionex) cartridge. The filtered extract was injected into the ion chromatograph after appropriate dilution.
2.4. Statistical Analysis
Data on FBY, DBY, DMC, and NO3− concentration for each trial and harvest date were subjected to analysis of variance (ANOVA) using the statistical software SAS version 9.2 (SAS Institute Inc., Cary, NC, USA, 2009). When normal distribution was not confirmed by the Shapiro–Wilk test, data were log-transformed before analysis. Tukey’s test was used to test for significant differences in variables among treatments with a p-value ≤ 0.05 as the threshold for statistical significance.
4. Discussion
Overall, we can report that in the present study, lettuce FBY and DBY were higher in the spring trial than in the autumn trial, corroborating previous studies [
14,
25] that showed that external seasonal climatic conditions (i.e., light intensity, photoperiod length, and air temperatures) may affect greenhouse growing conditions.
More in detail, findings on biomass yield (i.e., FBY and DBY) obtained with the autumn trial in the present study were not in agreement with the experimental hypothesis. Stopping the fertilization in treatments from T2 to T6 was indeed expected to decrease both FBY and DBY [
26]. Conversely, our results did not show a specific pattern, neither for FBY nor for DBY. In detail, the fertilization suspension during the last ten days of the cropping cycle was irrelevant for affecting lettuce yields in the autumn conditions. A possible explanation to these results lies in the fluctuating light-intensity pattern during the autumn trial, despite the regulating system inside the greenhouse. Light intensity indeed peaked several times during the growing season and was very low for the rest of time. Thus, such unfavorable growing conditions had detrimental effects on FBY, as previously shown in several studies. For instance, Burns et al. [
27] found that reducing the light intensity by 50% may lead to a halving of FBY in a 28-day trial. Delaide et al. [
28] confirmed these results. Also, variations observed in DMC and, consequently, on DBY seem to be directly correlated with scarce light intensity in the autumn trial. Specifically, the lowest dry matter content was found in the 28-DAT growing cycle, right after a severe drop in light intensity for 3 consecutive days immediately preceding the harvest. Therefore, based on our results, the best length of the autumn growing cycle for obtaining maximum fresh weight is 30 DAT, which led to a 51% increase in either FBY or DBY with a 5-day elongation of the cycle.
Similarly, during the spring trial, FBY increased by 52% as one passed from the 25-DAT to the 30-DAT growing cycle. This increase in FBY clearly indicates that the 30-DAT cycle could be suggested as a means to maximize fresh yield in lettuce also for spring growing cycles. Our results, additionally, suggest that stopping the fertilization from 6 to 10 days before the harvest in the spring cycle is not indicated for the purpose of maximizing FBY. Fresh biomass yield was indeed, on average, 40% lower for plants under T6 than under T1. Also, the increase in DBY was of about 37% passing from the 25- to 30-DAT growing cycle, while the interruption of fertilization in the last days of the cycle led to an average DBY decrease of about 13% passing from T1 to T6. This low reduction in DBY compared with the high one in FBY is probably related to different values of DMC of plants in different treatments. In detail, DMC increased as nitrogen fertilization decreased, passing from 4.6% on average under T1 to 6.7% under T6. These results could be explained by the osmotic effect caused by nitrate accumulation as reported by Tei et al. [
29] and corroborate earlier studies indicating that the leaf nitrate content is correlated positively with plant fresh weight, but negatively with dry matter content [
21,
30]. The lettuce DMC during the spring trial was not only affected by N fertilization but was directly correlated with light intensity. On average, during the spring cycle the dry matter accumulation in lettuce was about 40% higher than that observed during the autumn cycle, confirming previous results from a double-season experiment of Lopes et al. [
31], who found that lettuce during the spring trial accumulated around 30% more DM than during the autumn trial.
As regards NO
3− concentration, the spring trial showed much lower values that the autumn trial, probably due to more favorable light conditions, photoperiod length and air temperatures, as previously reported by Guadagnin et al. [
25] and Fallovo et al. [
14]. In any case, mean NO
3− concentration in the leaves for all cycles and fertilization treatments were always below 5000 mg NO
3− kg
−1, the value set by the European Union as the legislative threshold for winter lettuce. Tamme et al. [
32], in a previous greenhouse study on lettuce under similar conditions, reported that NO
3− concentration in leaves was lower in the summer cycle (on average 1952 mg NO
3−-kg
−1) than in the winter cycle (3024 mg kg
−1), though it never exceeded the EU threshold.
As reported by Kmecl et al. [
5], the NO
3− concentration in vegetables is highly correlated with a number of different factors, such as the substrate characteristics and the rate of fertilization, which could affect the intensity of metabolic processes in the different organs of plants and in leaves. Also when using other growing systems, such as the aeroponic system, the concentration of nitrogen compounds (NO
3-N, NH
4-N and total N) in lettuce (cv.
capitata) was shown to be highly affected by the NO
3− concentration in the nutrient solution and by the specific growing conditions [
33]. Marsic et al. [
33] indeed reported that in three different experiments, reducing NO
3− concentration in the nutrient solution reduced lettuce nitrate content, and that the highest nitrate concentration occurred in the most external leaves of plants. Conversely, Amr and Hadidi [
34] found that the growing cycle (autumn vs spring) had no effect on NO
3− concentration of vegetables grown in a greenhouse.
As for lettuce yield, reducing fertilization during the final growing days in our autumn trial did not lead to a clear decrease in NO
3− concentration, which was also contrary to our expectations. In some cases, an increase was even measured, although it was not statistically relevant. Instead, the NO
3− concentration during the spring trial across all cycle durations (25 DAT/30 DAT) showed that (i) stopping fertilization only 2 days before harvest may reduce NO
3− concentration in leaves, and (ii) combining T6 treatment with the 27-DAT cycle is the most effective strategy to minimize NO
3− concentration both on fresh and dry matter basis. This agrees with what suggested by Gonnella et al. [
19] who found that the substitution of the nutrient solution with water 2 days before harvesting decreased nitrate concentration in lettuce leaves (−17%).