Scion × Rootstock Response on Production, Mineral Composition and Fruit Quality under Heavy-Calcareous Soil and Hot Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Weather Conditions
2.3. Tree Mortality and Field Determinations
2.4. Fruit Quality Determinations
2.5. Flower and Leaf Mineral Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Weather Conditions
3.2. Tree Mortality
3.3. Production and Fruit Quality
3.4. Fruit Quality Determinations
3.4.1. Effect of Year on Fruit Quality Traits
3.4.2. Effect of Rootstock on Fruit Quality Traits
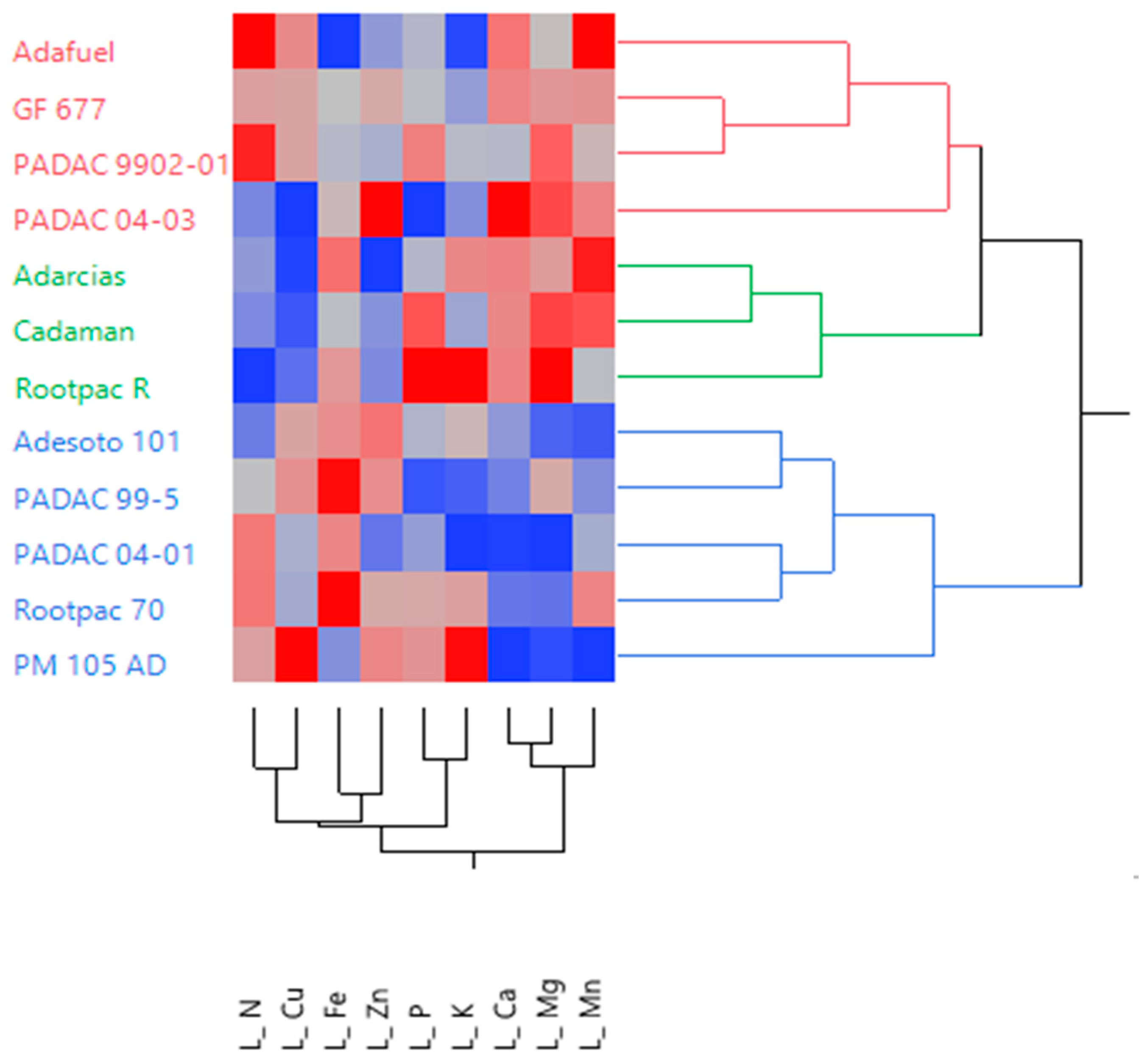
3.5. Flower and Leaf Mineral Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Reig, G.; Mestre, L.; Betrán, J.A.; Pinochet, J.; Moreno, M.A. Agronomic and physicochemical fruit properties of “Big Top” nectarine budded on peach and plum based rootstocks in Mediterranean conditions. Sci. Hortic. 2016, 210, 85–92. [Google Scholar] [CrossRef]
- Reig, G.; Alegre, S.; Cantín, C.M.; Gatius, F.; Puy, J.; Iglesias, I. Tree ripening and postharvest firmness loss of eleven commercial nectarine cultivars under Mediterranean conditions. Sci. Hortic. 2017, 219, 335–343. [Google Scholar] [CrossRef]
- Fernández, V.; Del Rio, V.; Pumarino, L.; Igartua, E.; Abadía, J.; Abadía, A. Foliar fertilization of peach (Prunus persica (L.) Batsch) with different iron formulations: Effects on re-greening, iron concentration and mineral composition in treated and untreated leaf surfaces. Sci. Hortic. 2008, 117, 241–248. [Google Scholar] [CrossRef]
- Paniagua, L.L.; García-Martín, A.; Moral, F.J.; Rebollo, F.J. Aridity in the Iberian Peninsula (1960–2017): Distribution, tendencies, and changes. Theor. Appl. Climatol. 2019, 138, 811–830. [Google Scholar] [CrossRef]
- Ozturk, T.; Zeynep, P.C.; Turkes, M.; Kurnaz, M.L. Projections of climate change in the Mediterranean Basin by using down scaled global climate model outputs. Int. J. Climatol. 2015, 35, 4276–4292. [Google Scholar] [CrossRef]
- Byrne, D.H.; Raseira, M.C.; Bassi, D.; Piagnani, M.C.; Gasic, K.; Reighard, G.L.; Moreno, M.A.; Pérez, S. Peach. In Fruit Breeding, Handbook of Plant Breeding 8; Badenes, M.L., Byrne, D.H., Eds.; Springer Science + Business Media, LLC: Düsseldorf, Germany, 2012; pp. 505–565. [Google Scholar]
- Reighard, G.; Loreti, F. Rootstock development. In The Peach: Botany, Production and Uses; Layne, D.R., Bassi, D., Eds.; CABI: Wallingford, UK, 2008; pp. 193–220. [Google Scholar]
- Moreno, M.A. Breeding and selection of Prunus rootstocks at the Aula Dei Experimental Station, Zaragoza, Spain. Acta Hortic. 2004, 658, 519–528. [Google Scholar] [CrossRef]
- Yahmed, J.B.; Ghrab, M.; Moreno, M.A.; Pinochet, J.; Mimoun, M.B. Performance of “Subirana” flat peach cultivar budded on different Prunus rootstocks in a warm production area in North Africa. Sci. Hortic. 2016, 206, 24–32. [Google Scholar] [CrossRef]
- Reig, G.; Garanto, X.; Mas, N.; Iglesias, I. Long-term agronomical performance and iron chlorosis susceptibility of several Prunus rootstocks grown under loamy and calcareous soil conditions. Sci. Hortic. 2020, 262, 109035. [Google Scholar] [CrossRef]
- Reig, G.; Alegre, S.; Gatius, F.; Iglesias, I. Adaptability of peach cultivars [Prunus persica (L.) Batsch] to the climatic conditions of the Ebro Valley, with the special focus on fruit quality. Sci. Hortic. 2015, 190, 149–160. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Reig, G.; Giménez, R.; Mignard, P.; Mestre, L.; Moreno, M.A. Sugars and organic acids profile and antioxidant compounds of nectarine fruits influenced by different rootstocks. Sci. Hortic. 2019, 248, 145–153. [Google Scholar] [CrossRef]
- Iglesias, I.; Giné-Bordonada, J.; Garanto, X.; Reig, G. Rootstock affects fruit quality and phytochemical composition of “Big Top” nectarine grown under hot climatic conditions. Sci. Hortic. 2019, 256, 108586. [Google Scholar] [CrossRef]
- Pinochet, J.; Calvet, C.; Hernández-Dorrego, A.; Bonet, A.; Felipe, A.; Moreno, M.A. Resistance of peach and plum rootstocks from Spain, France, and Italy to root-knot nematode Meloidogyne javanica. HortScience 1999, 34, 1259–1262. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Moreno, M.A. Influence of plum rootstocks on agronomic performance, leaf mineral nutrition and fruit quality of “Catherina” peach cultivar in heavy-calcareous soil conditions. Span. J. Agric. Res. 2017, 15, e0901. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.A.; Pinochet, J. Graft compatibility between peach cultivars and Prunus rootstocks. HortScience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- Salazar, A.E.; Torrents, J.; Bordas, M.; Val, J.; Moreno, M.A. Graft compatibility for new released Prunus rootstocks. Acta Hortic. 2018, 1228, 175–180. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Romero, J.; Gogorcena, Y.; Moreno, M.A.; Espada, J.L. Performance of peach and plum based rootstocks of different vigor on a late peach cultivar in replant and calcareous conditions. Sci. Hortic. 2011, 129, 58–63. [Google Scholar] [CrossRef]
- Iglesias, I. Costes de producción, sistemas de formación y mecanización en frutales, con especial referencia al melocotonero. Rev. Frutic. 2019, 69, 50–59. [Google Scholar]
- Orazem, P.; Stampar, F.; Hudina, M. Quality analysis of “Redhaven” peach fruit grafted on 11 rootstocks of different genetic origin in a replant soil. Food Chem. 2011, 124, 1691–1698. [Google Scholar] [CrossRef]
- Reighard, G.L.; Beckman, T.; Belding, R.; Black, B.; Byers, P.; Cline, J.; Cowgill, W.; Godin, R.; Johnson, R.S.; Kamas, J.; et al. Six-year performance of 14 Prunus rootstocks at 11 sites in the 2001 NC-140 peach trial. J. Am. Pomol. Soc. 2011, 65, 26–41. [Google Scholar]
- Remorini, D.; Fei, C.; Loreti, F.; Massai, R. Observations on nine peach rootstocks grown in a replant soil. Acta Hortic. 2015, 1085, 131–138. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gradziel, T.M.; Gogorcena, Y.; Moreno, M.A. Phenotypic diversity among local Spanish and foreign peach and nectarine [Prunus persica (L.) Batsch] accessions. Euphytica 2014, 197, 261–277. [Google Scholar] [CrossRef]
- Moreno, M.A.; Tabuenca, M.C.; Cambra, R. Performance of Adafuel and Adarcias as peach rootstocks. HortScience 1994, 29, 1271–1273. [Google Scholar] [CrossRef]
- Moreno, M.A.; Tabuenca, M.C.; Cambra, R. “Adesoto 101”, a plum rootstock for peaches and other stone fruit. HortScience 1995, 30, 1314–1315. [Google Scholar] [CrossRef]
- Edin, M.; Garcin, A. Un nouveau porte greffe du pêcher Cadaman-Avimag. L’Arboriculture Fruitière 1994, 475, 20–23. [Google Scholar]
- Felipe, A.J. “Felinem”, “Garnem” and “Monegro” almond × peach hybrid rootstock. HortScience 2009, 44, 196–197. [Google Scholar] [CrossRef]
- Bernhard, R.; Grasselly, C. Les pêchers × amandiers. L’Arboriculture Fruitière 1981, 328, 37–42. [Google Scholar]
- Nicotra, A.; Moser, L. Two new plum rootstocks for peach and nectarines: “Penta” and “Tetra”. Acta Hortic. 1997, 451, 269–271. [Google Scholar] [CrossRef]
- Pinochet, J. “Replantpac” (Rootpac® R), a plum-almond hybrid rootstock for replant situations. HortScience 2010, 45, 299–301. [Google Scholar] [CrossRef]
- Mestre, L.; Reig, G.; Betrán, J.A.; Pinochet, J.; Moreno, M.A. Influence of peach-almond hybrids and plum-based rootstocks on mineral nutrition and yield characteristics of “Big Top” nectarine in replant and heavy-calcareous soil conditions. Sci. Hortic. 2015, 192, 475–481. [Google Scholar] [CrossRef]
- Okie, W.R. Plum rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; John Wiley and Sons: New York, NY, USA, 1987; pp. 321–360. [Google Scholar]
- Salvador, R.; Martínez-Cob, A.; Cavero, J.; Playán, E. Seasonal on-farm irrigation performance in the Ebro basin (Spain): Crops and irrigation systems. Agric. Water Manag. 2011, 98, 577–587. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Watanabe, F.S. A method to determine a phosphorus adsorption maximum of soils as measured by the langmuir isotherm. Soil Sci. Soc. Am. Proc. 1957, 21, 144–149. [Google Scholar] [CrossRef]
- Gaucher, G. Traité de pédologie agricole: Le sol et ses caractéristiques agronomiques; Dunod: Paris, France, 1968; p. 578. [Google Scholar]
- Ahn, J.S.; Lee, Y.K. Color distribution of a shade guide in the value, chroma, and hue scale. J. Prosthet. Dent. 2008, 100, 18–28. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Gómez-Aparisi, J.; Betrán, J.A.; Moreno, M.A. Influence of almond x peach hybrids rootstocks on flower and leaf mineral concentration, yield and vigor of two peach cultivars. Sci. Hortic. 2005, 106, 502–514. [Google Scholar] [CrossRef]
- C.I.I. (Comité Inter-Institutos para el estudio de técnicas analíticas). Métodos de referencia para la determinación de elementos minerales en vegetales. Anales Edafología Agrobiología 1969, 38, 403–417. [Google Scholar]
- Montañés, L.; Heras, L.; Abadía, J.; Sanz, M. Plant analysis interpretation based on a new index: Deviation from optimum percentage (DOP). J. Plant Nutr. 1993, 16, 1289–1308. [Google Scholar] [CrossRef]
- Leece, D.R. Diagnostic leaf analysis for stone fruit. Peach (Prunus persica). Aust. J. Exp. Agric. Anim. Husb. 1975, 15, 138–139. [Google Scholar]
- Villar, P.; Villar, J.M. Guia de la Fertilitat dels Sòls i la Nutrició Vegetal en Producció Integrada; Consell Català de Producció Integrada: Lleida, Spain, 2016; p. 126.
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.A. Agronomical and fruit quality traits of two peach cultivars on peach-almond hybrid rootstocks growing on Mediterranean conditions. Sci. Hortic. 2012, 140, 157–163. [Google Scholar] [CrossRef]
- Reig, G.; Font i Forcada, C.; Mestre, L.; Jiménez, S.; Betrán, J.A.; Moreno, M.A. Horticultural, leaf mineral and fruit quality traits of two “Greengage” plum cultivars budded on plum based rootstocks in Mediterranean conditions. Sci. Hortic. 2018, 232, 84–91. [Google Scholar] [CrossRef]
- Tsipouridis, C.; Thomidis, T. Effect of 14 peach rootstocks on the yield, fruit quality, mortality, girth expansion and resistance to frost damages of “May Crest” peach variety and their susceptibility on Phytophthora citrophthora. Sci. Hortic. 2005, 103, 421–428. [Google Scholar] [CrossRef]
- Olmo-Vega, A.; García-Sánchez, F.; Simón, I.S.-G.; Lidón, V.; Manuel, N.; Martínez-Nicolás, J.J. Physiological responses of three pomegranate cultivars under flooded conditions. Sci. Hortic. 2017, 224, 171–179. [Google Scholar] [CrossRef]
- Ziegler, V.H.; Ploschuk, E.; Weibel, A.; Insausti, P. Short-term responses to flooding stress of three Prunus rootstocks. Sci. Hortic. 2017, 224, 135–141. [Google Scholar] [CrossRef]
- Massai, R.; Loreti, F. Preliminary observations on nine peach rootstocks grown in a replant soil. Acta Hortic. 2004, 658, 185–192. [Google Scholar] [CrossRef]
- Marra, F.P.; Lo Bianco, M.; La Mantia, L.; Caruso, T. Growth, yield and fruit quality of “Tropic Snow” peach on size-controlling rootstocks under dry Mediterranean climates. Sci. Hortic. 2013, 160, 274–282. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.A. Fruit sugar profile and antioxidants of peach and nectarine cultivars on almond × peach hybrid rootstocks. Sci. Hortic. 2013, 164, 563–572. [Google Scholar] [CrossRef]
- Lopestri, J.; Goodwin, I.; McGlasson, B.; Holford, P.; Golding, J. Variability in size and soluble solids concentration in peaches and nectarines. Hortic. Rev. 2014, 42, 253–311. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G. Differential effect of cultivar and harvest date on nectarine color: Quality and consumer acceptance. Sci. Hortic. 2009, 120, 41–50. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharv. Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Veberič, R.; Štampar, F. Quality changes during ripening of plums (Prunus domestica L.). Food Chem. 2008, 111, 830–836. [Google Scholar] [CrossRef]
- Daza, A.; García-Galavís, P.A.; Grande, M.J.; Santamaría, C. Fruit quality parameters of “Pioneer” Japanese plums produced on eight different rootstocks. Sci. Hortic. 2008, 118, 206–211. [Google Scholar] [CrossRef]
- Génard, M.; Souty, M.; Holmes, S.; Reich, M.; Breuils, L. Correlations among quality parameters of peach fruit. J. Sci. Food Agric. 1994, 66, 241–245. [Google Scholar] [CrossRef]
- Ruíz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Lewallen, K.S.; Marini, R.P. Relationship between flesh firmness and ground color in peach as influenced by light and canopy position. J. Am. Hortic. Sci. 2003, 128, 163–170. [Google Scholar]
- Jiménez, S.; Garín, A.; Gogorcena, Y.; Betrán, J.A.; Moreno, M.A. Flower and foliar analysis for prognosis of sweet cherry nutrition: Influence of different rootstocks. J. Plant Nutr. 2004, 27, 701–712. [Google Scholar] [CrossRef]
- El-Jendoubi, H.; Igartua, E.; Abadía, J.; Abadía, A. Prognosis of iron chlorosis in pear (Pyrus communis L.) and peach (Prunus persica L. Batsch) trees using bud, flower and leaf mineral concentrations. Plant Soil 2012, 354, 121–139. [Google Scholar] [CrossRef]
- Yahmed, J.B.; Ghrab, M.; Moreno, M.A.; Pinochet, J.; Mimoun, M.B. Leaf mineral nutrition and tree vigor of “Subirana” flat peach cultivar grafted on different Prunus rootstocks in a warm Mediterranean area. J. Plant Nutr. 2020, 43, 811–822. [Google Scholar] [CrossRef]
- Johnson, R.S.; Uriu, K. Mineral nutrition. In Growing and Handling for Fresh Market: Division of Agriculture Resource; Larue, J., Johnson, R.S., Eds.; University of California: Oakland, CA, USA, 1989; pp. 68–81. [Google Scholar]
- Moreno, M.A.; Adrada, R.; Aparicio, J.; Betrán, J.A. Performance of “Sunburst” sweet cherry grafted on different rootstocks. J. Hortic. Sci. Biotechnol. 2001, 76, 167–173. [Google Scholar] [CrossRef]
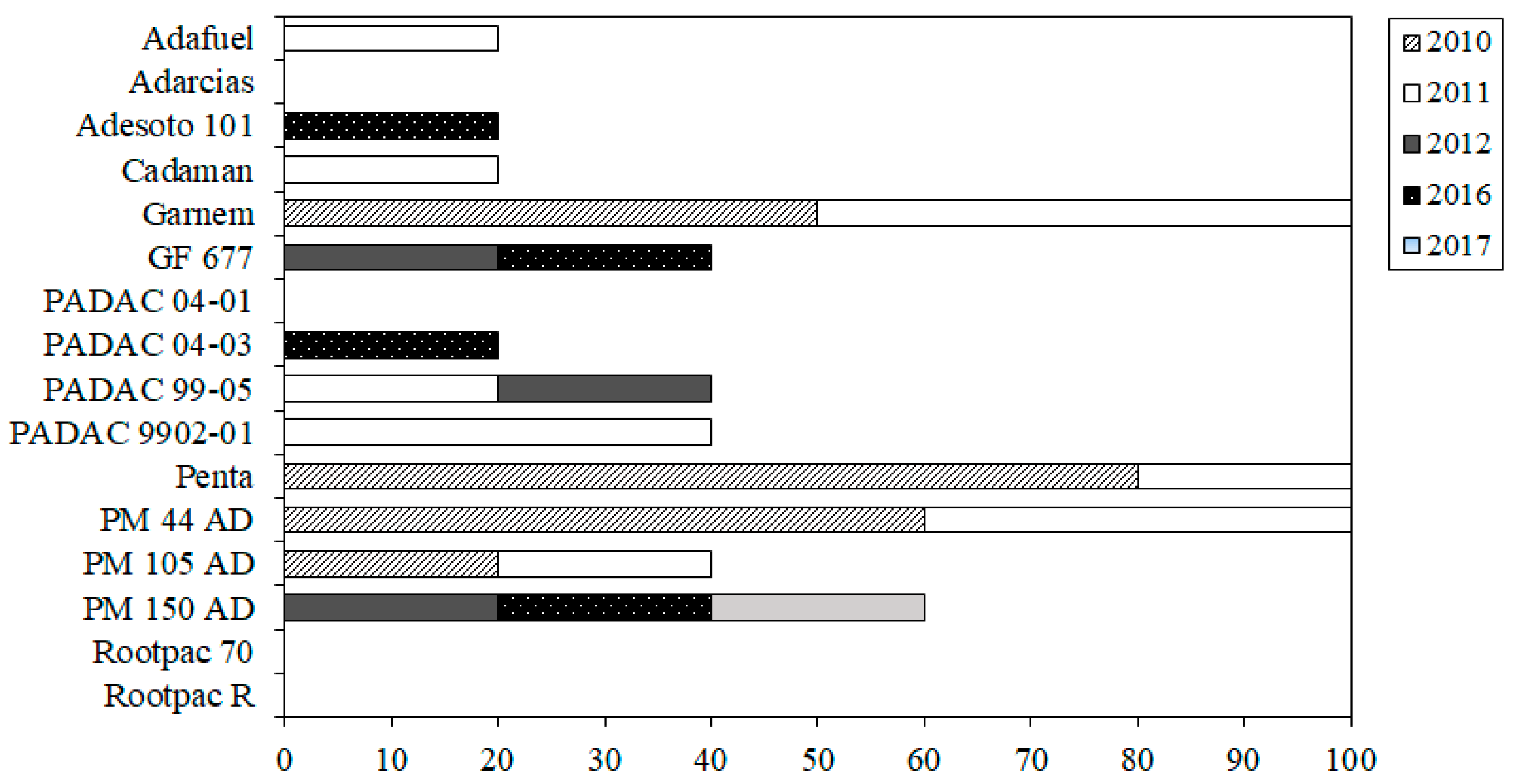



| Rootstock | Species | Type | Genetic Background | Origin a | Calcareous Soil T b | Waterlogging T b | References |
|---|---|---|---|---|---|---|---|
| “Adafuel®” | P. amygdalus × P. persica | Vigorous | Open-pollinated | CSIC, Spain | HT | S | Moreno et al. [26] |
| “Adarcias®” | P. amygdalus × P. persica | Semi-vigorous | Open-pollinated | CSIC, Spain | T | MT | Moreno et al. [26] |
| “Adesoto® 101” | P. insititia | Semi-vigorous | Open-pollinated | CSIC, Spain | HT | HT | Moreno et al. [27] |
| “Cadaman®” | P. persica × P. davidiana | Vigorous | Controlled cross | INRA, France | T | MT | Edin and Garcin [28] |
| “Garnem®” | P. amygdalus × P. persica | Vigorous | Controlled cross | CITA, Spain | HT | S | Felipe [29] |
| “GF 677” | P. amygdalus × P. persica | Vigorous | Open-pollinated | INRA, France | HT | S | Bernhard and Grasselly [30] |
| “PADAC 04-01” | P. cerasifera × (P. amygdalus × P. persica) | Vigorous | Controlled cross | CSIC, Spain | HT | HT | Moreno [9] |
| “PADAC 04-03” | P. cerasifera × (P. amygdalus × P. persica) | Vigorous | Controlled cross | CSIC, Spain | HT | HT | Moreno [9] |
| “PADAC 99-05” | P. cerasifera × (P. amygdalus × P. persica) | Vigorous | Controlled cross | CSIC, Spain | HT | HT | Moreno [9] |
| “PADAC 9902-01” | (P. amygdalus × P. persica) × (P. persica × P. davidiana) | Vigorous | Controlled cross | CSIC, Spain | HT | HT | Moreno [9] |
| “Penta” | P. domestica | Semi-vigorous | Open-pollinated | CRF, Italy | T | T | Nicotra and Moser [31] |
| “PM 44 AD” | P. insititia | Semi-vigorous | Open-pollinated | CSIC, Spain | T | T | Moreno [9] |
| “PM 105 AD” | P. insititia | Semi-vigorous | Open-pollinated | CSIC, Spain | T | T | Moreno [9] |
| “PM 150 AD” | P. insititia | Semi-vigorous | Open-pollinated | CSIC, Spain | T | T | Moreno [9] |
| “Rootpac® 70” | P. persica × (P. amygdalus × P. persica) | Vigorous | Controlled cross | AI, Spain | HT | MT | Jiménez et al. [20] |
| “Rootpac® R” | P. cerasifera × P. amygdalus | Vigorous | Controlled cross | AI, Spain | HT | HT | Pinochet [32] |
| Depth (cm) | Texture | E.C. (1:5) (dS/m) | pH | Organic Matter a (%) | P b (ppm) | K c (ppm) | NO3 d (ppm) | Mg c (mg/kg) | CaCO3 e (%) | Active Limestone f (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–30 | Silty-Clay-Sandy | 0.30 | 8.3 | 3.21 | 31 | 388 | 14 | 296 | 32 | 7.00 |
| 30–60 | Silty-Clay-Sandy | 0.30 | 8.4 | 1.76 | 10 | 168 | 6 | 208 | 32 | 6.78 |
| 60–90 | Silty-Clay-Sandy | 0.40 | 8.4 | 0.98 | 4 | 148 | 2 | 192 | 33 | 6.38 |
| Year | Tavr (°C) | Tmin (°C) | Tmax (°C) | Sr (W m−2) | Rainfall * (mm) |
|---|---|---|---|---|---|
| 2013 | 14.5 c | 8.1 b | 21.1 c | 268.2 ns | 151.0 |
| 2014 | 17.0 ab | 9.9 a | 24.3 ab | 272.1 | 105.0 |
| 2015 | 17.1 ab | 10.1 a | 25.0 ab | 257.3 | 108.6 |
| 2016 | 15.9 bc | 8.9 ab | 22.9 bc | 244.8 | 115.0 |
| 2017 | 17.8 a | 9.4 ab | 26.0 a | 273.0 | 91.0 |
| Trait | Units/Description | Variance Analysis | Variability a (%) | ||||
|---|---|---|---|---|---|---|---|
| Year (Y) | Rootstock (R) | Y x R | Year (Y) | Rootstock (R) | Y x R | ||
| TCSA | cm2 | *** | *** | ns | 34.9 | 59.7 | 5.4 |
| Yield | Kg/tree | *** | ** | ns | 75.8 | 12.0 | 12.2 |
| Average Fruit weight | grams | * | ns | ns | 23.5 | 31.4 | 45.1 |
| Soluble solid content (SSC) | ºBrix | *** | *** | ns | 56.1 | 28.0 | 15.9 |
| Titratable acidity (TA) | g malic acid per 100 g FW | *** | *** | *** | 36.6 | 25.2 | 38.3 |
| Flesh firmness | Newtons | *** | ns | ns | 85.4 | 4.9 | 9.7 |
| Brightness or Lightness angle (L) | Color parameter | *** | * | ns | 93.5 | 2.4 | 4.0 |
| Greenness or Redness (a*) | Color parameter | *** | * | * | 97.6 | 0.8 | 1.6 |
| Blueness or Yellowness (b*) | Color parameter | *** | ** | ns | 97.7 | 1.0 | 1.3 |
| Chroma (C*) | Color parameter | *** | ** | ns | 95.1 | 2.1 | 2.9 |
| Hue angle (h) | Color parameter | *** | ** | ns | 85.7 | 6.6 | 7.7 |
| Rootstock | Final TCSA (cm2) | Average Yield (kg/tree) | CY (kg/tree) | CYE (kg/cm2) | Average FW a (g) |
|---|---|---|---|---|---|
| “Adafuel” | 444.9 a | 39.5 a | 182.1 ns | 0.42 b | 171.1 ns |
| “Adarcias” | 187.4 b | 27.0 abc | 123.1 | 0.68 ab | 175.0 |
| “Adesoto 101” | 157.9 b | 21.7 c | 123.7 | 0.86 a | 177.5 |
| “Cadaman” | 274.3 b | 33.2 abc | 170.7 | 0.61 ab | 197.0 |
| “GF 677” | 303.3 ab | 34.1 abc | 176.4 | 0.62 ab | 176.3 |
| “PADAC 04-01” | 285.5 b | 33.3 abc | 165.9 | 0.59 ab | 173.0 |
| “PADAC 04-03” | 259.9 b | 27.2 abc | 137.1 | 0.52 ab | 180.4 |
| “PADAC 99-05” | 267.3 b | 30.1 abc | 160.4 | 0.61 ab | 183.6 |
| “PADAC 9902-01” | 279.7 b | 36.1 ab | 189.9 | 0.69 ab | 183.2 |
| “PM 105 AD” | 139.8 b | 21.0 c | 105.4 | 0.82 ab | 169.3 |
| “Rootpac 70” | 243.9 b | 30.0 abc | 141.8 | 0.57 ab | 182.1 |
| “Rootpac R” | 247.1 b | 28.9 abc | 141.4 | 0.59 ab | 169.2 |
| Rootstock | N a | Average SSC (ºBrix) | Average TA (g Malic Acid/100 FW) | Average FF (N) |
|---|---|---|---|---|
| “Adafuel” | 3 | 13.9 d | 0.58 cd | 40.4 ns |
| “Adarcias” | 5 | 15.7 abc | 0.63 abc | 42.1 |
| “Adesoto 101” | 5 | 16.0 ab | 0.69 a | 41.2 |
| “Cadaman” | 4 | 14.9 bcd | 0.64 abc | 44.2 |
| “GF 677” | 4 | 14.5 cd | 0.54 d | 44.3 |
| “PADAC 04-01” | 5 | 16.9 a | 0.53 d | 41.9 |
| “PADAC 04-03” | 5 | 15.3 bcd | 0.60 abc | 42.7 |
| “PADAC 99-05” | 4 | 16.4 ab | 0.64 abc | 43.7 |
| “PADAC 9902-01” | 3 | 14.2 d | 0.61 abc | 43.2 |
| “PM 105 AD” | 3 | 15.6 abcd | 0.66 ab | 41.5 |
| “Rootpac 70” | 4 | 14.2 d | 0.63 abc | 41.8 |
| “Rootpac R” | 5 | 16.2 ab | 0.68 a | 46.8 |
| Rootstock | n a | L* (Brightness/ Lightness) | a* (Greenness/ Redness) | b* (Blueness/ Yellowness) | C* (Chroma) | h (Hue Angle) |
|---|---|---|---|---|---|---|
| “Adafuel” | 3 | 37.3 ab | 37.3 a | 22.8 ab | 35.0 ns | 26.9 ab |
| “Adarcias” | 5 | 36.7 ab | 28.3 ab | 21.4 b | 32.8 | 25.1 b |
| “Adesoto 101” | 5 | 37.1 ab | 27.1 ab | 21.8 ab | 32.5 | 25.7 ab |
| “Cadaman” | 4 | 36.7 ab | 26.6 ab | 21.7 ab | 33.2 | 26.2 ab |
| “GF 677” | 4 | 37.7 ab | 27.1 ab | 22.7 ab | 33.0 | 26.8 ab |
| “PADAC 04-01” | 5 | 38.2 a | 26.3 ab | 23.7 a | 33.6 | 28.9 a |
| “PADAC 04-03” | 5 | 37.2 ab | 27.3 ab | 22.3 ab | 33.8 | 26.3 ab |
| “PADAC 99-05” | 4 | 37.6 ab | 27.6 ab | 23.1 ab | 34.4 | 27.9 ab |
| “PADAC 9902-01” | 3 | 37.5 ab | 27.6 ab | 22.7 ab | 34.3 | 27.2 ab |
| “PM 105 AD” | 3 | 35.8 b | 27.7 ab | 20.7 b | 32.4 | 25.1 b |
| “Rootpac 70” | 4 | 36.5 ab | 26.1 ab | 21.1 b | 32.6 | 25.1 b |
| “Rootpac R” | 5 | 37.3 ab | 26.9 b | 22.0 ab | 32.5 | 26.7 ab |
| Soluble Solids Content | Titratable Acidity | Leaf Mg | Leaf Mn | |
|---|---|---|---|---|
| Minimum temperature | 0.99 * | - | - | - |
| Maximum temperature | - | −0.91 ** | - | - |
| Hue angle | - | 0.30 * | - | - |
| Leaf Ca | - | - | 0.75 * | 0.60 * |
| Rootstock | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flower | Leaf | Flower | Leaf | Flower | Leaf | Flower | Leaf | Flower | Leaf | |
| “Adafuel” | 2.86 ab | 3.11 ns | 0.38 a | 0.20 ab | 1.96 ab | 1.88 c | 0.47 a | 2.07 ab | 0.20 a | 0.43 abc |
| “Adarcias” | 2.89 ab | 3.00 | 0.33 ab | 0.20 ab | 2.01 ab | 2.21 abc | 0.38 abc | 2.02 ab | 0.19 abc | 0.45 ab |
| “Adesoto 101” | 2.76 bc | 2.98 | 0.35 ab | 0.20 ab | 1.98 ab | 2.12 abc | 0.44 ab | 1.64 bc | 0.17 c | 0.37 bcd |
| “Cadaman” | 2.79 b | 2.99 | 0.33 ab | 0.22 ab | 1.97 ab | 2.04 abc | 0.41 abc | 2.00 ab | 0.19 abc | 0.47 a |
| “GF 677” | 2.86 ab | 3.05 | 0.37 a | 0.20 ab | 1.86 b | 2.03 abc | 0.38 abc | 2.01 ab | 0.18 abc | 0.45 ab |
| “PADAC 04-01” | 3.99 a | 3.06 | 0.37 a | 0.19 ab | 1.96 ab | 1.86 c | 0.32 d | 1.32 c | 0.17 c | 0.34 d |
| “PADAC 04-03” | 2.90 ab | 2.98 | 0.35 ab | 0.17 b | 1.97 ab | 2.00 abc | 0.39 abc | 2.42 a | 0.19 abc | 0.48 a |
| “PADAC 9902-01” | 2.74 bc | 3.10 | 0.36 a | 0.22 ab | 1.99 ab | 2.09 abc | 0.42 abc | 1.77 bc | 0.19 abc | 0.47 a |
| “PADAC 99-05” | 2.87 ab | 3.03 | 0.36 a | 0.18 ab | 1.95 ab | 1.92 c | 0.35 cd | 1.55 bc | 0.19 abc | 0.44 ab |
| “PM 105 AD” | 2.76 bc | 3.05 | 0.35 ab | 0.21 ab | 2.07 a | 2.43 ab | 0.43 ab | 1.29 c | 0.17 c | 0.36 cd |
| “Rootpac 70” | 2.62 c | 2.93 | 0.30 b | 0.24 a | 1.99 ab | 2.44 a | 0.41 abc | 2.02 ab | 0.19 abc | 0.51 a |
| “Rootpac R” | 2.86 ab | 3.06 | 0.35 ab | 0.21 ab | 1.96 ab | 2.16 abc | 0.37 abc | 1.51 bc | 0.17 c | 0.38 bcd |
| Adequate values a | - | 2.5–3.8 | - | 0.2–0.4 | - | 1.7–2.7 | - | 2.0–3.5 | - | 0.4–0.7 |
| Adequate values b | - | 3.0–3.5 | - | 0.14–0.25 | - | 2.0–3.0 | - | 1.8–2.7 | - | 0.3–0.8 |
| Rootstock | Fe (mg kg−1) | Cu (mg kg−1) | Mn (mg kg−1) | Zn (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Flower | Leaf | Flower | Leaf | Flower | Leaf | Flower | Leaf | |
| “Adafuel” | 144.7 ns | 50.6 ab | 47.0 c | 8.3 ab | 22.3 ab | 43.3 a | 45.3 abc | 19.0 ab |
| “Adarcias” | 147.0 | 76.7 ab | 65.3 bc | 6.3 cd | 25.3 ab | 42.5 a | 43.8 abc | 16.0 b |
| “Adesoto 101” | 164.3 | 75.0 ab | 75.0 ab | 8.0 abc | 22.0 ab | 27.7 bc | 47.0 abc | 22.5 ab |
| “Cadaman” | 135.5 | 71.5 ab | 67.5 bc | 6.5 abc | 28.3 a | 39.7 a | 45.0 abc | 18.7 ab |
| “GF 677” | 130.8 | 72.2 ab | 64.8 bc | 8.0 abc | 18.0 ab | 38.0 ab | 47.8 abc | 21.0 ab |
| “PADAC 04-01” | 135.4 | 75.4 ab | 72.2 b | 7.4 abc | 21.6 ab | 34.4 abc | 37.4 bc | 17.8 ab |
| “PADAC 04-03” | 133.5 | 72.7 ab | 64.8 bc | 6.2 d | 20.3 ab | 38.5 ab | 57.0 a | 25.5 a |
| “PADAC 9902-01” | 134.7 | 70.7 ab | 56.0 bc | 8.0 abc | 20.7 ab | 36.7 abc | 40.7 abc | 19.7 ab |
| “PADAC 99-05” | 129.8 | 81.5 a | 68.5 bc | 8.2 bcd | 20.3 ab | 32.0 abc | 52.0 ab | 21.7 ab |
| “PM 105 AD” | 146.7 | 64.0 b | 94.7 a | 10.0 a | 19.3 ab | 25.7 c | 44.0 abc | 22.0 ab |
| “Rootpac 70” | 150.0 | 74.5 ab | 62.0 bc | 6.8 abc | 17.3 b | 35.7 abc | 34.7 c | 18.5 ab |
| “Rootpac R” | 141.3 | 82.0 a | 68.8 bc | 7.3 abc | 19.7 ab | 38.5 ab | 38.3 bc | 21.0 ab |
| Adequate values a | - | 80–175 | - | 5–20 | - | 45–60 | - | 30–50 |
| Adequate values b | - | 100–250 | - | 5–16 | - | 40–160 | - | 20–50 |
| Rootstock | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOP a | DOP b | DOP a | DOP b | DOP a | DOP b | DOP a | DOP b | DOP a | DOP b | |
| “Adafuel” | −4.1 ns | −1.1 ns | 4.3 ns | −32.2 ns | −24.7 ns | −14.4 ns | −8.0 ab | −24.7 ab | −21.2 abc | −21.2 abc |
| “Adarcias” | −7.6 | −4.7 | 4.3 | −32.2 | −11.5 | 0.5 | −10.1 a | −26.5 ab | −18.5 abc | −18.5 abc |
| “Adesoto 101” | −8.3 | −5.4 | 3.8 | −32.5 | −15.1 | −3.5 | −27.0 ab | −40.2 ab | −32.7 bc | −32.7 bc |
| “Cadaman” | −8.0 | −5.1 | 14.5 | −25.6 | −18.1 | −6.9 | −11.0 ab | −27.2 ab | −11.8 ab | −11.8 ab |
| “GF 677” | −6.2 | −3.2 | 5.1 | −31.7 | −18.7 | −7.6 | −10.3 ab | −26.6 ab | −18.2 abc | −18.2 abc |
| “PADAC 04-01” | −5.7 | −2.7 | 1.5 | −34.0 | −25.5 | −15.4 | −41.5 b | −52.1 b | −37.1 c | −37.1 c |
| “PADAC 04-03” | −8.1 | −5.2 | −8.9 | −40.8 | −19.7 | −8.7 | 7.9 a | −11.7 a | −12.3 ab | −12.3 ab |
| “PADAC 99-05” | −6.8 | −3.8 | −6.4 | −39.2 | −23.1 | −12.6 | −30.9 ab | −43.4 ab | −19.5 abc | −19.5 abc |
| “PADAC 9902-01” | −4.5 | −1.5 | 111 | −27.8 | −16.5 | −5.1 | −21.3 ab | −35.6 ab | −13.9 abc | −13.9 abc |
| “PM 105 AD” | −6.3 | −3.3 | 9.4 | −28.9 | −2.7 | 10.6 | −42.4 b | −52.8 b | −35.1 bc | −35.1 bc |
| “Rootpac 70” | −9.7 | −6.8 | 20.5 | −21.7 | −2.2 | 11.1 | −10.2 ab | −26.5 ab | −7.3 a | −7.3 a |
| “Rootpac R” | −5.6 | −2.6 | 7.7 | −30.0 | −13.3 | −1.5 | −32.7 b | −44.9 b | −30.9 bc | −30.9 bc |
| Rootstock | Fe (mg kg−1) | Cu (mg kg−1) | Mn (mg kg−1) | Zn (mg kg−1) | ∑ DOP a | ∑ DOP b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOP a | DOP b | DOP a | DOP b | DOP a | DOP b | DOP a | DOP b | |||
| “Adafuel” | −58.3 ns | −42.7 ns | −20.6 ab | −33.3 ab | −56.7 a | −17.5 a | −45.7 ns | −52.5 ns | 259.8 ns | 239.6 ns |
| “Adarcias” | −56.2 | −39.9 | −39.7 b | −49.3 b | −57.5 a | −19.0 a | −54.3 | −60.0 | 277.7 | 268.2 |
| “Adesoto 101” | −57.1 | −41.2 | −23.8 ab | −36.0 ab | −72.2 ab | −47.1 ab | −35.7 | −43.7 | 285.7 | 290.2 |
| “Cadaman” | −59.1 | −43.9 | −38.1 b | −48.0 b | −59.5 ab | −22.8 ab | −46.4 | −53.1 | 269.3 | 247.5 |
| “GF 677” | −58.7 | −43.3 | −23.8 ab | −36.0 ab | −62.0 ab | −27.6 ab | −40.0 | −47.5 | 262.1 | 246.1 |
| “PADAC 04-01” | −56.9 | −40.8 | −29.5 ab | −40.8 ab | −65.6 ab | −34.5 ab | −49.1 | −55.5 | 322.9 | 314.6 |
| “PADAC 04-03” | −58.4 | −42.9 | −40.5 b | −50.0 b | −61.5 ab | −26.7 ab | −27.1 | −36.2 | 270.2 | 250.1 |
| “PADAC 9902-01” | −53.4 | −36.1 | −21.4 ab | −34.0 ab | −68.0 ab | −39.0 ab | −37.8 | −45.6 | 271.3 | 273.3 |
| “PADAC 99-05” | −59.6 | −44.6 | −23.8 ab | −36.0 ab | −63.3 ab | −30.1 ab | −43.8 | −50.8 | 259.7 | 247.0 |
| “PM 105 AD” | −63.4 | −49.8 | −4.8 a | −20.0 a | −74.3 b | −51.1 b | −37.1 | −45.0 | 283.6 | 297.3 |
| “Rootpac 70” | −57.4 | −41.6 | −35.7 b | −46.0 b | −64.2 ab | −31.9 ab | −47.1 | −53.7 | 260.4 | 248.7 |
| “Rootpac R” | −53.1 | −35.7 | −30.1 ab | −41.3 ab | −61.5 ab | −26.7 ab | −40.0 | −47.5 | 280.8 | 270.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font i Forcada, C.; Reig, G.; Mestre, L.; Mignard, P.; Betrán, J.Á.; Moreno, M.Á. Scion × Rootstock Response on Production, Mineral Composition and Fruit Quality under Heavy-Calcareous Soil and Hot Climate. Agronomy 2020, 10, 1159. https://doi.org/10.3390/agronomy10081159
Font i Forcada C, Reig G, Mestre L, Mignard P, Betrán JÁ, Moreno MÁ. Scion × Rootstock Response on Production, Mineral Composition and Fruit Quality under Heavy-Calcareous Soil and Hot Climate. Agronomy. 2020; 10(8):1159. https://doi.org/10.3390/agronomy10081159
Chicago/Turabian StyleFont i Forcada, Carolina, Gemma Reig, Lucía Mestre, Pierre Mignard, Jesús Ángel Betrán, and María Ángeles Moreno. 2020. "Scion × Rootstock Response on Production, Mineral Composition and Fruit Quality under Heavy-Calcareous Soil and Hot Climate" Agronomy 10, no. 8: 1159. https://doi.org/10.3390/agronomy10081159
APA StyleFont i Forcada, C., Reig, G., Mestre, L., Mignard, P., Betrán, J. Á., & Moreno, M. Á. (2020). Scion × Rootstock Response on Production, Mineral Composition and Fruit Quality under Heavy-Calcareous Soil and Hot Climate. Agronomy, 10(8), 1159. https://doi.org/10.3390/agronomy10081159





