Comparison of the Effects of Different Crop Production Systems on Soil Physico-Chemical Properties and Microbial Activity under Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Experimental Site
- Organic crop production system (ORG) based on a five field crop rotation: Potato + spring wheat + undersown—grass/clover mixture (1st year)—grass/clover mixture (2nd year)—winter wheat + catch crop mustard, and organic fertilizers only. Once during the complete crop rotation cycle, compost material made from plant residues was applied to the soil at a rate of 30 t ha−1 before potatoes were planted. No mineral fertilizers and/or chemicals for weed and pest control were used. Only P and K fertilizers were applied as a natural rock, according to soil requirements. The weed control was based mainly on mechanical treatments.
- The conventional crop production system (CON) was based on a three field crop rotation (winter oilseed rape, winter wheat and spring wheat), high mineral fertilization and full chemical plant protection, according to the high-input recommendations generally used in Poland. Most herbicides were applied on crops, and only a few based on glyphosate were sprayed on stubble and incorporated into soil.
- Permanent grass (PRG) is an unbroken continuation of the original permanent pasture on the site., There is a mixture of grasses: Festuca pratensis (Festuca pratensis Huds.)—20%; Festuca rubra (Festuca rubra L.)—20%; White clover (Trifolium repens L.)—15%; Perennial ryegrass (Lolium perenne L.)—10%; Dactylis glomerata (Dactylis glomerata L.)—10%; Kentucky bluegrass (Poa pratensis L.)—10%; Black Medic (Medicago lupulina L.)—10%; Timothy grass (Phleum pratense L.)—5%.
2.2. The Weather Conditions
2.3. Physical, Chemical and Microbiological Analysis of Soil
2.3.1. The Particle Size Distribution
2.3.2. Total Porosity
2.3.3. Physical-Chemical Properties
2.3.4. Total Organic Carbon (TOC) and Organic Matter (OM) in Soil
2.3.5. Particulate Organic Matter (POM)
2.3.6. Humic Substances (HS)
2.3.7. Cold Water Extractable Carbon (CWEC) and Hot Water Extractable Carbon (HWEC)
2.3.8. Microbial Biomass Carbon (MBC)
2.3.9. Dehydrogenase Activity (DH)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effects on Total Porosity
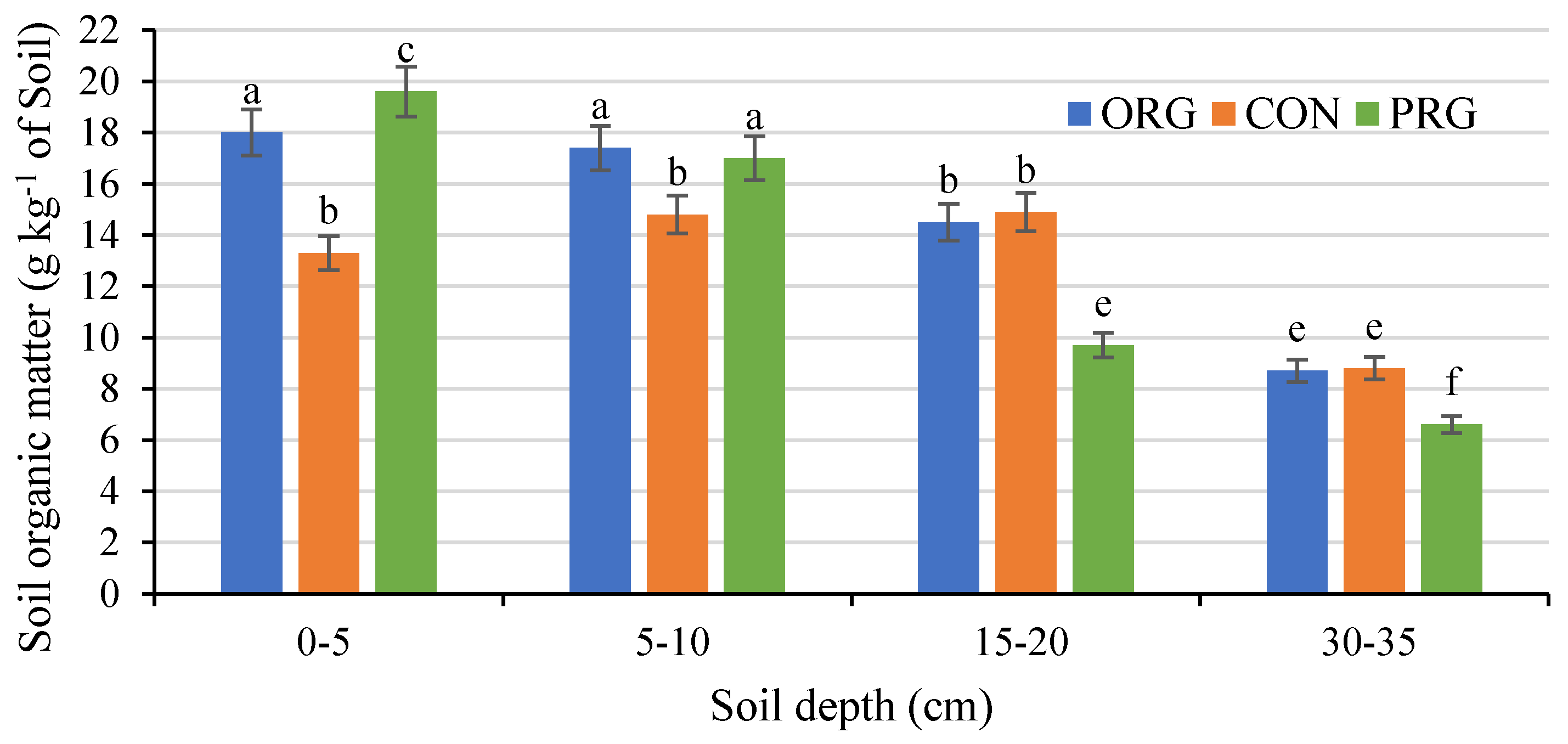
3.2. Effects on Soil Organic Matter
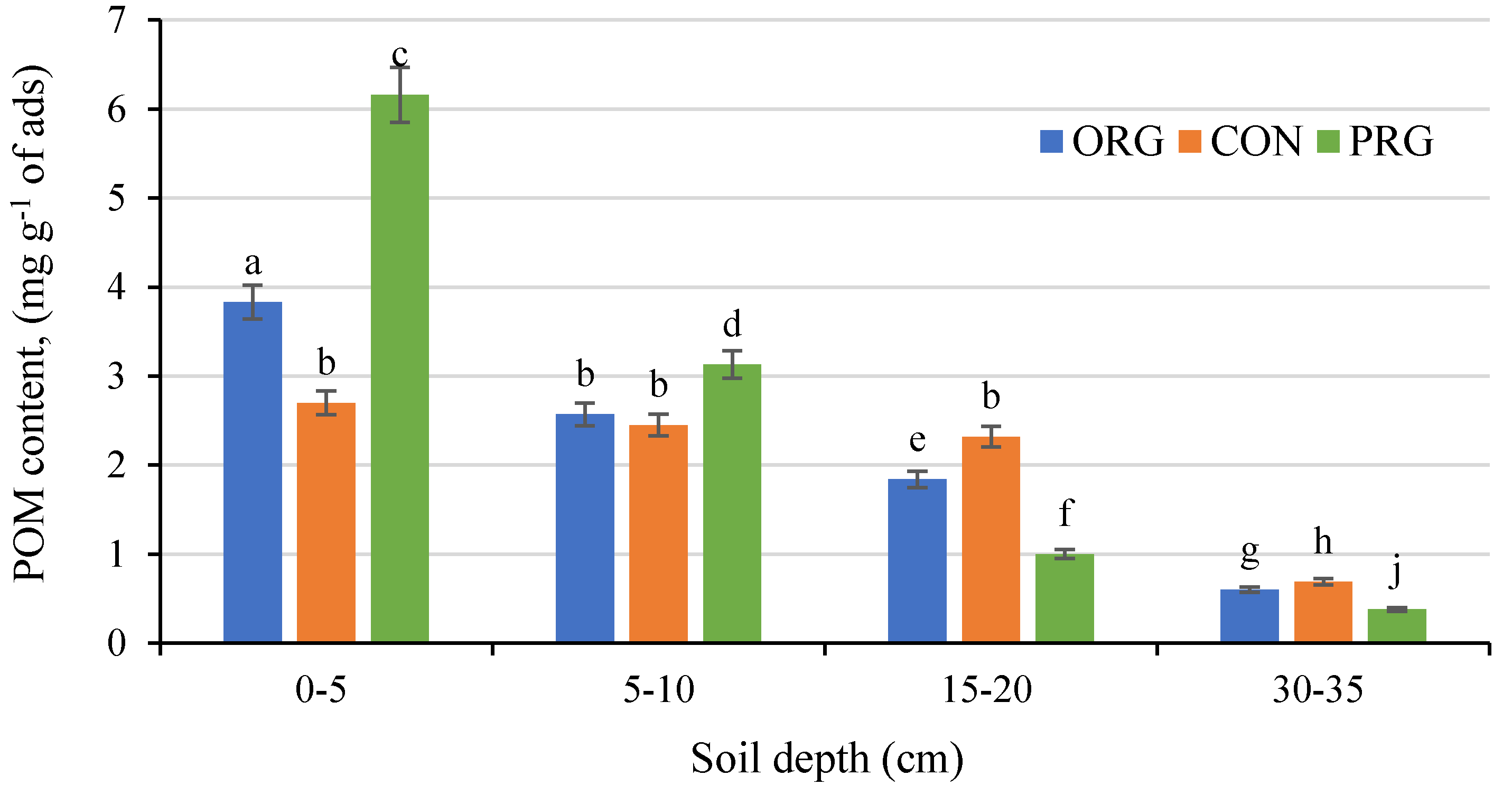
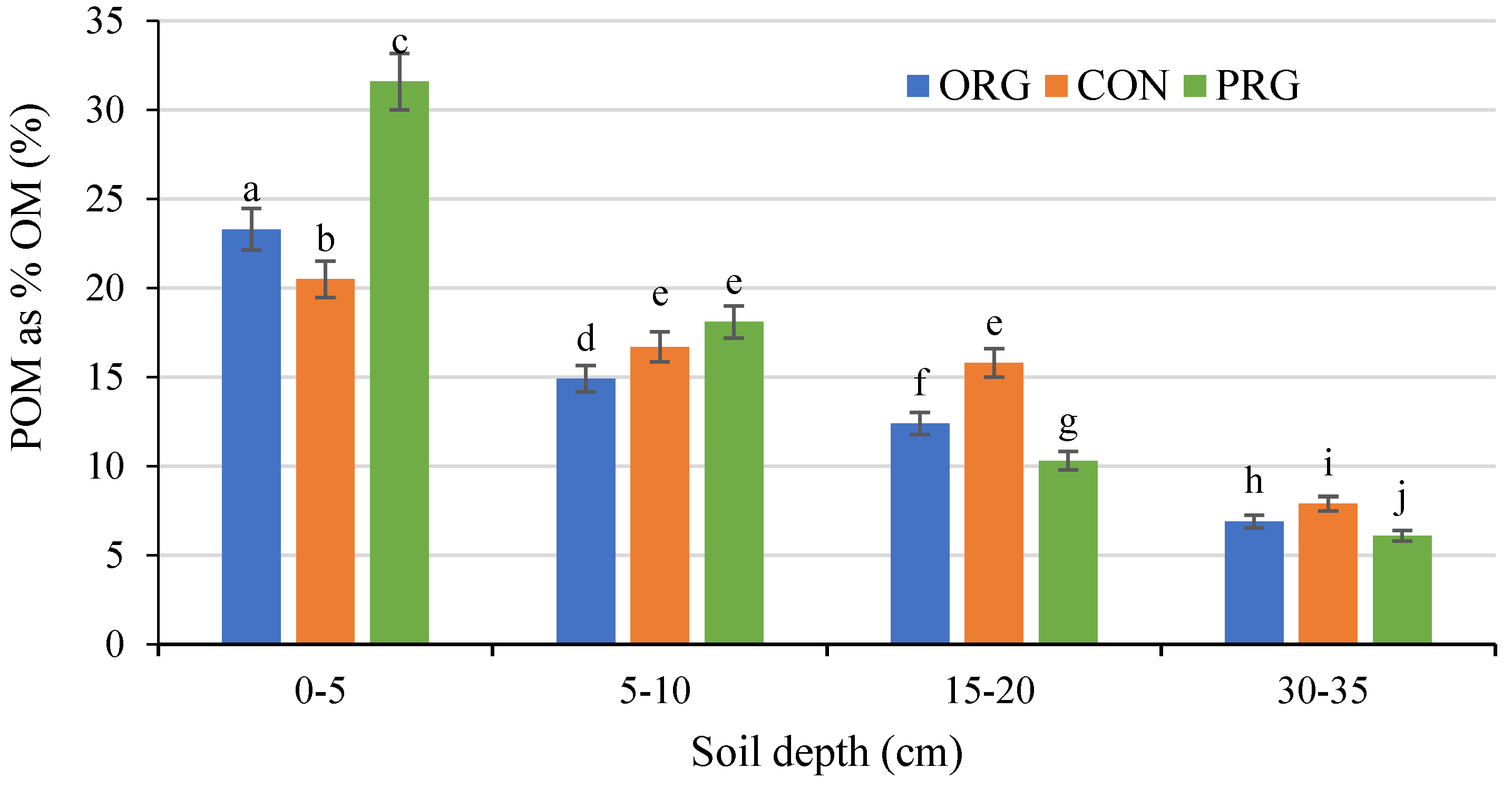
3.3. Effects on Particle Organic Matter
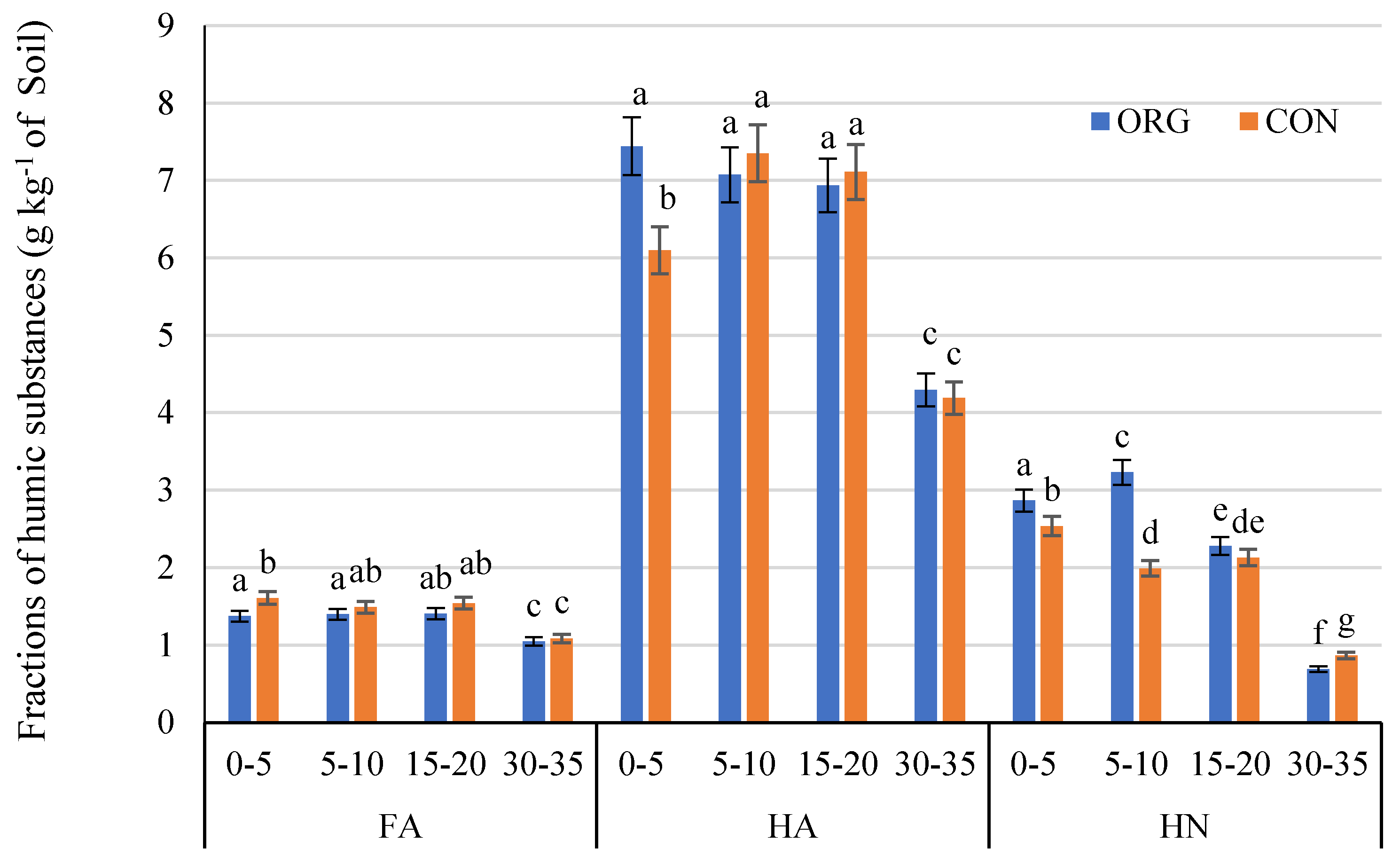
3.4. Effects on Humic Substances
3.5. Effects on Water Extractable Carbon Fraction
3.6. Effects on Microbial Biomass Carbon
3.7. Effects on Soil Dehydrogenases Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doran, J.W.; Sarrantonio, M.; Liebig, M. Soil health and sustainability. Adv. in Agron. 1996, 56, 1–54. [Google Scholar]
- Lal, R.; Delgado, J.A.; Groffman, P.M.; Millar, N.; Dell, C.; Rotz, A. Management to mitigate and adapt to climate change. J. Soil and Water Conserv. 2011, 66, 276–285. [Google Scholar] [CrossRef]
- Doran, J.W.; Liebig, M.A.; Santana, D.P. Soil health and global sustainability. In Proceedings of the 16th World Congress of Soil Science, Montpellier, France, 20–26 August 1998. [Google Scholar]
- Dexter, A.R. Soil physical quality. Part I: Theory, effects of soil texture, density and organic matter, and effects on root growth. Geoderma 2004, 120, 201–214. [Google Scholar] [CrossRef]
- Dexter, A.R. Soil physical quality. Part II: Friability, tillage, tilth and hard-setting. Geoderma 2004, 120, 215–226. [Google Scholar] [CrossRef]
- Dexter, A.R. Soil physical quality, Part III: Unsaturated hydraulic conductivity and general conclusions about S-theory. Geoderma 2004, 120, 227–239. [Google Scholar] [CrossRef]
- Gajda, A.M.; Czyż, E.A.; Furtak, K.M.; Jończyk, K. Effects of crop production practices on soil characteristics and metabolic diversity of microbial communities under winter wheat. Soil Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Klimkowicz-Pawlas, A.; Smreczak, B. Characterization of organic matter fractions in the top layer of soils under different land uses in Central-Eastern Europe. Soil Use and Manag. 2019, 35, 595–606. [Google Scholar] [CrossRef]
- Gajda, A.M.; Ewa, A.; Czyż, E.A.; Dexter, A.R.; Furtak, K.M.; Grządziel, J.; Stanek-Tarkowska, J. Effects of different soil management practices on soil properties and microbial diversity. Int. Agrophys. 2018, 32, 81–91. [Google Scholar] [CrossRef]
- Stanek-Tarkowska, J.; Czyż, E.A.; Dexter, A.; Sławiński, C. Effect of reduced and traditional tillage on soil properties and diversity of diatoms under winter wheat. Int. Agrophys. 2018, 32, 403–409. [Google Scholar] [CrossRef]
- Czyż, E.A. Effects of traffic on soil aeration, bulk density and growth of spring barley. Soil Tillage Res. 2004, 79, 153–166. [Google Scholar] [CrossRef]
- Keller, T.; Arvidsson, J.; Dexter, A.R. Soil structures produced by tillage as affected by water content and the physical quality of soil. Soil Tillage Res. 2007, 92, 45–52. [Google Scholar] [CrossRef]
- Dexter, A.R.; Richard, G.; Arrouays, D.; Czyż, E.A.; Jolivet, C.; Duval, O. Complexed organic matter controls soil physical properties. Geoderma 2008, 144, 620–627. [Google Scholar] [CrossRef]
- Czyż, E.A.; Dexter, A.R. Soil physical properties as affected by traditional, reduced and no-tillage for winter wheat. Int. Agrophys. 2009, 23, 319–326. [Google Scholar]
- Dexter, A.R.; Czyż, E.A. Applications of S-theory in the study of soil physical degradation and its consequences. Land Degrad. Dev. 2007, 18, 369–381. [Google Scholar] [CrossRef]
- Ordoñez-Morales, K.D.; Cadena-Zapata, M.; Zermeño-González, A.; Campos-Magaña, S. Effect of tillage systems on physical properties of a clay loam soil under Oats. Agriculture 2019, 9, 62. [Google Scholar] [CrossRef]
- Boydaş, M.G.; Turgut, N. Effect of tillage implements and operating speeds on soil physical properties and wheat emergence. Turk. J. Agric. For. 2007, 31, 399–412. [Google Scholar]
- Małecka, I.; Blecharczyk, A.; Sawińska, Z.; Swędrzyńska, D.; Piechota, T. Winter wheat yield and soil properties response to long-term non-inversion tillage. J. Agr. Sci. Tech. 2015, 17, 1571–1584. [Google Scholar]
- Ukalska-Jaruga, A.; Debaene, G.; Smreczak, B. Particle and structure characterization of fulvic acids -from agricultural soils. J. Soil. Sediment. 2018, 18, 2833–2843. [Google Scholar] [CrossRef]
- Baldock, J.; Nelson, P. Soil organic matter. In Handbook of Soil Science; Sumner, E., Ed.; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2000; pp. 25–72. [Google Scholar]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M. Qualitative and quantitative differences in particulate organic matter fractions in organic and conventional farming systems. Soil Biol. Biochem. 2006, 38, 1527–1536. [Google Scholar] [CrossRef]
- Semenov, V.M.; Lebedeva, T.N.; Pautova, N.B. Particulate organic matter in noncultivated and arable soils. Eurasian Soil Sc. 2019, 52, 396–404. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Methods for physical separation and characterization of soil organic matter fractions. Geoderma 1993, 56, 449–457. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Ccomposition, Reactions; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Mandeep, S.; Binoy, S.; Sabir, H.; Yong, S.O.; Bolan, N.S.; Gordon, J.C. Influence of physico-chemical properties of soil clay fractions on the retention of dissolved organic carbon. Environ. Geochem. Health 2017, 39, 1335–1350. [Google Scholar]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Louis, B.P.; Maron, P.; Viaud, V.; Leterme, P.; Menasseri-Aubry, S. Soil C and N models that integrate microbial diversity. Environ. Chem. Lett. 2016, 14, 331–344. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Hamkalo, Z.; Bedernichek, T. Total, cold and hot water extractable organic carbon in soil profile: Impact of land-use change. Zemdirbyste-Agriculture 2014, 101, 125–132. [Google Scholar] [CrossRef]
- Landgraf, D.; Leinweber, P.; Makeschin, F. Cold and hot water extractable organic matter as indicators of litter decomposition in forest soils. J. Plant Nutr. Soil Sc. 2006, 169, 76–82. [Google Scholar] [CrossRef]
- Chen, H.; Hou, R.; Gong, Y.; Li, H.; Fan, M.; Kuzyakov, Y. Effects of 11 years of conservation tillage on soil organic matter fractions in wheat monoculture in Loess Plateau of China. Soil Tillage Res. 2009, 106, 85–94. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fert. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An under-ground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Hazra, K.K.; Venkatesh, M.S.; Nath, C.P.; Singh, J.; Nadarajan, N. Increasing soil organic carbon through crop diversification in cereal–cereal rotations of Indo-Gangetic Plain. Proc. Nat. Acad. Sci. India Sec. B Biol. Sci. 2019, 89, 429–440. [Google Scholar] [CrossRef]
- García-Orenes, F.; Roldán, A.; Morugán-Coronado, A.; Linares, C.; Cerdà, A.; Caravaca, F. Organic fertilization in traditional Mediterranean grapevine orchards mediates changes in soil microbial community structure and enhances soil fertility. Land Degrad. Dev. 2016, 27, 1622–1628. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports No.103; FAO: Rome, Italy, 2007. [Google Scholar]
- Polish Society of Soil Science. Particle Size Distribution and Textural Classes of Soils and Mineral Materials—Classification of Polish Society of Soil Science; (PTG in Polish); Soil Science Annual, PTG: Warsaw, Poland, 2009; Volume 60, pp. 5–16. [Google Scholar]
- Kuś, J.; Jończyk, K. Influence of organic and conventional crop production system on some parameters of soil fertility. J. Res Appl Agric Eng. 2008, 53, 161–165. [Google Scholar]
- Gajda, A.M.; Czyż, E.A.; Dexter, A.R. Effect of long-term use of different farming systems on some physical, chemical and microbiological properties of soil quality. Int. Agrophys. 2016, 30, 165–172. [Google Scholar] [CrossRef]
- PN-ISO 11277: Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation; Polish Committee for Standardization: Warsaw, Poland, 2020.
- ISO 17892-3: Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 3: Determination of Particle Density; ISO Central Secretariat: Geneva, Switzerland, 2015.
- ISO 10390: International Standardization Organization (ISO), 2005. Soil Quality—Determination of pH; ISO: Geneva, Switzerland, 2020.
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- PN-R-04028:1997. Chemical-agricultural Analysis of Soil—Sampling Method and Determination of Nitrate and Ammonium Ions in Mineral Soils; Polish Committee for Standardization: Warsaw, Poland, 1997. (In Polish)
- PN-ISO 14235: 2003. Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation; Polish Committee for Standardization: Warsaw, Poland, 2003.
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Estimation of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1991; pp. 59–61. (In Polish) [Google Scholar]
- Gajda, A.M.; Doran, J.W.; Kettler, T.A.; Wienhold, B.J.; Pikul, J.L., Jr.; Cambardella, C.A. Soil quality evaluations of alternative conventional management systems in the great plains. In Assessment Methods for Soil Carbon; Lal, R., Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 381–400. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and gypsum. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- ISO 12782-4: Soil Quality—Parameters for Geochemical Modelling of Leaching and Speciation of Constituents in Soils and Materials—Part 4: Extraction of Humic Substances from Solid Samples; ISO Central Secretariat: Geneva, Switzerland, 2012.
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis, Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar]
- Haynes, R.J.; Francis, G.S. Changes in microbial biomass C, soil carbohydrate composition and aggregate stability induced by growth of selected crop and forage species under field conditions. J. Soil Sc. 1993, 44, 665–675. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. In Soil Biochemistry; Paul, E.A., Ladd, J.N., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1981; pp. 415–471. [Google Scholar]
- PN-EN ISO 23753-1: Determination of Dehydrogenase Activity in Soil—Part 1: Method Using 2,3,5-triphenyltetrazolium Chloride (TTC); ISO: Geneva, Switzerland, 2011.
- Martinez, E.; Fuentes, J.P.; Silva, P.; Valle, S.; Acevedo, E. Soil physical properties and wheat root growth as affected by no-tillage and conventional tillage systems in a Mediterranean environment of Chile. Soil Tillage Res. 2008, 99, 232–244. [Google Scholar] [CrossRef]
- Głąb, T.; Kulig, B. Effect of mulch and tillage on soil porosity under wheat (Triticum aestivum L.). Soil Till. Res. 2008, 99, 169–178. [Google Scholar] [CrossRef]
- Pagliai, M.; Vignozzi, N. The soil pore system as an indicator of soil quality. Adv. Geoecol. 2002, 35, 69–80. [Google Scholar]
- Paluszek, J. Criteria for assessing the physical quality of Poland’s arable soils. In Acta Agrophys; Institute of Agrophysics, Polish Academy of Sciences: Lublin, Poland, 2011; pp. 1–138. (In Polish) [Google Scholar]
- Sarkar, I.; Zulfikar Khan, M.Z.; Hanif, M. Soil organic fractions in cultivated and uncultivated soils of Costal area in Bangladesh. J. Agric. Chem. Environ 2019, 8, 129–144. [Google Scholar] [CrossRef]
- Souza, G.P.; Figueiredo, C.C.; Sousa, D.M.G. Relationships between labile soil organic carbon fractions under different soil management systems. Sci. Agric. 2016, 73, 535–542. [Google Scholar] [CrossRef]
- Alvaro-Fuentes, J.; López, M.V.; Cantero-Martínez, C.; Arrúe, J.L. Tillage effects on soil organic carbon fractions in Mediterranean dryland agroecosystems. Soil Sci. Soc Am. J. 2008, 72, 541–547. [Google Scholar] [CrossRef]
- Sequeira, C.H.; Alley, M.M.; Jones, B.P. Evaluation of potentially labile soil organic carbon and nitrogen fractionation procedures. Soil Biol. Biochem. 2011, 43, 438–444. [Google Scholar] [CrossRef]
- Sun, C.Y.; Liu, J.S.; Wang, Y.; Zheng, N.; Wu, Q.X.; Liu, Q. Effect of long-term cultivation on soil organic carbon fraction and metal distribution in humic and fulvic acid in black soil, Northeast China. Soil Res. 2012, 50, 562–569. [Google Scholar] [CrossRef]
- Seddaiu, G.; Porcu, G.; Ledda, L.; Roggero, P.; Agnelli, A.; Corti, G. Soil organic matter content and composition as influenced by soil management in a semi-arid Mediterranean agro-silvo-pastoral system. Agr. Ecosyst. Environ. 2013, 167, 1–11. [Google Scholar] [CrossRef]
- Liu, H. Relationship between organic matter humification and bioavailability of sludge- borne copper and calcium during long-term sludge amendment to soil. Sci. Total Environ. 2016, 566–567, 8–14. [Google Scholar] [CrossRef]
- You, M.; Burger, M.; Li, L.; Zou, W.; Li, N.; Qiao, Y.; Han, X. Changes in soil organic carbon and carbon fractions under different land use and management practices after development from parent material of Mollisols. Soil Sci. 2014, 179, 205–210. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; Silva, T.O.; Silva, T.L.; Dias, N.; Matias, M.I. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Tillage Res. 2013, 126, 177–182. [Google Scholar]
- Moraes, G.M.; Xavier, F.A.; Mendonça, E.S.; Araujo Filho, J.A.; Oliveira, T.S. Chemical and structural characterization of soil humic substances under agroforestry and conventional systems. Brazilian J. of Soil Sci. 2011, 35, 1597–1608. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment. Principles and Controversies; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 1–495. [Google Scholar]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-term effects of no-tillage management practice on soil organic carbon and its fractions in the northern China. Geoderma 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Yang, X.; Sun, B. Effects of soil management regimes on biochemical properties of a loess soil. J. Soil Sci. Plant Nutr. 2015, 15, 711–725. [Google Scholar] [CrossRef]
- Haynes, R.J. Soil organic matter quality and the size and activity of the microbial biomass: Their significance to the quality of agricultural soils. In Soil Mineral Microbe-organic Interactions; Huang, Q., Huang, P.M., Violante, A., Eds.; Springer: Heidelberg, Germany, 2008; pp. 201–231. [Google Scholar]
- Yang, X.; Ren, W.; Sun, B.; Shulan Zhang, S. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177–178, 49–56. [Google Scholar] [CrossRef]
- Lopes, M.M.; Salviano, A.A.C.; Araujo, A.S.F.; Nunes, L.A.P.L.; Oliveira, M.E. Changes in soil microbial biomass and activity in different Brazilian pastures. Span. J. Agric. Res. 2010, 8, 1253–1259. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 107–124. [Google Scholar]
- Stamenov, D.; Durić, S.; Hajnal Jafari, T.; Ćirić, V.; Manojlović, M. Microbiological activity in the soil of various agricultural crops in organic production. Contemp. Agricult. 2018, 67, 34–39. [Google Scholar] [CrossRef]
- Bergstrom, D.W.; Monreal, C.M.; Tomlin, A.D.; Miller, J.J. Interpretation of soil enzyme activities in a comparison of tillage practices along a topographic and textural gradient. Can. J. Soil Sci. 2000, 80, 71–79. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Gallardo-Lancho, J.F. Organic matter properties in cultivated versus set-aside arable soils. Agric. Ecosyst. Environ. 1998, 67, 267–274. [Google Scholar] [CrossRef]
- Caravaca, F.; Masciandaro, G.; Ceccanti, B. Land use in relation to soil chemical and biochemical properties in a semiarid Mediterranean environment. Soil Tillage Res. 2002, 68, 23–30. [Google Scholar] [CrossRef]

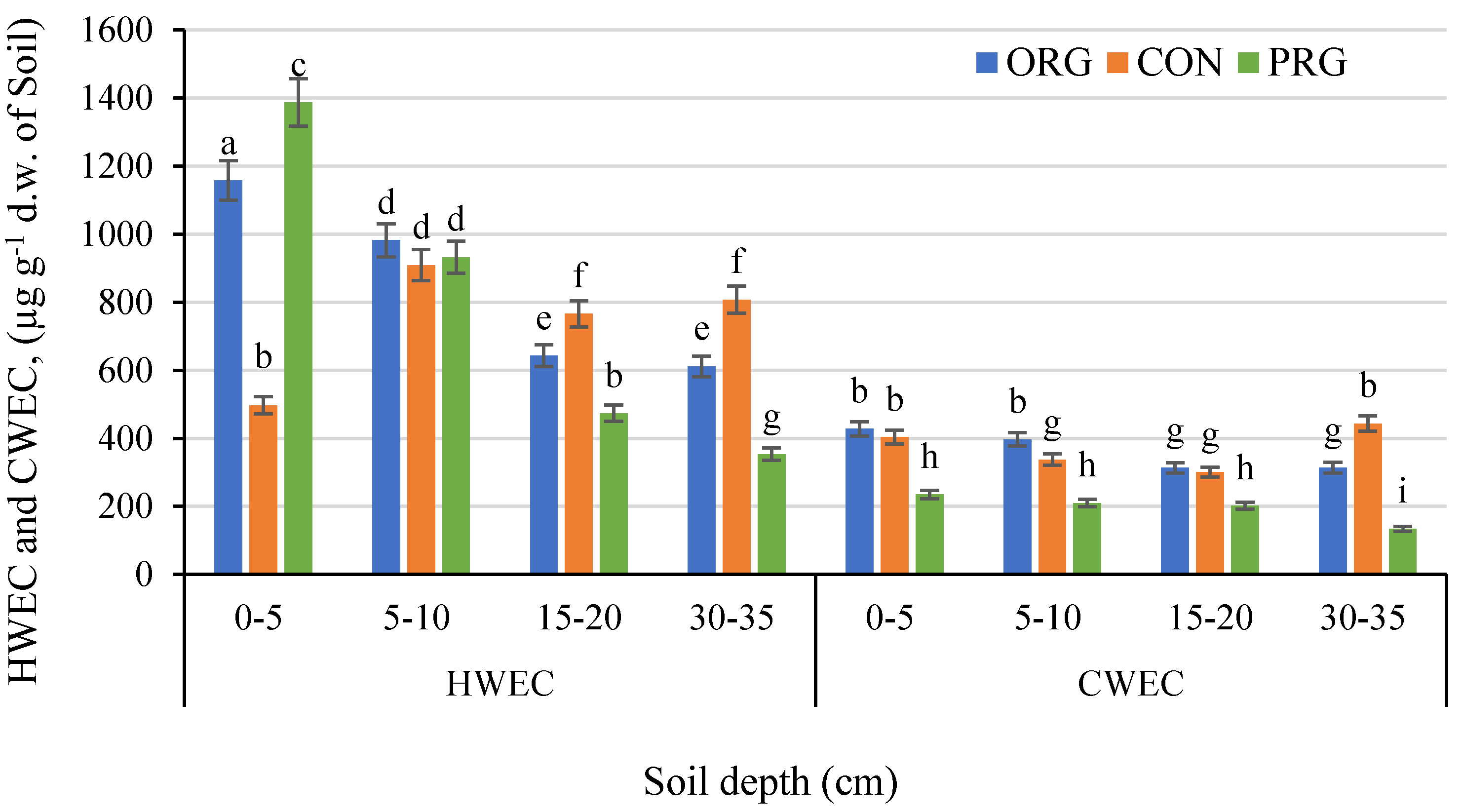
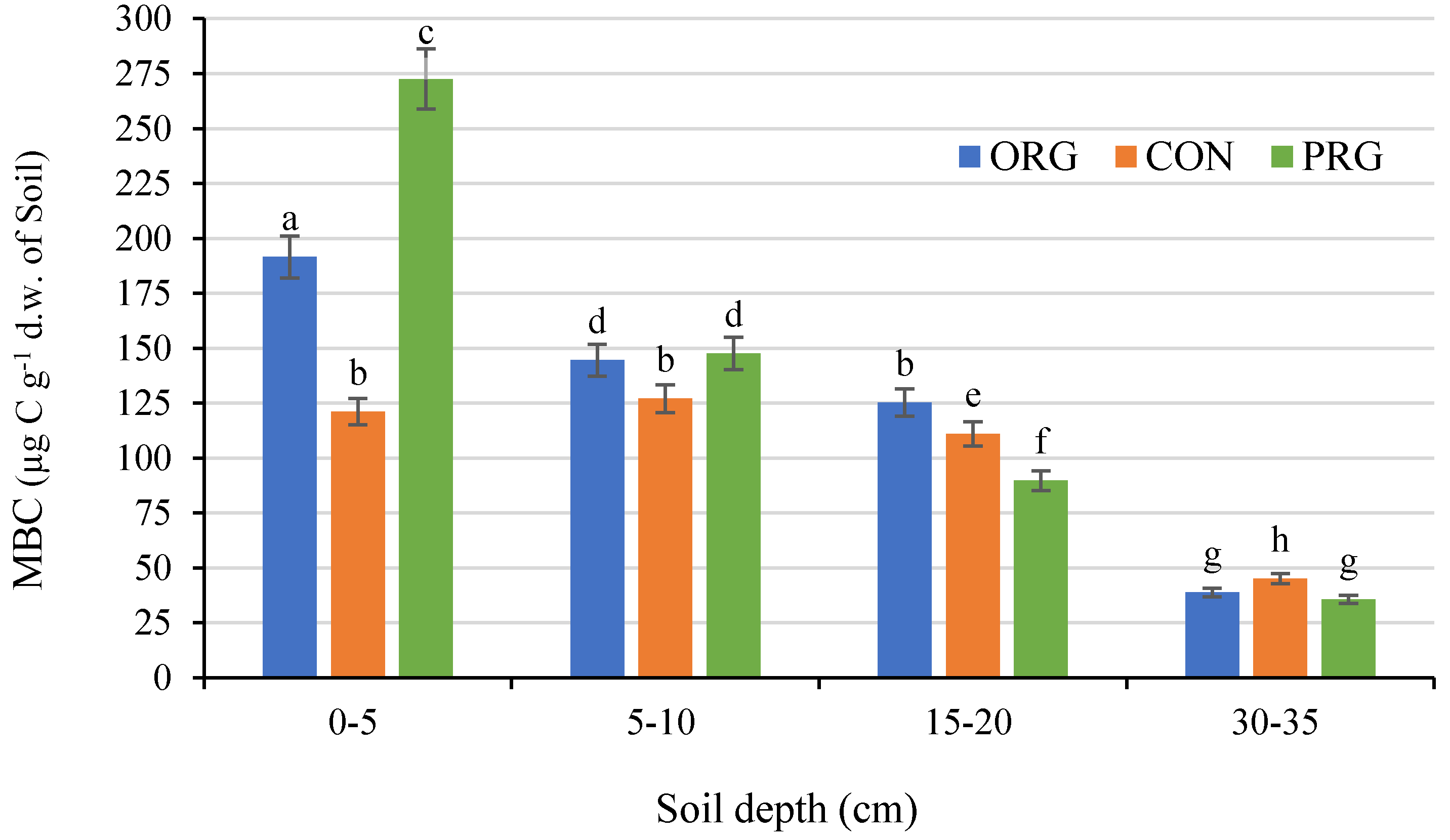
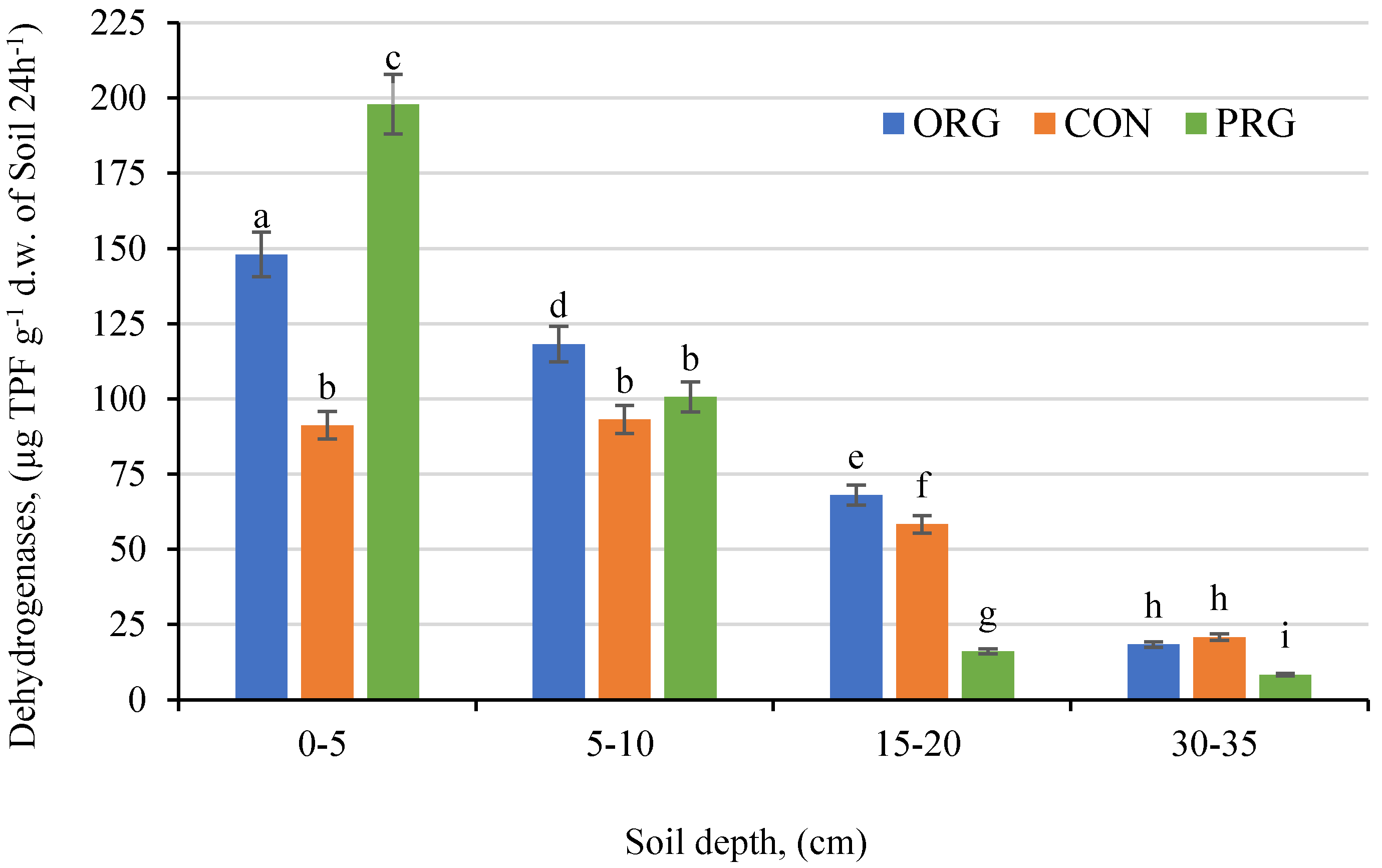
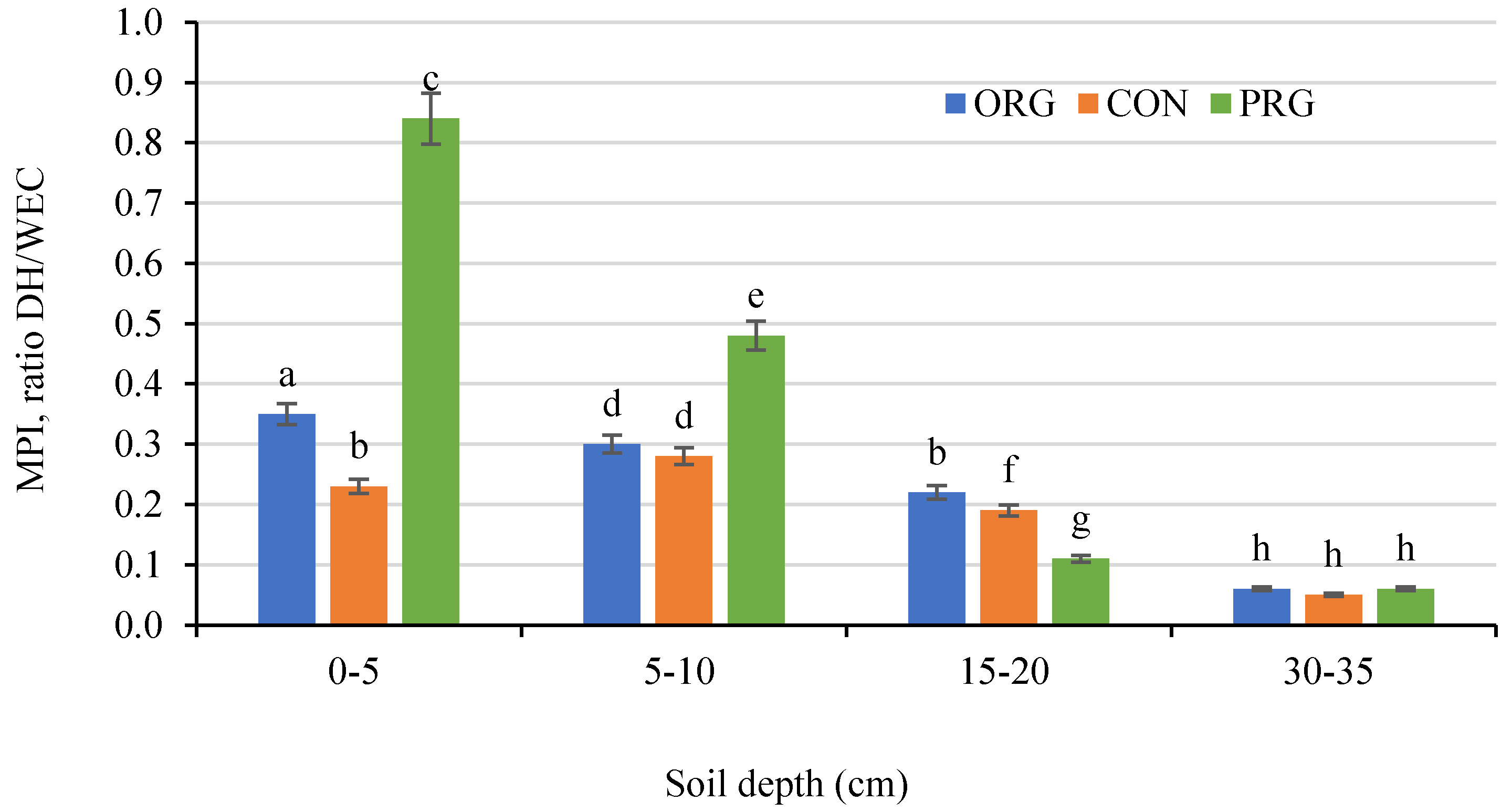
| Crop Production System | OM | PEgner | KEgner | MgSchatschstshabel | Nmin. | |
|---|---|---|---|---|---|---|
| pH KCl | (g kg−1 of Soil) | (mg kg−1 of Soil) | (kg ha−1) | |||
| ORG | 5.8 a | 17.7 a | 67 a | 130 a | 80 a | 60.5 a |
| CON | 6.2 a | 14.1 b | 89 b | 191 b | 33 b | 48.7 b |
| PRG | 4.6 b | 18.3 a | 72 c | 133 a | 38 b | 59.2 a |
| HSD (0.05) | 0.7 | 0.5 | 4.1 | 35.1 | 5.2 | 10.5 |
| HSD (0.05) For Years—Significant Differences | ||||||
| HSD (0.05) For Interaction (Organic System × Conventional System × Permanent Grass)—Significant Differences | ||||||
| HSD (0.05) For Interaction (Organic System × Conventional System × Permanent Grass × Years)—Mot Significant Differences | ||||||
| Management | Crop Production Systems | Permanent Grass (PRG) | |
|---|---|---|---|
| Organic (ORG) | Conventional (CON) | ||
| Soil tillage | Mouldboard Ploughing | Mouldboard Ploughing | 0 |
| Crop Rotation | Potato Spring wheat + Undersown Clover and Grass—1st year Clover and Grass—2nd year Winter Wheat + Catch Crop Mustard | Winter Rape Winter Wheat Spring Barley | Grass |
| Organic Fertilization | Under Potato Compost (30 t·ha−1) + Catch Crop | Winter Rape Straw Winter Wheat Straw | 0 |
| Mineral Fertilization (kg·ha−1) | Natural P and K Fertilizers: | ||
| N | 0 | 140 | 120 |
| P2O5 | 42 | 60 | 50 |
| K2O | 75 | 80 | 60 |
| Weed Control | Weeder Harrow 2–3 × | Herbicides 2–3 × | Herbicides 1 × |
| Retardants | 0 | 2 × | 0 |
| Fungicides | 0 | 2–3 × | 0 |
| Month | 1984–2014 | 2015/2016 | 2016/2017 | 2017/2018 |
|---|---|---|---|---|
| IX | 13.5 | 15.3 | 15.6 | 14.1 |
| X | 8.3 | 7.0 | 7.7 | 9.5 |
| XI | 3.5 | 5.2 | 3.2 | 4.6 |
| XII | −0.4 | 4.0 | 0.8 | 2.5 |
| I | −1.7 | −3.4 | −4.6 | 0.4 |
| II | −0.5 | 3.7 | −1.2 | −3.4 |
| III | 3.0 | 4.3 | 6.0 | 0.4 |
| IV | 8.8 | 9.6 | 7.6 | 13.6 |
| V | 14.1 | 15.5 | 13.6 | 17.2 |
| VI | 17.2 | 19.8 | 18.1 | 18.8 |
| VII | 19.4 | 20.0 | 18.6 | 20.7 |
| VIII | 18.6 | 18.7 | 19.6 | 20.7 |
| t | 8.7 a | 10.0 | 8.8 | 9.9 |
| d | - | +1.3 | +0.1 | +1.2 |
| Month | 1984–2014 | 2015/2016 | 2016/2017 | 2017/2018 |
|---|---|---|---|---|
| IX | 58 | 126 | 21 | 105 |
| X | 41 | 30 | 100.0 | 95 |
| XI | 39 | 47 | 45.0 | 54 |
| XII | 31 | 25 | 65 | 20 |
| I | 27 | 33 | 30.2 | 17 |
| II | 27 | 65 | 44.1 | 17 |
| III | 33 | 53 | 32.0 | 31 |
| IV | 40 | 38 | 65 | 30 |
| V | 59 | 72 | 62 | 59 |
| VI | 62 | 28 | 31 | 38 |
| VII | 83 | 87 | 105 | 122 |
| VIII | 68 | 42 | 96 | 28 |
| r | 568 b | 646 | 696 | 616 |
| d | - | +78 | +131 | +48 |
| Crop Production Systems | Humification Index [HI = HA/FA] | ||||
|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 15–20 cm | 30–35 cm | 0–35 cm | |
| ORG | 5.4 a | 5.1 a | 4.9 a | 4.1 a | 4.9 a |
| CON | 3.8 b | 4.9 a | 4.6 a | 3.9 a | 4.3 b |
| Transformation Degree of HS (DT = (HA + FA/HN)) | |||||
| 0–5 cm | 5–10 cm | 15–20 cm | 30–35 cm | 0–35 cm | |
| ORG | 3.1 a | 2.6 a | 3.6 a | 7.7 a | 4.2 a |
| CON | 3.0 a | 4.4 b | 4.1 b | 6.1 b | 4.4 a |
| Related Parameter | Correlation Coefficient | ||
|---|---|---|---|
| Crop Production Systems | Permanent Grass (PRG) | ||
| Organic (ORG) | Conventional (CON) | ||
| TP | 0.915 ** | 0.889 * | 0.984 ** |
| POM | 0.934 ** | 0.910 ** | 0.942 ** |
| FA | 0.930 ** | 0.984 ** | n/a |
| HA | 0.919 ** | 0.7979 * | n/a |
| HN | 0.981 ** | 0.836 * | n/a |
| HS | 0.983 ** | 0.990 ** | n/a |
| HWEC | 0.962 ** | 0.826 * | 0.969 ** |
| CWEC | 0.819 * | 0.792 * | 0.878 * |
| MBC | 0.969 ** | 0.943 ** | 0.939 ** |
| DH | 0.964 ** | 0.793 * | 0.945 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajda, A.M.; Czyż, E.A.; Ukalska-Jaruga, A. Comparison of the Effects of Different Crop Production Systems on Soil Physico-Chemical Properties and Microbial Activity under Winter Wheat. Agronomy 2020, 10, 1130. https://doi.org/10.3390/agronomy10081130
Gajda AM, Czyż EA, Ukalska-Jaruga A. Comparison of the Effects of Different Crop Production Systems on Soil Physico-Chemical Properties and Microbial Activity under Winter Wheat. Agronomy. 2020; 10(8):1130. https://doi.org/10.3390/agronomy10081130
Chicago/Turabian StyleGajda, Anna Maria, Ewa Antonina Czyż, and Aleksandra Ukalska-Jaruga. 2020. "Comparison of the Effects of Different Crop Production Systems on Soil Physico-Chemical Properties and Microbial Activity under Winter Wheat" Agronomy 10, no. 8: 1130. https://doi.org/10.3390/agronomy10081130
APA StyleGajda, A. M., Czyż, E. A., & Ukalska-Jaruga, A. (2020). Comparison of the Effects of Different Crop Production Systems on Soil Physico-Chemical Properties and Microbial Activity under Winter Wheat. Agronomy, 10(8), 1130. https://doi.org/10.3390/agronomy10081130






